Abstract
Background:
Tetracycline (TET) has been found to have both antibiotic and anti-inflammatory properties. The anti-inflammatory effect of topical TET on atopic dermatitis (AD) has not been reported. The purpose of this study was to explore the potential role of topical TET and its anti-inflammatory effects in a mouse model of AD.
Methods:
The 2% TET was applied topically to ears of MC903-induced AD-like BALB/c mice once a day. AD-like symptoms and severity were evaluated by assessing skin scoring of dermatitis, ear thickness, and frequency of scratching. Serum IgE and thymic stromal lymphopoietin (TSLP) levels were measured by enzyme-linked immunosorbent assay. Western blot was used for analyzing the expressions of TSLP, protease-activated receptor 2 (PAR2), and nuclear factor-kappa B (NF-κB) in skin lesions. Real-time polymerase chain reaction was performed to assess the mRNA levels of TSLP and inflammatory cytokines including interleukin (IL)-4, IL-13, tumor necrosis factor (TNF)-α, and IL-1β in skin lesions.
Results:
Scoring of dermatitis (9.00 ± 0.63 vs. 6.67 ± 1.03, P = 0.001), ear thickness (0.44 ± 0.02 mm vs. 0.40 ± 0.03 mm, P = 0.018), and serum IgE level (421.06 ± 212.13 pg/ml vs. 244.15 ± 121.39 pg/ml, P = 0.047) were all improved in the 2% TET treatment group compared with AD group. Topical TET significantly reduced the serum level of TSLP (119.04 ± 38.92 pg/ml vs. 65.95 ± 54.61 pg/ml, P = 0.011) and both mRNA and protein expressions of TSLP in skin lesions compared with AD group (P = 0.003 and 0.011, respectively), and NF-κB and PAR2 expression in skin lesions were also suppressed (P = 0.016 and 0.040, respectively). Furthermore, expressions of inflammatory cytokines IL-4, IL-13, and TNF-α in skin lesions were down-regulated in 2% TET group compared with AD group (P = 0.035, 0.008, and 0.044, respectively).
Conclusions:
Topical TET exerted anti-inflammatory effects through suppression of TSLP and inflammatory cytokines in AD mouse model, suggesting TET as a potential agent for the topical treatment of AD in the future.
Keywords: Atopic Dermatitis, Tetracycline, Thymic Stromal Lymphopoietin
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease with severe pruritus. It usually starts in early infancy and lasts to affect children and adults, with a prevalence of 3–10%.[1,2] Patients with AD are likely to develop other allergic diseases such as allergic rhinitis and/or asthma.[3]
It is well known that T helper 2 (Th2) predominance plays a critical role in inflammatory progress in AD, while thymic stromal lymphopoietin (TSLP) has recently been considered as a key molecule involved in the development of AD and even atopic march. TSLP has been found overexpressed in patients with acute and chronic AD and in mouse AD models[4,5] and it could also induce downstream dendritic cell (DC)-derived Th2-type response in skin inflammation, with increased interleukin (IL)-4, IL-13, and tumor necrosis factor (TNF)-α mRNA expressions.[6] Several clinical trials targeting TSLP have been performed for the treatment of AD.[6,7]
Although topical glucocorticosteroid and calcineurin inhibitors are mainstay treatments for AD, they are sometimes associated with adverse effects. Therefore, safe and effective treatment is needed. Tetracyclines (TETs) have been demonstrated to present exceptional antimicrobial properties, such as anti-inflammation, proteolysis inhibition, and anti-angiogenesis properties. TETs have been successfully used in the treatment of some inflammatory skin diseases.[8] Recently, topical TET has also been tested to help to treat asthma and other atopic diseases.[9] However, the molecular mechanism of TET in inflammatory diseases has not been fully elucidated. It has been reported that activated protease-activated receptor 2 (PAR2) could induce the expression of TSLP,[10] while TET could modulate PAR2 mediated inflammatory response in keratinocytes.[11] This study aimed to evaluate the therapeutic effects of topical TET using an AD mouse model and to investigate its effect on TSLP expression.
