Abstract
OBJECTIVE
The objective of the study was to investigate whether group prenatal care (Centering Pregnancy Plus [CP+]) has an impact on pregnancy weight gain and postpartum weight loss trajectories and to determine whether prenatal depression and distress might moderate these trajectories.
STUDY DESIGN
This was a secondary analysis of a cluster-randomized trial of CP+ in 14 Community Health Centers and hospitals in New York City. Participants were pregnant women aged 14–21 years (n = 984). Medical record review and 4 structured interviews were conducted: in the second and third trimesters and 6 and 12 months postpartum. Longitudinal mixed modeling was utilized to evaluate the weight change trajectories in the control and intervention groups. Prenatal distress and depression were also assessed to examine their impact on weight change.
RESULTS
There were no significant differences between the intervention and control groups in baseline demographics. Thirty-five percent of the participants were overweight or obese, and more than 50% had excessive weight gain by Institute of Medicine standards. CP+ was associated with improved weight trajectories compared with controls (P < .0001): women at clinical sites randomized to group prenatal care gained less weight during pregnancy and lost more weight postpartum. This effect was sustained among women who were categorized as obese based on prepregnancy body mass index (P < .01). Prenatal depression and distress were significantly associated with higher antepartum weight gain and postpartum weight retention. Women with the highest levels of depression and prenatal distress exhibited the greatest positive impact of group prenatal care on weight trajectories during pregnancy and through 12 months postpartum.
CONCLUSION
Group prenatal care has a significant impact on weight gain trajectories in pregnancy and postpartum. The intervention also appeared to mitigate the effects of depression and prenatal distress on antepartum weight gain and postpartum weight retention. Targeted efforts are needed during and after pregnancy to improve weight gain trajectories and overall health.
Keywords: excessive gestational weight gain, group prenatal care, postpartum weight loss
In the United States, approximately one-quarter of high school females are overweight or obese and by ages 20–39 years, one-third are obese.1,2 More than half of pregnant women are overweight or obese.3 In 2009, the Institute of Medicine (IOM) published target gestational weight gain recommendations to address gestational weight gain based on prepregnancy body mass index (BMI).4
The increased risk of maternal and fetal complications associated with obesity and excessive gestational weight gain are well documented and include fetal anomalies, gestational diabetes, preeclampsia, cesarean delivery, macro-somia, asphyxia, and stillbirth.5-8 Since these recommendations were published, there has been increasing evidence that most women do not have appropriate gestational weight gain or postpartum weight loss within the IOM guidelines.9 In a previous study, we conducted among young mothers aged 14–25 years, almost two thirds exceeded recommendations for weight gain during pregnancy, and by 1 year post-partum, 68.5% were overweight or obese.10
Given the compounding effects of repeated pregnancies with excessive weight gain and inadequate postpartum weight loss, there has been focused research attention investigating lifestyle and dietary interventions for reducing gestational weight gain and postpartum weight retention.11 Most studies are small and have different primary outcomes making the development of evidence-based recommendations difficult. Several metaanalyses of interventions demonstrate that antepartum dietary counseling and physical activity may significantly reduce maternal weight gain.12-14 However, few studies include women who are obese, and the effect of such interventions among overweight and obese women is equivocal.15,16
Studies of antepartum interventions on postpartum weight loss are even more limited. Postpartum interventions that utilize both exercise and dietary interventions have had some, although not uniform, success.17,18 Disparate results in both antepartum and postpartum interventions are due in part to heterogeneity of populations and treatments, social norms of pregnancy weight gain, and nonadherence to study protocols, especially in the postpartum period. There are few randomized controlled trials, limiting our ability to make evidence-based recommendations in pregnancy and postpartum.
Prenatal depression and distress have been linked to preterm birth and low birthweight, and there is emerging literature on the effects of chronic stress on weight gain.19-22 Stress increases circulating levels of cortisol, ghrelin, insulin, and proinflammatory cytokines, which affect appetite, satiety, energy expenditure, adipose storage, and weight gain.23 Similar neuroendocrine and in-flammatory processes also have been theorized as potential mechanisms in the pathophysiology of preterm birth and low birthweight. However, few studies target depression or distress in pregnancy as a mechanism to improve weight trajectories or birth outcomes.
