Abstract
Background
Esophageal anastomotic leaks following cancer surgery remain a major cause of morbidity and mortality. Endoscopic interventions, including covered metal stents (cSEMS), clips, and direct percutaneous endoscopic jejunostomy (dPEJ) tubes are increasingly used despite limited published data regarding their utility in this setting. This study aimed to determine the efficacy and safety of a multi-modality endoscopic approach to anastomotic leak management following surgery for esophageal or gastric cancer.
Methods
We performed a retrospective review of prospectively maintained databases of gastric and esophageal operations at our hospital between January 2003 and December 2012. Included patients had surgery for esophageal or gastric cancer, demonstrated evidence of an anastomotic leak at the esophageal anastomosis, and underwent attempted endoscopic therapy. Healing was defined as clinical and radiographic leak resolution.
Results
Forty-nine patients with leaks underwent endoscopic management. Of the 49 patients, 31 (63%) received cSEMS, 40 (82%) had dPEJ tubes inserted, and 3 (6%) received clips. Twenty-three (47%) patients underwent a combined approach. Overall, 88% of patients achieved healing in a median of 83 days. Twenty-two of 23 patients (96%) who underwent a multi-modality endoscopic approach healed. Only one patient had a major complication associated with stent erosion into the pulmonary artery, which was successfully treated with operative repair.
Conclusions
Esophageal anastomotic leaks following esophageal and gastric cancer surgery can be managed successfully and safely with endoscopic therapy. Combining cSEMS for leak control and dPEJ tube placement for nutrition support was highly effective in achieving healing, without need for surgical repair.
Keywords: Esophageal injury/perforation, stents, Endoscopy/endoscopic procedures, Cancer, Esophagus
Despite recent technical advances in gastroesophageal cancer surgery, post-operative anastomotic leaks remain a major cause of morbidity and mortality. Resulting respiratory and infectious complications can be significant, and frequently require intensive care unit admission and prolonged hospitalization [1–4]. Historically, these leaks have been managed with a combination of surgical repair, radiographically-guided drainage, broad-spectrum antibiotic therapy, nasogastric tube drainage, nil per os, and total parenteral nutrition [5–7]. Morbidity remains significant, however, with reported rates as high as 50–60% [8,9]. In an attempt to limit these complications, endoscopic interventions such as covered self-expanding metal stents (cSEMS), jejunal feeding tubes, and over-the-scope clips are increasingly used to manage patients with anastomotic leaks [10–12]. Despite the more frequent use of endoscopic interventions, there is a dearth of published data regarding their utility and safety in this setting. Prior studies included relatively few patients with anastomotic leaks and rarely employed a multi-modality approach of cSEMS for leak control and direct percutaneous endoscopic jejunostomy (dPEJ) tubes for nutrition support [10, 13–15]. In addition, endoscopic therapy in this setting has not been specifically examined in cancer patients. The primary objective of this study was to determine the efficacy and safety of endoscopic management of esophageal anastomotic leaks following surgery for esophageal or gastric cancer.
Patients and Methods
We retrospectively reviewed all patients with an esophageal anastomotic leak. Cases were identified from a prospectively maintained database. The study period was from January 2003 to December 2012. Inclusion criteria were age greater than 18 years, esophagogastric surgery performed for esophageal or gastric cancer, radiographic evidence of anastomotic leak at the esophagogastric or esophagojejunal anastomosis on esophagram or CT scan with oral contrast, and referral by the primary surgeon for attempted endoscopic therapy with cSEMS, over-the-scope clip, and/or dPEJ tube placement. Exclusion criteria were prior history of esophageal surgery, operation performed at another institution, incomplete medical records, and loss to follow-up.
Records were reviewed for demographics, procedures, radiological findings, clinical outcomes, and complications. Endoscopic therapy was performed by an experienced gastroenterologist with anesthesia support for each case. Healing was defined as radiographic resolution of the anastomotic leak on follow-up esophagram or CT scan with oral contrast after cSEMS removal. This study was approved by the Institutional Review Board.