METHODS
Animals
BALB/c mice weighing 15–18 g (6–8 weeks old, female) were purchased from Vital River Laboratory Animal Company (Beijing, China). All mice were healthy and housed in a specific pathogen-free environment in 12 h light-dark cycle. The mice were fed with a laboratory diet and water. Animal experiments were strictly performed in accordance with the Guide for Care and Use of Laboratory Animals and were approved by the Ethics Committee of Peking University People's Hospital, Beijing, China (Permit number: 2013-74).
Atopic dermatitis mouse models and treatment
A total of 18 mice were randomly divided into three groups: naïve control (NC) group, AD group, and 2% TET treatment group, with 6 animals per group. The AD mouse model was established by repeated topical application of MC903 (Cayman, MI, USA), according to a published protocol.[12] Briefly, 2 nmol MC903 (20 μl, dissolved in ethanol) was topically applied to dorsal side of each ear in AD group and 2% TET group once a day for 14 days (4 nmol in total per day), while NC group received 20 μl ethanol on the dorsal side of each ear as solvent control.[12,13] After AD models were successfully induced (day 0 was defined), MC903 was still applied to both AD group and 2% TET group in the morning. In the afternoon, 2% TET group received 20 μl 2% TET topically (dissolved in ethanol, Sigma-Aldrich, MA, USA), while AD group received 20 μl solvent topically for the following 14 days.
Scoring of dermatitis, ear thickness measurement, and frequency of scratching
On the day 0, 1, 4, 7, 10, and 14, the severity of AD, including scoring of dermatitis, ear thickness measurement, and scratching behavioral test were performed in each group. Scoring of dermatitis with a total of 16 scores described by Matsuda et al.[14] was performed with a minor modification, including erythema/hemorrhage (0–4), scale/dryness (0–4), edema (0–4), and excoriation/erosion (0–4). The scores were classified as 0 (none), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe). The ear thickness (mm) was evaluated in the same region of the ear using vernier caliper. Frequency of scratching was observed by two investigators alternately with counting scratching numbers within 10 min in the morning before application of MC903.
Sample collection
On day 14, all mice were sacrificed to collect blood samples. Serum was collected after centrifugation and stored at −80°C. The left and right ears were obtained and then divided into three parts. Two parts were stored at −80°C immediately for extracting total RNA and protein separately, and the rest was fixed with 4% paraformaldehyde for histopathology and immunohistochemistry study.
Evaluation of serum IgE and thymic stromal lymphopoietin levels
Serum total IgE and TSLP levels were measured using mouse serum enzyme-linked immunosorbent assay Ready-Set-Go! Kits (eBioscience, CA, USA) according to the manufacturer's protocols.
Histopathological examination and immunohistochemistry
The fixed ear tissues were embedded in paraffin and cut into 5 μm thin sections, stained with hematoxylin and eosin. For immunohistochemistry, the section slices were washed in phosphate buffer saline and then boiled in 0.01 mmol citrate buffer (pH = 6.0) for antigen retrieval, followed by 3% hydrogen peroxide incubation and 5% bovine serum albumin blockade. Then, the sections were incubated with mouse anti-TSLP antibody (1:200, Abcam, Cambridge, UK) overnight at 4°C. Isotype antibody (rabbit polyclonal anti-IgG, Abcam, Cambridge, UK) was applied as a negative control. After incubated with secondary antibody, diaminobenzidine/hematoxylin staining results were observed under the microscopy (Leica, Solms, Germany). The images were recorded for further analysis.
Western blot analysis
A small piece of ear tissue was lysed with RIPA Lysis Buffer (Solarbio, Beijing, China) using Qiagen TissueLyser II (Qiagen, HK, China) with shaking at 20 Hz for 5 min and standing on ice for 5 min. The aforementioned procedure was repeated twice. Then, the lysates were centrifuged at 13,000 ×g for 10 min. Concentrations of total protein were determined using a bicinchoninic acid kit (Solarbio). Subsequently, an equal amount of protein sample was loaded in each well and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for electrophoresis and then transferred onto polyvinylidene difluoride membrane. After blocking with 5% skim milk, the membrane was incubated overnight at 4°C with the following primary antibodies: mouse anti-TSLP (1:1000, Abcam), mouse anti-nuclear factor-kappa B (NF-κB) p65 (1:1000, Cell Signaling Technology, MA, USA), and mouse anti-PAR2 (1:1000, Abcam); anti-β-actin antibody was used as a loading control. The other day, the membrane was incubated with corresponding secondary antibodies after washing. Finally, specific bands were observed by ImageQuant 350 (GE, MA, USA) with standard chemiluminescence (Thermo, IL, USA). The band intensity was calculated by ImageJ software (National Institutes of Health, MD, USA) compared with β-actin.