Group prenatal care has been shown to improve obstetric outcomes with a 33% lower rate of preterm delivery as well as improved psychological parameters.24,25 High-stress women who were randomized to group prenatal care reported significantly increased self-esteem and decreased stress and social conflict in the third trimester of pregnancy compared with the control group; social conflict and depression were significantly decreased at 1 year postpartum. Group time consists of discussion, education, and skills building to address explicit learning objectives in prenatal care, childbirth preparation, and postpartum care. It addresses common pregnancy stressors encountered by the group. The intervention directly addresses healthy nutritional choices and exercise, although these are a minor focus, and at each session participants are involved in self-care activities such as taking and charting their weight and blood pressure.
The objectives of this analysis are to investigate whether group prenatal care has an impact on pregnancy weight gain and postpartum weight loss trajectories and to determine whether prenatal depression and distress might moderate these trajectories.
Materials and Methods
This is a secondary analysis of data from a cluster-randomized trial in 14 community health centers and hospitals in New York City aimed at evaluating whether improved reproductive outcomes resulted from group prenatal care compared with usual individual care.
Clinical sites with a minimum of 300 pregnant women annually and that serve predominantly low-income and minority women were selected. Clinical sites were recruited and randomized in stratified blocks to account for lags in recruitment and time required for training and implementation support. Sites were also matched by relative size and type (ie, smaller community health centers vs larger hospital-based clinics). Enrollment began in 2008, with clinical follow-up 1 year postpartum completed in 2012. After patient enrollment was completed, sites randomized to individual care were offered training to implement group prenatal care.
Young women aged 14–21 years attending an initial prenatal care visit at a participating clinical site were referred to the study by a health care provider or recruited directly by research staff. Inclusion criteria consisted of the following: (1) pregnancy before 24 weeks’ gestation; (2) no severe medical problems at time of enrollment that would require individualized assessment and tracking as a high-risk pregnancy; (3) English or Spanish speaking; and (4) agreed to receive group prenatal care if offered at their site.
Women completed structured interviews at 4 time points: during pregnancy at trimester two (M = 18.72 weeks’ gestational age, SD, 3.29) and trimester three (M = 29.99 weeks’ gestational age, SD, 5.28) as well as postpartum at 6 months (M = 26.07 weeks, SD, 5.21) and 12 months (M = 57.30 weeks’ gestational age, SD, 13.50). Interviews were completed in English (77.7%) or Spanish (22.3%) using audio handheld-assisted personal interview technology.26 Participants were paid $20 for each interview. Systematic review of maternal and child medical records by trained research staff was also conducted.
All procedures were approved by the Institutional Review Boards at Yale University, Clinical Directors Network, and at each clinical site. Participants provided written informed consent.
Intervention
The intervention was implemented at the practice (ie, cluster) level. Participants at sites randomized to the intervention condition received CenteringPregnancy Plus (CP+) group prenatal care (Centering Healthcare Institute, Boston, MA), whereas those at sites randomized to the delayed intervention condition received standard individual prenatal care.
Described in detail previously, group prenatal care begins with a standard clinical intake (history/physical) conducted individually.24 Thereafter all care occurs within the group except health concerns requiring privacy and cervical assessments late in pregnancy. Groups include 8–12 women of the same gestational age and are facilitated by 2 health providers: physician or midwife and an assistant.
There are ten 120 minute sessions, scheduled to follow clinical guidelines from the American College of Obstetrics and Gynecology.27 There is a manualized curriculum to include skills and information designated by clinical guidelines as central to prenatal care. When participants arrive, they engage in self-care activities including taking their own weight and blood pressure, charting progress in their health records, and completing brief surveys. Fundal height and heart rate monitoring are completed by the clinician.
The majority of group time consists of facilitated discussion, education, and skills building to address explicit learning objectives in prenatal care, childbirth preparation, and postpartum care. Nutrition counseling occurs at the first group session.
Measures
Prepregnancy BMI (kilograms per square meter) was calculated from self-reported prepregnancy height and weight. Weight during pregnancy was obtained from medical record review as recorded at each prenatal care visit. Gestational age was estimated using ultrasound, and time was designated as a continuous variable representing weeks since conception to account for variability in the timing of delivery and interviews.