The cSEMS used in this study included Cook Medical Evolution (Bloomington, IN), Boston Scientific Polyflex (Natick, MA), Boston Scientific Wallflex, and Alveolus AliMAXX-E (Charlotte, NC) esophageal stents. The choice of cSEMS was at the discretion of the endoscopist at the time of the procedure. Covered SEMS were placed under fluoroscopy with guidewire-assisted deployment. The middle portion of the cSEMS was positioned to cover the anastomotic leak. Stents were not routinely secured using clips or suturing devices. A post-procedural chest x-ray was performed to document stent positioning. Patients who required dPEJ tube had the Boston Scientific Microvasive 20 French feeding tube placed directly into the jejunum via the pull technique with subsequent endoscopic visual confirmation of appropriate placement as previously described [11]. In select patients who underwent endoscopic closure of the anastomotic leak, the Ovesco OTSC (Los Gatos, CA) over-the-scope clip was used. Percutaneous drainage, when required, was performed by an experienced interventional radiologist.
Results
We identified 145 patients with esophageal anastomotic leaks during the study period. Forty-one patients did not have radiographic evidence of an esophageal leak seen on esophagram or CT scan with oral contrast, underwent surgery for benign disease, reported prior history of esophageal surgery, underwent surgery at other institutions, had incomplete records, or were lost to follow-up and were therefore excluded from the study. Of the remaining 104 patients, 49 were referred for endoscopic therapy at the discretion of the primary surgeon if the leak failed to heal with conservative management (e.g. nil per os, parenteral nutrition), or if the anastomotic defect was judged to be too large to successfully heal on its own. These 49 patients were included in the final analysis.
The 49 total patients had a median age of 63 years (range 38–84) (Table 1). Forty patients (82%) were men. Forty patients (82%) had esophageal adenocarcinoma, 4 (8%) had esophageal squamous cell carcinoma, and 5 (10%) had gastric adenocarcinoma. Forty-two patients (86%) underwent esophagectomy, 4 patients (8%) had total gastrectomy, 2 patients (4%) had esophagogastrectomy, and 1 patient (2%) had esophagogastrectomy with jejunal interposition.
Table 1.
Patient Characteristics
|
Total number of patients undergoing endoscopic management |
49 |
| Median age (years) | 63 (range 38 – 84) |
| Sex | |
| Male | 40 (82%) |
| Female | 9 (18%) |
| Type of Cancer | |
| Esophageal adenocarcinoma | 40 (82%) |
| Esophageal squamous cell carcinoma | 4 (8%) |
| Gastric adenocarcinoma | 5 (10%) |
| Type of Surgery | |
| Ivor-Lewis esophagectomy | 42 (86%) |
| Total gastrectomy | 4 (8%) |
| Esophagogastrectomy | 2 (4%) |
|
Esophagogastrectomy with jejunal interposition |
1 (2%) |
| Median time to leak (days) | 7 (range 3 – 27) |
| Median time from leak to cSEMS (days) | 10 (range 0 – 53) |
cSEMS = covered self-expanding metal stent
Anastomotic leaks were identified on radiologic imaging a median of 7 days after surgery (range 3–27). Of the 32 patients who had leak size quantified on esophagram, 15 patients (47%) had a small to moderate leak, 12 patients (38%) had a moderate to large leak, and 5 patients (16%) had a large leak. Time from leak identification to cSEMS placement was a median of 10 days (range 0–53).