RNA isolation and real-time polymerase chain reaction analysis
The ear tissue was put into 300 μl buffer RLT (Qiagen RNeasy Fibrous Tissue Mini Kit, CA, USA) and homogenized using Qiagen TissueLyser II (Qiagen, CA, USA) at 20 Hz shaking for 5 min twice. Total RNA was extracted from the homogenized tissue. RNA quality and quantity were then determined with a NanoVue plus spectrophotometer (GE, MA, USA). The cDNA was synthesized in 20 μl reaction systems with 1000 ng RNA using a reverse transcription kit (Takara, Shiga, Japan). Real-time polymerase chain reaction (PCR) was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA) by a SYBR Premix Ex Taq Kit (Takara, Shiga, Japan). Expression data were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase. The primer sequences (Invitrogen, CA, USA) are summarized in Table 1.
Table 1.
Primer set list for mRNAs
| Gene name | Primer sequence |
|---|---|
| GAPDH | Forward: 5’-GGTGAAGGTCGGTGTGAACG-3’ Reverse: 5’-CTCGCTCCTGGAAGATGGTG-3’ |
| TSLP | Forward: 5’-ATCGAGGACTGTGAGAGCAAGCCAG-3’ Reverse: 5’-GTGAAGGGCAGCCAGGGATAGGA-3’ |
| IL-4 | Forward: 5’-CCCCCAGCTAGTTGTCATCC-3’ Reverse: 5’-AGGACGTTTGGCACATCCAT-3’ |
| IL-13 | Forward: 5’-TGCCATCTACAGGACCCAGA-3’ Reverse: 5’-CTCATTAGAAGGGGCCGTGG-3’ |
| TNF-α | Forward: 5’-CCCACGTCGTAGCAAACCAC-3’ Reverse: 5’-GCAGCCTTGTCCCTTGAAGA-3 |
| IL-1β | Forward: 5’-TGCCACCTTTTGACAGTGATG-3’ Reverse: 5’-AAGGTCCACGGGAAAGACAC-3’ |
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; TSLP: Thymic stromal lymphopoietin; IL: Interleukin; TNF-α: Tumor necrosis factor-α.
Shuttle PCR Fast protocol included holding stage at 95°C for 30 s, followed by cycling stage with 40 cycles of amplification at 95°C for 3 s and 60°C for 30 s. Relative expression ratio was measured using the comparative threshold cycle (Ct) and 2−ΔΔCt method.
Statistical analysis
Data were expressed as a mean ± standard error of mean. SPSS version 19.0 (IBM Corp., NY, USA) was used for statistical analysis. The comparisons between two groups were analyzed using Student's t-test. One-way analysis of variance was used to determine statistical differences among three groups. A P < 0.05 was considered statistically significant. GraphPad Prism 5 (GraphPad Software Inc., CA, USA) was used to plot the graphs.