All psychosocial and behavioral factors were measured using valid and reliable scales. Baseline depressive symptoms were assessed using a 15 item version of the Centers for Epidemiologic Study-Depression, which is a measure of depressive symptomatology exhibiting good sensitivity and specificity as well as high internal consistency with both nonpatient and patient populations. Exhibiting good reliability in our sample (alpha = .82), the 15 item version omitted the 5 psychophysiological items (eg, appetite, sleep disturbance) because of the strong colinearity with common pregnancy symptoms.
Also demonstrating good reliability in our sample (alpha = .86), the 17-item Prenatal Distress Questionnaire was administered at baseline and asked participants to rate on a 3 point scale from not at all to very much, how much they were bothered, worried, or upset about various aspects of pregnancy (eg, low energy, physical symptoms such as nausea or backaches).28-30
Nutrition was assessed at baseline using a modified versions of the REAP (Rapid Eating Assessment for Patients), a 10-item measure that asks about a range of nutrition-related behaviors (eg, skipping breakfast, eating meals out). Responses are based on a frequency scale of 0 to 4 from never to every day, with higher scores denoting poorer nutrition (range, 0–40). Baseline physical activity was assessed using a 4 item version of the WAVE (Weight, Activity, Variety, Excess), which utilizes the same 5 point response scale as the REAP and assesses frequency of moderate activity, playing an organized sport, building exercise into daily activities, and sedentary behavior such as 2 hours or more per day of watching television or playing video games.31 Demographic factors were obtained by self-report at the baseline interview. Race was categorized as African-American/black, Latina/Hispanic, or other.
Statistical analyses
For demographic comparisons, the study population was subdivided according to prepregnancy BMI category, using the published IOM-defined cut points and excessive weight gain cutoffs.3 Nonindependence of the repeated weight change scores required the use of multilevel models to examine weight change trajectories. Multilevel analyses were performed using PROC MIXED with maximum likelihood estimation and an unstructured covariance structure using SAS version 9.3 (SAS Institute, Cary, NC). Because of a significant P value for variance estimates, both the model intercept and gestational age (ie, time) were treated as random effects in the models. Because all other predictors were person-level effects (ie, level 2), they were treated as fixed.
Per multilevel modeling convention, the trajectory model building process began with testing of the random intercept model, followed by the unconditional growth model.32 Three iterations of growth models were sequentially tested: linear, quadratic, and cubic. Comparison of model fit using a χ2 difference test of the –2 log likelihood statistic indicated that the cubic model, which includes the linear, quadratic, and cubic terms in the model, represented the best fit of the data. Next, each main effect of interest was tested with only the linear, quadratic, and cubic time effects entered as covariates. All significant main effects were then tested in a combined effects model that included time effects. Interactions terms of theoretical interest were then entered in the model.
To maximize generalizability of the model, maternal age, racial/ethnic group, prepregnancy BMI (obese/nonobese), smoking during pregnancy, and intervention group were predetermined to be included in final models as covariates, regardless of statistical significance. Prenatal depression and distress were retained in the model as they were tested as moderators of weight loss trajectories. All other predictors and interactions terms were retained only if P < .05.
Results
Participant characteristics and comparison between intervention and control conditions
Of 1538 young women eligible for the study, 1233 enrolled (80.2%). Of the 1233 young women who participated in the study, those with any of the following issues were excluded from all multilevel analyses: multiple births (n = 12), history of heart disease (n = 31), history of hypertension (n = 31), history of diabetes (n = 16), usable BMI data not provided (n = 80), and completely missing or invalid weight data (n = 63), yielding a working model of n = 1012.
Because of a small amount of missing data for the other predicators and covariates, the analytic sample informing the combined effects weight trajectory model was n = 984. χ2 tests revealed that there were no significant differences between condition for any of the exclusionary health conditions or for invalid prepregnancy BMI data; however, women in the control group were more likely to have missing or invalid pregnancy weight data as compared with the intervention group (9% of control sample vs 1% in the intervention sample; P < .05).
Participant demographics are presented in Table 1. Comparing women at clinical sites randomized to group vs individual care, there were no significant differences in demographic, behavioral, or clinical characteristics, with the exception of the baseline nutritional assessment. Although the women in the intervention condition reported slightly better eating habits using the REAP (14.0 ± 5.3 vs 15.0 ± 5.8 vs P < .05), both groups demonstrated relatively good habits overall (range, 0–40). It is notable that half of the participants exceeded gestational weight gain amounts based on IOM guidelines. There was no significant difference in the total number of prenatal care visits with women in group care averaging 9.3 total visits compared with 8.9 in individual care.