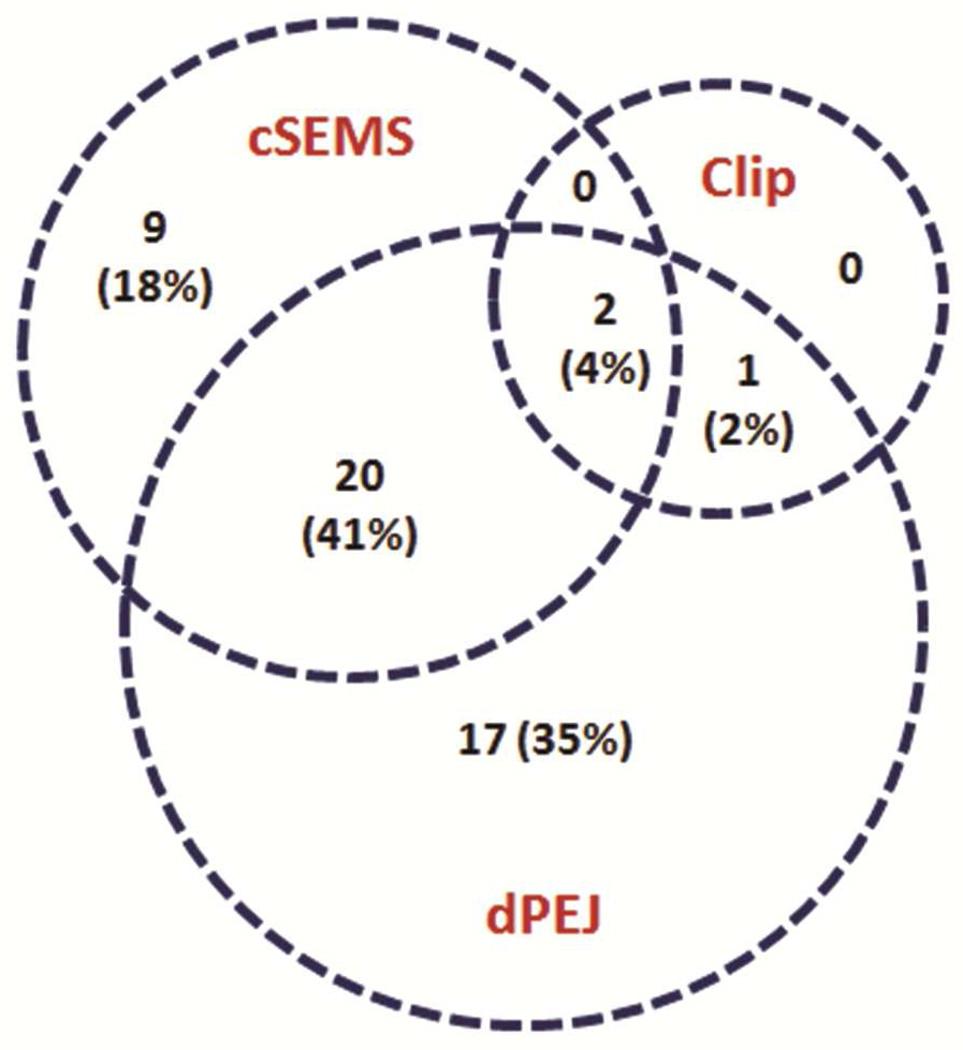
Endoscopic placement of cSEMS was performed in 31 of 49 patients (63%), over-the-scope clips in 3 patients (6%), and dPEJ tubes in 40 patients (82%) (Figure 1). Twenty-two patients (45%) were treated with a combination of cSEMS and dPEJ tube.
Figure 1.
Number of patients (%) who underwent one or a combination of endoscopic interventions.
clip = over-the-scope clip
cSEMS = covered self-expanding metal stent
dPEJ = direct percutaneous endoscopic jejunostomy tube
Nineteen out of 49 patients (39%) required percutaneous drainage of an infected cavity. Of the 40 patients who had dPEJ tubes placed, 15 (38%) had a percutaneous drainage performed, while 15 out of 31 patients (48%) with cSEMS also underwent percutaneous drainage. Of the 22 patients who had both a dPEJ and cSEMS placed, 11 (50%) had a percutaneous drainage.