RESULTS
Topical tetracycline attenuated skin inflammation in MC903-induced atopic dermatitis mouse model
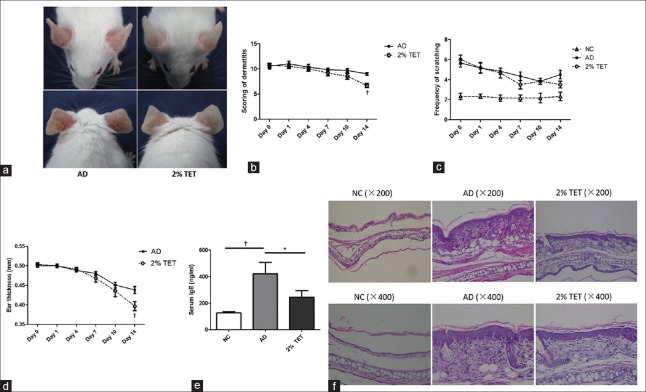
After topical application of MC903 for 2 weeks, the ears of the mice became red, scaly, swollen, and crusted. Those symptoms, including erythema, swelling, and scale, were significantly ameliorated following the topical treatment of 2% TET for 14 days [Figure 1a]. Compared with AD group, the total scoring of dermatitis and the ear thickness in 2% TET group decreased with time, and these changes decreased significantly at day 14 (P = 0.001 and 0.018, respectively) [Figure 1b and 1c]. The frequency of scratching also decreased after the topical treatment of 2% TET, while there was no significant difference (P = 0.098) [Figure 1d]. Serum IgE levels was also significantly decreased in 2% TET group compared with AD group (P = 0.047) [Figure 1e]. Moreover, application of 2% TET alleviated epidermal and dermal thickening and hyperkeratosis induced by MC903 compared with AD group [Figure 1f].
Figure 1.
Topical application of 2% TET inhibited AD-like symptoms induced by MC903 in BALB/c mice. (a) The ears of mice in AD group (left) and 2% TET group (right) at day 14. (b–d) Scoring of dermatitis (b), frequency of scratching (c), and ear thickness (d) at days 0, 1, 4, 7, 10, and 14. (e) Serum IgE levels in NC group, AD group, and 2% TET group. (f) Histopathology of the skin lesions was stained with H and E staining. Data were expressed as a mean ± SEM (*P < 0.05, †P < 0.01). NC: Naïve control; AD: Atopic dermatitis; TET: Tetracycline; SEM: Standard error of mean.
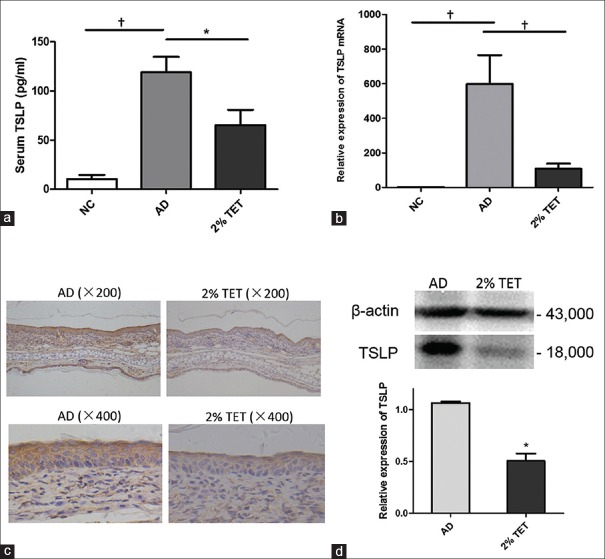
Topical tetracycline decreased serum thymic stromal lymphopoietin level and down-regulated thymic stromal lymphopoietin expression in skin lesions
As TSLP is the key biomarker of AD, we investigated the effect of TET on TSLP production in AD mouse models. Compared with NC group, serum TSLP level and lesional TSLP mRNA expression were significantly elevated in AD group (P < 0.001 and P = 0.001, respectively) [Figure 2a and 2b]. Following topically treated with 2% TET, the serum TSLP level was reduced significantly compared with AD group (P = 0.011) [Figure 2a]. Moreover, 2% TET strongly inhibited TSLP mRNA and protein expression in skin lesions compared with AD group (P = 0.003 and 0.011, respectively) [Figure 2b and 2d]. Compared with the AD group, TSLP expression in the epidermis was also attenuated following treatment with 2% TET [Figure 2c].
Figure 2.