TABLE 1.
Demographics
| Variable | Group care (n = 495) | Individual care (n = 489) | Total (n = 984) |
|---|---|---|---|
| Age, M (SD) | 18.7 (1.79) | 18.6 (1.69) | 18.7 (1.74) |
| Race, % (n) | |||
| Latina | 63.6 (315) | 63.4 (310) | 63.5 (625) |
| Black, non-Latina | 30.9 (153) | 33.3 (163) | 32.1 (316) |
| Other | 5.5 (27) | 3.3 (16) | 4.4 (43) |
| Parity, % (n) | |||
| 0 | 84.8 (407) | 85.9 (413) | 85.3 (820) |
| 1 | 13.8 (66) | 11.4 (55) | 12.6 (121) |
| Education, % (n) | |||
| Less than high school | 29.4 (143) | 28.0 (136) | 28.7 (279) |
| High school graduate/in school | 50.8 (247) | 50.1 (243) | 50.5 (490) |
| Some college or more | 19.8 (96) | 21.9 (106) | 20.8 (202) |
| Depression, M (SD) | 12.8 (8.3) | 12.3 (8.9) | 12.5 (8.6) |
| Prenatal distress, M (SD) | 13.0 (6.8) | 13.3 (6.7) | 13.1 (6.7) |
| Prepregnancy weight, % (n) | |||
| Underweight | 11.7 (58) | 11.5 (56) | 11.6 (114) |
| Healthy weight | 49.9 (247) | 56.6 (277) | 53.3 (524) |
| Overweight | 21.8 (108) | 17.2 (84) | 19.5 (192) |
| Obese | 16.6 (82) | 14.7 (72) | 15.7 (154) |
| Excessive weight gain, % (n) | 48.8 (205) | 51.6 (215) | 50.2 (420) |
| REAP, M (SD)a | 14.0 (5.3) | 15.0 (5.8) | 14.5 (5.6) |
| WAVE, M (SD) | 5.67 (2.8) | 5.81 (2.8) | 5.74 (2.8) |
| Attempted breast-feeding, % (n) | 88.1 (267) | 87.2 (231) | 87.7 (498) |
ANOVA with Tukey correction for continuous outcomes and χ2 test for categorical outcomes were used to examine between-group differences.
Indicates a significant difference between groups with P < .05.
Impact of group prenatal care on pregnancy weight gain and postpartum weight loss trajectories
Statistically significant and nonsignificant main effects from the multilevel model and their beta weights, SEs, and P values are presented in Table 2. In addition to several significant main effect, the multilevel model showed a significant difference in weight trajectories between the intervention and control groups over time (P < .0001). Women randomized to group prenatal care gained less weight during pregnancy and retained less weight 12 months postpartum. Furthermore, 12 months postpartum, the intervention group trajectory mean weight gain was within the guidelines of retaining <10 pounds postpartum (Figure 1, A).
TABLE 2.
Pregnancy and postpartum weight change model estimates
| Combined effects depression interaction modela |
Combined effects prenatal distress interaction modelb |
|||||
|---|---|---|---|---|---|---|
| Predictor | b | P value | SE | B | P value | SE |
| Intercept | –29.9 | c | 2.76 | –29.5 | c | 2.75 |
| Level 1 (time-varying measures) | ||||||
| Time, weeks from conception based on GAd | ||||||
| GA, linear time effect (random) | 2.53 | c | 0.05 | 2.49 | c | 0.05 |
| GA2, quadratic time effect | –0.04 | c | 0.001 | –0.04 | c | 0.001 |
| GA3, cubic time effect | 0.00016 | c | .000006 | 0.0002 | c | 0.000005 |
| Level 2 (non–time-varying measures) | ||||||
| Demographic variables | ||||||
| Intervention (0 = control group; 1 = intervention group) | 2.75 | e | 1.21 | 2.45 | e | 1.20 |
| Race (reference group is Other race category)d | ||||||
| African American | –2.98 | 1.62 | –2.90 | 1.61 | ||
| Latino/Hispanic | –3.26 | e | 1.57 | –3.17 | e | 1.55 |
| Maternal age, grand mean centered | 0.44 | 0.23 | 0.43 | 0.23 | ||
| Health-related variables | ||||||
| BMI group (0, nonobese; 1, obese) | 0.48 | 1.39 | –0.09 | 1.39 | ||
| Smoking during first 24 wks of pregnancy (0, no; 1, yes) | 2.28 | 1.80 | 2.29 | 1.80 | ||
| Depression (CES-D), grand mean centered | –0.02 | 0.10 | 0.06 | 0.05 | ||
| Prenatal distress, grand mean centered | 0.01 | 0.07 | –0.07 | 0.14 | ||
Model intraclass correlation = 0.56; unadjusted estimates for education, income, and primigravida were not significantly predictive of weight change, so these effects were excluded from the combined effects models.