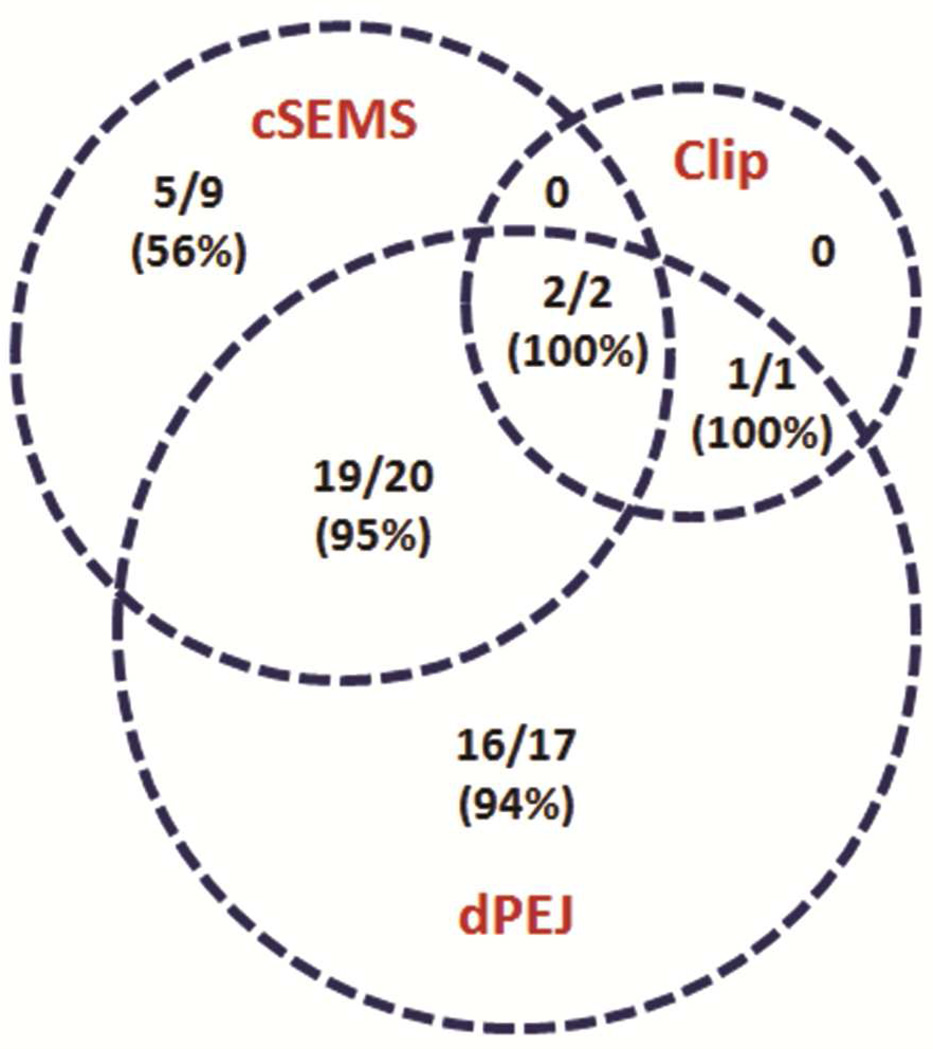
Of the 31 patients who received cSEMS, the stents were in place for a median of 55 days (range 12–170) (Table 2). Twenty-six patients (84%) treated with cSEMS achieved documented healing (Figure 2). Of the 40 patients who received dPEJ tubes, the feeding tubes were in place for a median of 73 days (range 18–358). Thirty-eight patients (95%) treated with dPEJ tubes achieved documented healing. Among these two endoscopic treatment groups, 22 patients were treated with a combination of cSEMS and dPEJ tube. Overall, 21 patients (95%) treated with a combination of cSEMS and dPEJ tube achieved documented healing.
Table 2.
Outcomes of Endoscopic Management
| Median duration of cSEMS (days) | 55 (range 12 – 170) |
| Median duration of dPEJ (days) | 73 (range 18 – 358) |
|
Median time to healing with endoscopic management (days) |
83 (range 3 – 337) |
cSEMS = covered self-expanding metal stent
dPEJ = direct percutaneous endoscopic jejunostomy tube
Figure 2.