Topical application of 2% TET suppressed TSLP expression. (a) Serum TSLP levels in NC group, AD group, and 2% TET group (n = 6 in each group). (b) Relative mRNA expression of TSLP in different groups and results were normalized to the expression of GAPDH (n = 6 in each group). (c) TSLP expression in AD lesion (left) and in 2% TET treatment lesion (right). (d) Expression of TSLP protein in the AD group and 2% TET group (western blot). The results were normalized to the expression of β-actin expression (n = 3 in each group). Data were expressed as a mean ± SEM (*P < 0.05, †P < 0.01). TSLP: Thymic stromal lymphopoietin; NC: Naïve control; AD: Atopic dermatitis; TET: Tetracycline; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; SEM: Standard error of mean.
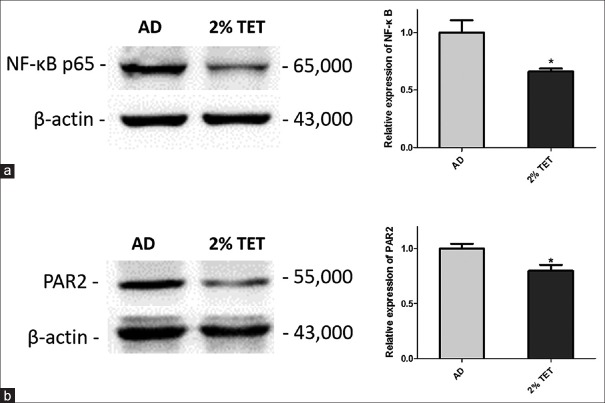
Topical tetracycline suppressed the expressions of nuclear factor-kappa B and protease-activated receptor 2 in skin lesions of atopic dermatitis mouse models
As NF-κB and PAR2 are associated with TSLP in skin inflammation, we further investigated the expressions of NF-κB and PAR2 in skin lesions by Western blot. As shown in Figure 3, PAR2 and NF-κB expression were both significantly inhibited in 2% TET group compared with AD group (P = 0.016 and 0.040, respectively).
Figure 3.
Downregulation of PAR2 and NF-κB by topical application of 2% TET. Relative expressions of PAR2 protein (a) and NF-κB protein (b) in AD group and 2% TET group (Western blot). The results were normalized to the expression of β-actin expression (n = 3 in each group). Data were expressed as a mean ± SEM (*P < 0.05). NC: Naïve control; AD: Atopic dermatitis; TET: Tetracycline; NF-κB: Nuclear factor-kappa B; PAR2: Protease-activated receptor 2; SEM: Standard error of mean.
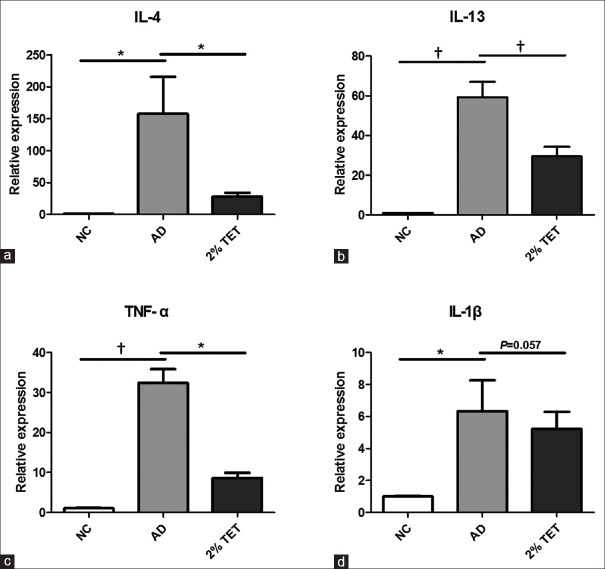
Topical tetracycline treatment inhibited inflammatory cytokines expression in skin lesions
As inflammatory cytokines play a critical role in the pathogenesis of AD, we detected the expressions of IL-4, IL-13, TNF-α, and IL-1β in skin lesions. As shown in Figure 4, relative mRNA expressions of IL-4, IL-13, TNF-α, and IL-1β were significantly up-regulated in AD group, compared with NC group (P = 0.017, P < 0.001, P < 0.001, and P = 0.025, respectively). The 2% TET significantly decreased the mRNA levels of IL-4, IL-13, and TNF-α (P = 0.035, 0.008, and 0.044, respectively) [Figure 4a–4c]. IL-1β mRNA level was also down-regulated in 2% TET group, although there was no significant difference (P = 0.057) [Figure 4d].