BMI, body mass index; CES-D, Centers for Epidemiologic Study-Depression; GA, gestational age.
The estimates in this column are adjusted for the following significant (P < .05) interactions, including all required lower-order interaction terms: GA2 × BMI group; GA3 × intervention, CESD × intervention; GA3 × CESD; and GA3 × CES-D × intervention
The estimates in this column are adjusted for the following significant (P < .05) interactions, including all required lower-order interaction terms: GA2 × BMI group GA3 × intervention, PD × intervention; GA3 × PD, and GA3 × PD × intervention
Variables subsumed under this term tested together as a block
P < .0001
P < .05.
FIGURE 1.
Weight change over time by intervention condition and obese group status
There were no substantive differences in the multilevel models between underweight, normal weight, and over-weight groups; therefore, these categories were combined in multilevel models such that women were classified as either nonobese (BMI <30.0 kg/m2) or obese (BMI 30.0 kg/m2) based on prepregnancy BMI. The difference in weight gain trajectories between the intervention and control groups persisted when stratified by obesity status (P < .01). As illustrated in Figure 1, B, the primary effect of the group prenatal care intervention was upheld, and in addition, there was a main effect of BMI group such that women who were categorized as obese based on prepregnancy BMI gained less weight during pregnancy and lost more weight postpartum than those who were not obese.
Prenatal depression and distress as mediators of weight change trajectories
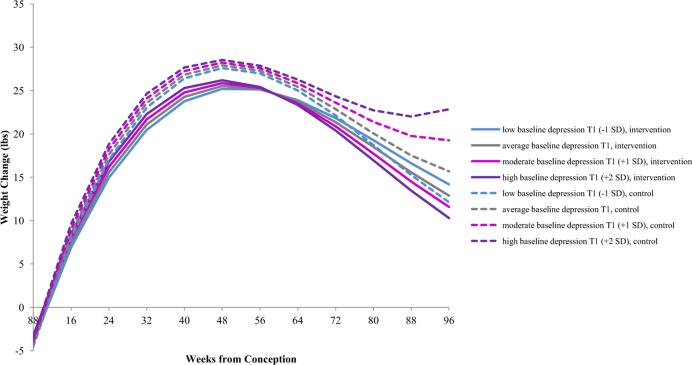
Figure 2 illustrates that there was a significant difference in weight change between the intervention and control groups as a function of baseline depressive symptomatology. Women who were at clinical sites randomized to group prenatal care had similar (and generally favorable) trajectories of weight loss, regardless of depressive symptoms. An interaction was observed, such that women at clinical sites randomized to individual prenatal care with moderate and high baseline depressive symptoms had significantly more weight gain in pregnancy and significantly less weight loss throughout the postpartum period (P < .0001). For example, 12 months postpartum, those in the control group with high depression (+2 SD) retained an average of 22.0 pounds, compared with those in group prenatal care with high depression who retained an average of 13.5 pounds (Table 3).
FIGURE 2.
Weight change over time as predicted by intervention condition × depression
TABLE 3.