Number of patients (%) who achieved documented healing after endoscopic management.
clip = over-the-scope clip
cSEMS = covered self-expanding metal stent
dPEJ = direct percutaneous endoscopic jejunostomy tube
Of the 3 patients who received endoscopic over-the-scope clip placement, 2 were treated in combination with both cSEMS and dPEJ tube and 1 in combination with dPEJ tube only. All 3 of these patients (100%) achieved documented healing.
Overall, of the 49 total patients who received endoscopic treatment, 43 (88%) achieved documented anastomotic healing. Median time to healing was 83 days (range 3–337). Of the 6 patients (12%) who did not achieve anastomotic healing, 2 were treated with surgical anastomotic revision, and 1 was treated with esophageal exclusion. Two died of multiple surgical complications, and 1 died at another facility with cSEMS in place.
Of the 31 patients who received cSEMS, stent migration was seen in 11 patients (35%), and all 11 were managed successfully with endoscopic revision (Table 3). One patient (3%) who received a cSEMS had stent erosion into the pulmonary artery and was treated with intra-operative stent removal, oversewing of the bleeding artery, esophageal exclusion, and completion esophagectomy and gastrectomy. This patient had previously undergone an Ivor-Lewis esophagectomy complicated by an esophagogastric leak that was treated with a Boston Scientific PolyFlex 120mm × 21/25mm stent. The cSEMS was in place for a total of 54 days prior to operative removal. The patient ultimately developed metastatic disease and died of septic shock one year later.
Table 3.
Adverse Events
| cSEMS migration | 11/31 (35%) |
| cSEMS erosion into major vessel | 1/31 (3%) |
|
Initial failed dPEJ placement requiring second attempt |
4/40 (10%) |
|
dPEJ insertion site infection and persistent fistula |
1/40 (3%) |
cSEMS = covered self-expanding metal stent
dPEJ = direct percutaneous endoscopic jejunostomy tube
Of the 40 patients who received dPEJ tube, 4 (10%) failed an initial attempt at feeding tube placement and required a second procedure for successful insertion. One patient (3%) who received a dPEJ tube developed a dPEJ insertion site infection and persistent fistula after the feeding tube was removed. The patient was treated successfully with antibiotics and dPEJ tube reinsertion.
Of the 3 patients who received over-the-scope clip placement, there were no documented complications related to clipping.
Comment
In this study, we examined the efficacy and safety of endoscopic management of esophageal anastomotic leaks in 49 patients following surgery for esophageal or gastric cancer, using cSEMS and over-the-scope clip placement for leak control, in conjunction with dPEJ tube insertion for enteral nutrition support. Of the 49 patients, 31 received a cSEMS, 40 had a dPEJ tube inserted, and 3 received an over-the-scope clip. Twenty-three of these patients underwent a combination of the above approaches. Overall, 88% of patients achieved documented anastomotic leak healing in a median of 83 days. Twenty-two of 23 patients (96%) who underwent a multi-modality endoscopic approach achieved anastomotic healing. The most common adverse event related to cSEMS placement was stent migration, which was managed successfully with endoscopic revision in all cases. Only one cSEMS patient had a major complication associated with stent erosion into the pulmonary artery, which was successfully treated with operative vascular repair and esophageal exclusion. Adverse events related to dPEJ tube placement were rare, and none were related to over-the-scope clip placement.
Several recent studies have examined the role of endoscopic management of esophageal leaks; however, few patients with post-operative anastomotic leaks were included [10, 13–15]. David et al. analyzed 30 patients who had cSEMS placed for treatment of intrathoracic esophageal leakage [15]. Of these 30 patients, only 5 had anastomotic leaks. Blackmon et al. evaluated 25 patients, 23 of whom had cSEMS placed for treatment of esophageal or gastric leakage.
However, only 13 of these 23 patients had anastomotic leaks [13]. Similarly, other authors have also examined small numbers of patients with anastomotic leaks treated with cSEMS, and very few of these studies have focused specifically on a cancer population [10,14].