Figure 4.
The mRNA expressions of IL-4, IL-13, TNF-α, and IL-1β were inhibited by topical application of 2% TET in skin lesions. (a–d) Relative mRNA expression of IL-4 (a), IL-13 (b), TNF-α (c), and IL-1β (d) in NC group, AD group, and 2% TET group. The results were normalized to the expression of GAPDH. Data were expressed as a mean ± SEM (*P < 0.05, †P < 0.01). NC: Naïve control; AD: Atopic dermatitis; TET: Tetracycline; IL: Interleukin; TNF-α: Tumor necrosis factor-α; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; SEM: Standard error of mean.
DISCUSSION
In this study, topical application of TET could alleviate the AD-like symptoms by improving erythema, scale, ear swelling, ear thickness, and scratching behavior as well as decreasing the elevated serum IgE level. Moreover, topical TET also suppressed serum TSLP level and inhibited the mRNA and protein expressions of TSLP through PAR2-NF-κB pathway in skin lesions.
TET and its derivatives have been an emerging treatment for various inflammatory skin diseases such as acne, rosea, autoimmune bullous disease, neutrophil diseases, and perioral dermatitis[8,15] due to their effects of inhibiting production of IL-1 and TNF-α, inflammatory cell trafficking, and lymphocyte proliferation.[16] Recently, TET has also been used to treat allergic diseases like asthma.[17,18] However, TET treatment in AD is limited because most Staphylococcus aureus and streptococcus are resistant to TET. Meanwhile, systematic TET has some side effects. Therefore, TET has been an alternative anti-infectious treatment in AD by topical application. However, topical TET alone using its anti-inflammatory property to treat AD has not been reported yet.
AD is characterized by skin inflammation, with dry skin, eczema-like lesions, and itching.[2] Our results demonstrated that topical TET could ameliorate the clinical severity in MC903-induced AD mouse model. Scoring of dermatitis and ear thickness were important indexes to evaluate the severity of dermatitis inflammation in an experimental model. Compared with the AD group, topical TET treatment inhibited the inflammation significantly. Scoring of dermatitis and ear thickness significantly decreased. A study attempted to evaluate the efficacy of addition of TET to triamcinolone acetonide in the AD treatment, but the result did not show a significant improvement of TET for AD.[9] However, our study showed a therapeutic effect of topical TET on AD symptoms.
We found that topical TET suppressed both serum TSLP level and TSLP expression in skin lesions. TSLP is a critical cytokine involved in the initiation and progression of atopic march in both mice and humans.[19] The highest levels of TSLP are expressed in lung-derived epithelial cells and epidermal keratinocytes. Higher TSLP expression was found in the lesional epidermis than in nonlesional epidermis in AD.[5] Overexpression of TSLP could induce AD-like inflammation in mice.[20,21] In AD, TSLP can be induced by various processes and molecules, and then trigger mainly Th2 inflammatory response via activating DCs.[22,23] Thus, TSLP is considered as a critical factor interacting with both environmental responses outside and inflammatory responses inside. Our results suggested that TET might play an important role in reducing the skin inflammation through TSLP pathway. Therefore, TET exhibits the potential to be a topical therapeutic agent for AD.
It has been reported that TET exerts its anti-inflammatory effects via PAR2 and NF-κB pathway. TSLP expression can also be induced by PAR2 and NF-κB activation.[10,11,24] PAR2 has been found to be involved in skin inflammation, including itch generation and epidermal barrier homeostasis,[25] which mainly occurs in the epidermis, especially in the stratum granulosum.[26,27] In keratinocytes, various pathways including PAR2 and transient receptor potential cation channel subfamily V member 1 (TRPV1) could induce NF-κB activation, leading to overexpression of TSLP.[28,29,30] In our previous study, we did not find the difference of TRPV1 expression between 2% TET group and AD group. In addition, TRPV1 is associated with pruritus in epidermis.[31] The negative result of TRPV1 expression might be consistent with the negative result of frequency of scratching. Our present results demonstrated that TET could block PAR2 activation in vivo, which was similar to the findings by Ishikawa and his colleagues.[11] In this present study, NF-κB activation was suppressed by topical TET, indicating that the suppression of TSLP production might be through PAR2-NF-κB pathway.