Weight change model estimates for intervention × depression group interaction, adjusted for model covariates (pounds)
| Group prenatal care |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks from conception |
–1 SD depression |
M depression |
+1 SD depression |
+2 SD depression |
–1 SD depression |
M depression |
+1 SD depression |
+2 SD depression |
| 12 | 1.80 | 2.21 | 2.61 | 3.01 | 2.18 | 2.63 | 3.08 | 3.53 |
| 20 | 11.3 | 11.9 | 12.4 | 13.0 | 12.9 | 13.5 | 14.1 | 14.7 |
| 40 | 23.8 | 24.3 | 24.8 | 25.3 | 26.4 | 26.8 | 27.3 | 27.7 |
| 6 wks’ postpartum | 25.0 | 25.4 | 25.8 | 26.1 | 27.5 | 27.8 | 28.3 | 28.5 |
| 6 mo postpartum | 23.9 | 23.7 | 23.5 | 23.3 | 25.0 | 25.4 | 25.9 | 26.3 |
| 12 mo postpartum | 16.6 | 15.6 | 14.5 | 13.5 | 15.3 | 17.5 | 19.8 | 22.0 |
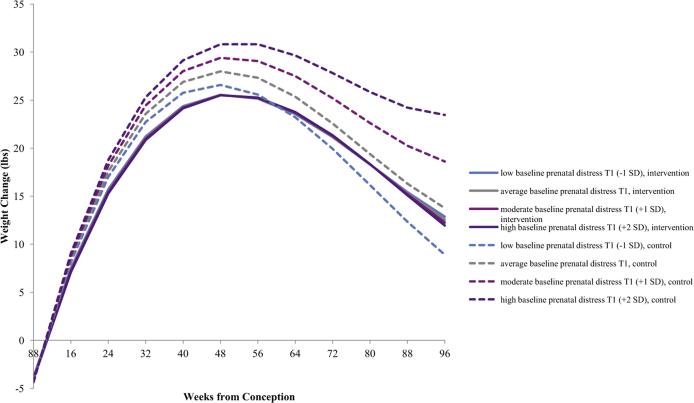
Figure 3 illustrates similar trajectories for varying levels of prenatal distress (P < .0001), whereby those with moderate and high levels of prenatal distress gained the most weight during pregnancy and retained the most weight postpartum. Using the same comparison as above, at 12 months postpartum, those in the control group with high prenatal distress (+2 SD) retained an average of 24.2 pounds, compared with those in group prenatal care with high prenatal distress who retained an average of 15.1 pounds (Table 4).
FIGURE 3.
Weight change over time as predicted by intervention condition × prenatal distress
TABLE 4.
Weight change model estimates for intervention × prenatal distress group interaction, adjusted for model covariates (pounds)
| Group prenatal care |
Control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Weeks from conception | –1 SD prenatal distress | M prenatal distress | +1 SD prenatal distress | +2 SD prenatal distress | –1 SD prenatal distress | M prenatal distress | +1 SD prenatal distress | +2 SD prenatal distress |
| 12 wks | 2.25 | 2.14 | 2.03 | 1.92 | 2.45 | 2.58 | 2.71 | 2.84 |
| 20 | 12.0 | 11.8 | 11.7 | 11.5 | 13.0 | 13.5 | 13.9 | 14.4 |
| 40 | 24.4 | 24.3 | 24.2 | 24.2 | 25.8 | 26.9 | 28.0 | 29.2 |
| 6 wks’ postpartum | 25.4 | 25.4 | 25.4 | 25.3 | 26.6 | 27.9 | 29.2 | 30.6 |
| 6 mo postpartum | 23.6 | 23.6 | 23.7 | 23.8 | 23.2 | 25.4 | 27.5 | 29.7 |
| 12 mo postpartum | 15.4 | 15.3 | 15.2 | 15.1 | 12.3 | 16.3 | 20.3 | 24.2 |
Stratified weight differences for the full range of depression and prenatal distress are shown in Tables 3 and 4, respectively. These results demonstrate that women with the highest levels of depression and prenatal distress exhibited the greatest positive impact of group prenatal care on weight trajectories during pregnancy and through 12 months postpartum.
Comment
Our results demonstrate that group prenatal care has a significant positive effect on pregnancy weight gain trajectories and postpartum weight loss that extends to 12 months postpartum. This finding is further accentuated among women with prepregnancy obesity. Few studies to date have demonstrated an effect of lifestyle interventions on either gestational weight gain or postpartum weight loss, especially among those who are obese.12-14
Group prenatal care is not a weight-loss intervention. It is an innovative model of group medical care that provides more direct contact with providers (ie, 20 hours in the group setting) and deeply engages women and their family members, providing education and skills to have a healthy pregnancy. Participants discuss healthy nutrition and exercise in the first session as well as personally monitor their weight gain at each visit throughout the pregnancy. We believe that this intensive interaction with health care providers and self-care is likely the reason for these favorable outcomes.