Our study included 49 patients with endoscopically managed esophageal anastomotic leaks following surgery for esophageal or gastric cancer at a specialized cancer center, representing a larger, more homogenous patient population. It is notable that treatment of a substantial portion of these patients (82%) included dPEJ tube placement, with 95% achieving anastomotic healing. Taken together with prior reports of its effectiveness in a similar population [11], dPEJ tube placement may be an underutilized safe and effective endoscopic treatment option for providing enteral nutrition support while allowing for anastomotic leak healing in these patients.
Thirty-five percent of patients who underwent cSEMS placement experienced stent migration in this study. In our institution’s experience, routine endoscopic clipping in an effort to secure these stents has not been effective. The recent development of novel endoscopic suturing devices holds significant promise as a potential means of safely and effectively securing esophageal stents and preventing migration [16].
There are several limitations to our study. It is a retrospective review of medical records and is therefore limited by the absence of prospective or randomized data. The decision to refer patients for endoscopic therapy was made at the discretion of the primary surgeon, and further investigation is needed to determine which factors make patients ideal candidates for such an approach.
Our results do suggest that esophageal anastomotic leaks following esophageal and gastric cancer surgery can be managed successfully and safely with endoscopic therapy. A combination of esophageal cSEMS to control the leak and dPEJ tube placement for nutrition support was highly effective in achieving anastomotic healing without the need for high-risk surgical repair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation:
N/A
References
- 1.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–1669. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 2.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–372. doi: 10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesphageal cancer: a multicentre, open-label, randomised control trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 4.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel L, Grillo HC, Malt RA. Operative and nonoperative management of esophageal perforation. Ann Surg. 1981;194:57–63. doi: 10.1097/00000658-198107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eroglu A, Turkyilmaz A, Aydin Y, Yekeler E, Karaoglanoglu N. Current management of esophageal perforation: 20 years experience. Dis Esophagus. 2009;22:374–380. doi: 10.1111/j.1442-2050.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- 7.Maroney TP, Ruiz EJ, Gordon RL, Pellegrini CA. Role of interventional radiology in the management of major esophageal leaks. Radiology. 1989;170:1055–1057. doi: 10.1148/radiology.170.3.2916056. [DOI] [PubMed] [Google Scholar]
- 8.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–222. doi: 10.1016/s0003-4975(02)04368-0. [DOI] [PubMed] [Google Scholar]
- 9.Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90:936–942. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009;23:1526–1530. doi: 10.1007/s00464-009-0432-4. [DOI] [PubMed] [Google Scholar]
- 11.Bueno JT, Schattner MA, Barrera R, Gerdes H, Bains M, Shike M. Endoscopic placement of direct percutaneous jejunostomy tubes in patients with complications after esophagectomy. Gastrointest Endosc. 2003;57:536–540. doi: 10.1067/mge.2003.155. [DOI] [PubMed] [Google Scholar]
- 12.Mennigen R, Colombo-Benkmann M, Senninger N, Laukoetter M. Endoscopic closure of postoperative gastrointestinal leakages and fistulas with Over-the-Scope Clip (OTSC) J Gastrointest Surg. 2013;17:1058–1065. doi: 10.1007/s11605-013-2156-y. [DOI] [PubMed] [Google Scholar]
- 13.Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931–937. doi: 10.1016/j.athoracsur.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Chopra SS, Kneif S, Hünerbein M. Management of esophageal anastomotic leaks, perforations, and fistulae with self-expanding plastic stents. J Thorac Cardiovasc Surg. 2011;141:1213–1217. doi: 10.1016/j.jtcvs.2010.07.096. [DOI] [PubMed] [Google Scholar]
- 15.David EA, Kim MP, Blackmon S, et al. Esophageal salvage with removable covered selfexpanding metal stents in the setting of intrathoracic esophageal leakage. Am J Surg. 2011;202:796–801. doi: 10.1016/j.amjsurg.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Sharaiha RZ, Kumta NA, DeFillipis EM, et al. A Large Multicenter Experience with Endoscopic Suturing for Management of Gastrointestinal Defects and Stent Anchorage in 122 Patients: A Retrospective Review. J Clin Gastroenterol. 2015 doi: 10.1097/MCG.0000000000000336. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]