TET is also able to affect Th2 inflammatory response by inhibition of IL-4, IL-13, TNF-α, and IL-1β in skin lesions. These cytokines were found at high levels in AD group in our study. Interestingly, IL-4, IL-13, TNF-α, and IL-1β can also induce TSLP expression in vitro, suggesting a feed-forward inflammatory cascade.[32] Our results suggested that TET could suppress TSLP-induced Th2-driven skin inflammation. Increased serum IgE level is also a hallmark of AD, which has several effects on skin inflammation.[33] In this study, elevated total serum IgE was reduced significantly after topical TET treatment. Joks et al. have also reported that systematic TET and its derivative minocycline could suppress ongoing human and murine IgE responses, therefore suggestive of a new treatment for IgE-mediated allergic diseases.[16,18] The findings are in consistent with our results that TET may help to control skin inflammation in AD.
To date, there is still a large unmet need for effective therapeutics of AD. Compared with glucocorticoid and calcineurin inhibitors, the mainstay topical treatment, topical TET has no steroid-associated adverse effects such as atrophy, hyperpigmentation, telangiectasia, withdrawal effects, and irritation. In our study, no adverse effects were observed, suggesting that topical TET has a good safety and efficacy profile. Further study is expected to elucidate a deeper insight into the mechanism of TET in vitro, as well as the relationship between the patients’ phenotype and effectiveness of TET.
In conclusion, the present study demonstrates a beneficial effect of topical TET on AD-like inflammation by attenuating TSLP and inhibition of inflammatory cytokines. The PAR2-NF-κB signaling pathway might be involved in this process. These results indicate that topical TET treatment might be a potential therapy for AD.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81301354) and Peking University People's Hospital Research and Development Funds (No. RDB2015-14).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank the staff at the Laboratory Animal Unit of Peking University People's Hospital for their valuable assistance.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Bieber T. Atopic dermatitis 2.0: From the clinical phenotype to the molecular taxonomy and stratified medicine. Allergy. 2012;67:1475–82. doi: 10.1111/all.12049. doi: 10.1111/all.12049. [DOI] [PubMed] [Google Scholar]
- 2.Noda S, Suárez-Fariñas M, Ungar B, Kim SJ, de Guzman Strong C, Xu H, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–64. doi: 10.1016/j.jaci.2015.08.015. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. doi: 10.1016/j.jaad.2007.06.041. doi: 10.1016/j.jaad.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol. 2013;171:330–7. doi: 10.1111/cei.12021. doi: 10.1111/cei.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 6.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324–36. doi: 10.1016/j.jaci.2014.11.015. doi: 10.1016/j.jaci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H) 22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–65. doi: 10.1016/j.jaad.2005.10.004. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Schuttelaar ML, Coenraads PJ. A randomized, double-blind study to assess the efficacy of addition of tetracycline to triamcinolone acetonide in the treatment of moderate to severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22:1076–82. doi: 10.1111/j.1468-3083.2008.02716.x. doi: 10.1111/j.1468-3083.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 10.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa C, Tsuda T, Konishi H, Nakagawa N, Yamanishi K. Tetracyclines modulate protease-activated receptor 2-mediated proinflammatory reactions in epidermal keratinocytes. Antimicrob Agents Chemother. 2009;53:1760–5. doi: 10.1128/AAC.01540-08. doi: 10.1128/AAC.01540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical Vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebane A, Runnel T, Aab A, Maslovskaja J, Rückert B, Zimmermann M, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. 2014;134:836–47. e11. doi: 10.1016/j.jaci.2014.05.022. doi: 10.1016/j.jaci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–6. doi: 10.1093/intimm/9.3.461. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- 15.Mokos ZB, Kummer A, Mosler EL, Ceovic R, Basta-Juzbašic A. Perioral dermatitis: Still a therapeutic challenge. Acta Clin Croat. 2015;54:179–85. [PubMed] [Google Scholar]