Group prenatal care focuses on positive lifestyle choices to promote a healthier pregnancy and also has demonstrated to have improved birth outcomes such as preventing preterm birth, rapid repeat pregnancy, and better mental health outcomes.24,25,33 Moreover, although one might presume that the time commitment for group prenatal care would be a barrier for participation, we have generally found that most patients report that the amount of time they are typically required to spend in a waiting room in a traditional care setting, in combination with the time of their individual care appointment, is often fairly equivalent to the time investment required in the group care context.
A second reason that the group prenatal care may lead to less weight gain is that it appears to reduce stress for those high baseline psychosocial distress, as we know from our previous research,25 and thus, it may reduce stress-related eating and weight gain.21,22 This study demonstrates that group prenatal care has a significant impact of mitigating the negative effects of depression and prenatal distress on weight gain trajectories, especially in the postpartum period.
The effect is both statistically significant and clinically meaningful, with weight gain differentials throughout the perinatal and postpartum periods. One year postpartum weight loss differentials are as much as 8–10 pounds. Both depression and stress are associated with weight gain, binge eating, and an increased intake of high caloric, nonnutritious food.22,23 Addressing psychosocial factors in pregnancy and postpartum may lead to more sustained and beneficial results.11,34-37
This is a large cohort followed up longitudinally for 12 months post-partum. Participants are young and primarily ethnic minority; therefore, results may not be generalizable to women of different socioeconomic backgrounds. Although there was no apparent bias in the final sample analyzed, 20% of the original sample was excluded. The missing women, who were excluded because of health and pregnancy conditions, which may affect weight gain in pregnancy, or missing data may not have had as favorable weight outcomes. However, there was no difference in the groups at randomization or in the reason for patient exclusion by intervention. The results highlight the importance of incorporating weight control at a young age. More than one third of our cohort of young predominantly nulliparous women aged 14–21 years were overweight or obese at conception, and half gained weight excessively during pregnancy.
Pregnancy is a window of opportunity to improve health outcomes because women are highly motivated to incorporate healthy behaviors to improve the outcomes for themselves and their children. Group prenatal care may be creating healthy social norms that might override the predominant cultural norms.38,39 It creates a new social network along with information about what is healthy weight gain, social support, and peer role models for healthy weight. Given the burgeoning rates of obesity as well as the importance of perinatal health for the health of future generations, it is time to implement a more holistic approach to the obstetric care of women.
ACKNOWLEDGMENT
The study had the ClinicalTrials.gov identifier of NCT00628771.
This study was supported by the National Institute of Mental Health as a linked R01 to Yale University (grant R01 MH074399; J.R.I., principal investigator) and to Clinical Directors Network (grant R01 MH07394; J.N.T., principal investigator).
Footnotes
The National Institute of Mental Health had no role in the design or conducting of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
S.S.R. is Founder of the Centering Healthcare Institute and serves on the Board of Directors. The other authors report no conflict of interest.
Cite this article as: Magriples U, Boynton MH, Kershaw TS, et al. The impact of group prenatal care on pregnancy and postpartum weight trajectories. Am J Obstet Gynecol 2015;213:688.e1-9.
REFERENCES
- 1.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993e2003. Obesity. 2007;15:986–93. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention [April 21, 2015];Youth Risk Behavior Survey. 2011 Available at: www.cdc.gov/yrbs.