- 16.Joks R, Smith-Norowitz T, Nowakowski M, Bluth MH, Durkin HG. Tetracycline-mediated IgE isotype-specific suppression of ongoing human and murine IgE responses in vivo and murine memory IgE responses induced in vitro. Int Immunol. 2010;22:281–8. doi: 10.1093/intimm/dxq004. doi: 10.1093/intimm/dxq004. [DOI] [PubMed] [Google Scholar]
- 17.Daoud A, Gloria CJ, Taningco G, Hammerschlag MR, Weiss S, Gelling M, et al. Minocycline treatment results in reduced oral steroid requirements in adult asthma. Allergy Asthma Proc. 2008;29:286–94. doi: 10.2500/aap.2008.29.3121. doi: 10.2500/aap.2008.29.3121. [DOI] [PubMed] [Google Scholar]
- 18.Joks R, Durkin HG. Effect of tetracyclines on IgE allergic responses and asthma. Recent Pat Inflamm Allergy Drug Discov. 2011;5:221–8. doi: 10.2174/187221311797264919. doi: 10.2174/1−1311797264919. [DOI] [PubMed] [Google Scholar]
- 19.Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292–8. doi: 10.1183/09059180.00004314. doi: 10.1183/09059180.00004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS One. 2010;5:e9258. doi: 10.1371/journal.pone.0009258. doi: 10.1371/journal.pone.0009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Zhang LJ, Guha G, Li S, Kyrylkova K, Kioussi C, et al. Selective ablation of Ctip2/Bcl11b in epidermal keratinocytes triggers atopic dermatitis-like skin inflammatory responses in adult mice. PLoS One. 2012;7:e51262. doi: 10.1371/journal.pone.0051262. doi: 10.1371/journal.pone.0051262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitajima M, Ziegler SF. Cutting edge: Identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol. 2013;191:4903–7. doi: 10.4049/jimmunol.1302175. doi: 10.4049/jimmunol.1302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takai T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 24.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–34. doi: 10.4049/jimmunol.0900904. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–86. doi: 10.1038/sj.jid.5700351. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- 26.Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammationin vivo. FASEB J. 2003;17:1871–85. doi: 10.1096/fj.02-1112com. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- 27.Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: Tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–94. doi: 10.1111/j.1600-0625.1999.tb00383.x. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 28.Takai T, Chen X, Xie Y, Vu AT, Le TA, Kinoshita H, et al. TSLP expression induced via toll-like receptor pathways in human keratinocytes. Methods Enzymol. 2014;535:371–87. doi: 10.1016/B978-0-12-397925-4.00021-3. doi: 10.1016/B978-0-12-397925-4.00021-3. [DOI] [PubMed] [Google Scholar]
- 29.Hau CS, Kanda N, Watanabe S. Suppressive effects of antimycotics on thymic stromal lymphopoietin production in human keratinocytes. J Dermatol Sci. 2013;71:174–83. doi: 10.1016/j.jdermsci.2013.04.023. doi: 10.1016/j.jdermsci.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Jia X, Zhang H, Cao X, Yin Y, Zhang B. Activation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca2+/NFAT pathway in airway epithelial cells. FEBS Lett. 2014;588:3047–54. doi: 10.1016/j.febslet.2014.06.018. doi: 10.1016/j.febslet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: Getting the itch out? Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-015-8488-5. Epub ahead of print. doi: 10.1007/s12016-015-8488-5. [DOI] [PubMed] [Google Scholar]
- 32.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, et al. Cutting edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–7. doi: 10.4049/jimmunol.178.6.3373. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 33.Belloni B, Ziai M, Lim A, Lemercier B, Sbornik M, Weidinger S, et al. Low-dose anti-IgE therapy in patients with atopic eczema with high serum IgE levels. J Allergy Clin Immunol. 2007;120:1223–5. doi: 10.1016/j.jaci.2007.08.060. doi: 10.1016/j.jaci.2007.08.060. [DOI] [PubMed] [Google Scholar]