- 3.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine . Weight gain during pregnancy: re-examining the guidelines. National Academy of Sciences; Washington, DC: 2009. [Google Scholar]
- 5.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Pre-pregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 6.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287, 213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JL, Malone FD, Emig D, et al. Obesity, obstetric complications and cesarean delivery rate—a population-based screening study. FASTER Research Consortium. Am J Obstet Gynecol. 2004;190:1091–7. doi: 10.1016/j.ajog.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Persson M, Johansson S, Villamor E, Cnattingius S. Maternal overweight and obesity and risks of severe birth-asphyxia-related complications in term infants: a population-based cohort study in Sweden. PLoS Med. 2014;11:e1001648. doi: 10.1371/journal.pmed.1001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine Guidelines. Obstet Gynecol. 2013;121:969–75. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould Rothberg BE, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Gestational weight gain and subsequent postpartum weight loss among young, low-income, ethnic minority women. Am J Obstet Gynecol. 2011;204:52, e1–11. doi: 10.1016/j.ajog.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skouteris H, Hartley-Clark L, McCabe M, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev. 2010;11:757–68. doi: 10.1111/j.1467-789X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- 12.Muktabhant B, Lumbiganon P, Ngamjarus C, Dowswell T. Interventions for preventing excessive weight gain during pregnancy. Cochrane Database Syst Rev. 2012;4:CD007145. doi: 10.1002/14651858.CD007145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. Am J Clin Nutr. 2010;92:678. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 14.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furber CM, McGowan L, Bower P, Kontopantelis E, Quenby S, Lavender T. Ante-natal interventions for reducing weight in obese women for improving pregnancy outcome (Review). Cochrane Database of Syst Rev. 2013:CD009334. doi: 10.1002/14651858.CD009334.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for over-weight or obese pregnant women: a systematic review of randomised trials. BJOG. 2010;117:1316. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 17.Agha M, Agha RA, Sandell J. Interventions to reduce and prevent obesity in preconceptual and pregnant women: a systematic review and meta-analysis. PLoS One. 2014;9:e95132. doi: 10.1371/journal.pone.0095132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN. The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis. Int J Obes. 2014;38:626–35. [Google Scholar]
- 19.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mother, children, research and practice. Curr Opin Psychiatry. 2012;25:141–8. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunkel Schetter C. Psychological science on pregnancy: stress processes, bio-psychosocial models and emerging research issues. Annual Rev Psychol. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 21.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol. 2014;5:434. doi: 10.3389/fpsyg.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groesz LM, McCoy S, Carl J, et al. What is eating you? Stress and the drive to eat. Appetite. 2012;58:717–21. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007;110(2 Pt 1):330–9. doi: 10.1097/01.AOG.0000275284.24298.23. Erratum in: Obstet Gynecol 2007;110:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ickovics JR, Reed E, Magriples U, Westdahl C, Schindler Rising S, Kershaw TS. Effects of group prenatal care on psychosocial risk in pregnancy: results from a randomised controlled trial. Psychol Health. 2011;26:235–50. doi: 10.1080/08870446.2011.531577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner CF, Ku L, Rodgers SM, et al. Adolescent sexual behavior, drug use and violence: Increased reporting with computer survey technology. Science. 1998;280:867–73. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists . Guidelines for Perinatal Care. 7th ed. American College of Obstetricians and Gynecologists; Washington, DC: 2012. [Google Scholar]
- 28.Lobel M. The Revised Pregnancy Distress Questionnaire (NUPDQ) State University of New York at Stony Brook; Stony Brook, NY: 1996. [Google Scholar]
- 29.Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J of Psychosom Obstet Gynecol. 1999;20:39–52. doi: 10.3109/01674829909075575. [DOI] [PubMed] [Google Scholar]
- 30.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 31.Gans KM, Ross E, Barner CW, Wylie-Rosett J, McMurray J, Eaton C. REAP and WAVE: new tools to rapidly assess/discuss nutrition with patients. J Nutr. 2003;133:556S–62S. doi: 10.1093/jn/133.2.556S. [DOI] [PubMed] [Google Scholar]
- 32.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press; London (UK): 2003. [Google Scholar]
- 33.Kershaw TS, Magriples U, Westdahl C, Rising SS, Ickovics JR. Pregnancy as a window of opportunity for HIV prevention: effects of an HIV intervention delivered within prenatal care. Am J Public Health. 2009;99:2079–86. doi: 10.2105/AJPH.2008.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116:1191–5. doi: 10.1097/AOG.0b013e3181f60da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogaerts AFL, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BRH. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes. 2013;37:814–21. doi: 10.1038/ijo.2012.162. [DOI] [PubMed] [Google Scholar]
- 36.Phillips J, King R, Skouteris H. The influence of psychological factors on post-partum weight retention at 9 months. Br J Health Psychol. 2014;19:751–66. doi: 10.1111/bjhp.12074. [DOI] [PubMed] [Google Scholar]
- 37.Hemmingsson E. A new model of the role of psychological and emotional distress in promoting obesity: conceptual review with implications for treatment and prevention. Obes Rev. 2014;15:769–79. doi: 10.1111/obr.12197. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Xue H, Chen HJ, Igusa T. Examining social norm impacts on obesity and eating behaviors among US school children based on agent-based model. BMC Public Health. 2014;14:923. doi: 10.1186/1471-2458-14-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]