Abstract
Recent evidence has suggested that the N-methyl-D-aspartate receptor antagonist ketamine shows significant therapeutic effects in major depression and bipolar disorder. This effect is especially important in treatment-resistant depression and depression with suicidal ideation. In this review we explain the mechanism of action, drug efficacy, and the side effects of ketamine; the antidepressive effects of ketamine; the individual effects of ketamine isomers, R(–) ketamine and S(+) ketamine; the effects of the combination of ketamine with electroconvulsive therapy; and the possible use of ketamine in treating depression.
Keywords: depression, electroconvulsive therapy, ketamine
Introduction
Major depressive disorder (MDD) is a debilitating mental illness that affects millions of people worldwide leading to severe health and socioeconomic consequences [Kessler et al. 2003]. Although many antidepressant treatments are available, there is a need for more effective treatments, especially for treatment-resistant depression (TRD) and depression with suicidal ideation. Moreover, most antidepressant treatments require a period of several weeks before improvements in mood are experienced. Therefore, therapeutic options that are able to bypass the lag period for improvements in symptoms are needed.
The majority of approved antidepressant medications for MDD act through monoaminergic mechanisms [Iadarola et al. 2015]. The concept is that depression is related to some imbalance in neurotransmitters such as serotonin, dopamine and norepinephrine. However, there is evidence that other neurotransmitters may also play a role. The hippocampal-prefrontal circuit (HC-PFC) is one of the key pathways mediating cognition and memory. In major depression, this circuit shows a decrease in functional connectivity [Thierry et al. 200]. The HC-PFC pathway uses both excitatory and inhibitory neurotransmitter receptors, N-methyl-D-aspartate (NMDA) receptors and γ-aminobutryic acid type A (GABAA) receptors respectively [Thierry et al. 2000]. In order to search for a drug that is a more effective, quicker acting antidepressant, particular attention must be placed on the glutamatergic and GABAergic systems. Recent research suggests that low-dose ketamine can act as a novel, rapid-acting antidepressant [Naughton et al. 2014]. In fact, a single subanesthetic dose infusion of ketamine has rapid and potent antidepressant effects in treatment-resistant major depression and bipolar depression [Iadarola et al. 2015]. Ketamine as an antidepressant agent is of great interest as an alternative to the delayed onset to efficacy, repeated administration and unwanted side effects of current pharmacotherapeutics, behavioral therapies and electroconvulsive therapy (ECT).
Drug information
Ketamine hydrochloride, a chiral arylcyclohexylamine derivative, synthesized in 1962, has been used clinically as an intravenous anesthetic since 1970. It produces dissociative anesthesia and effective analgesia. Although it renders the patient pain free, it does not induce sedation or hypnosis seen with other general anesthetics. The patient’s eyes may open, the patient may retain some normal reflexes such as coughing and swallowing, and the patient’s extremities may move without purpose. However, when patients recover from ketamine anesthesia, they have no memory of the surgery or their movements. Ketamine has a rapid onset of action (<1 min) and is metabolized quickly so that patients regain full orientation within 30 min. The analgesic effect of ketamine, however, lasts longer than the dissociative anesthesia. Indeed, subanesthetic doses of ketamine are analgesic. Unlike opiates, ketamine does not depress respiration [Vuyk et al. 2015].
The first recognized primary mechanism of action of ketamine is inhibition of NMDA receptors. These receptors are located at synapses where glutamine is the neurotransmitter. They are involved in learning and memory [Rezvani, 2006]. Inhibition of NMDA receptors decreases excitation in some areas of the nervous system. In the cortex, this allows for inhibitory circuits (with GABA as the neurotransmitter) to have a greater influence. In addition, ketamine has been shown to potentiate certain forms of the GABAA receptor [Hevers et al. 2008], similar to most general anesthetics. It has become clear, however, that ketamine has many other effects as a neuromodulator and a modulator for gene expression [Sleigh et al. 2014]. In the spinal cord, inhibition of NMDA receptors leads to decreased release of acetylcholine leading to analgesia [Lydic and Baghdoyan, 2002].
Ketamine is a desirable anesthetic because of its short half-life (180 min) and the lack of respiratory depression [Clemens et al. 1982]. Similar to phencyclidine, when abused at subanesthetic doses, ketamine produces dissociative and hallucinogenic effects. It also distinguishes itself from conventional antidepressants through its rapid effect and its efficacy. In fact, low doses of ketamine (1–4.5 mg/kg intravenously or 6.5–13 mg/kg intramuscularly) take about 30–40 min to show mild psychotomimetic and dissociative effects that then completely dissipate by 80 min [Zarate et al. 2006].
Some of the side effects of ketamine include perceptual disturbances, confusion, elevations in blood pressure, euphoria, dizziness and increased libido [Zarate et al. 2006]. However, the majority of side effects cease within 80 min after the infusion. Chronic side effects include ulcerative cystitis, cognitive and memory impairments, liver damage and structural brain damage. Also, there is a high potential for abuse. Ketamine, or ‘Special K’ is one of the several ‘club drugs’ that is abused to produce a dissociative state of relaxed wellbeing. Animal studies show a high level of self administration, which can be translated to the drug being highly reinforcing. This is in part due to the activating effect on midbrain dopaminergic cell firing and the stimulation of dopamine release, particularly in the prefrontal cortex [Tan et al. 2012].
Stereoisomerism
Stereoisomers have the same atoms connected by the same bonds, but contain different three-dimensional orientation. Stereoisomerism in drugs is often due to chirality or ‘handedness’; that is, the presence of right-handed (R) and left-handed (S) forms of drugs, which are nonsuperimposable mirror images (‘enantiomers’). Approximately 60% of anesthetic agents are chiral drugs; some of these are administered as single enantiomers [Calvey, 1995]. However, many synthetic chiral drugs are equal mixtures of (R) and (S) isomers, and there are often important differences in their activity and pharmacokinetics [Calvey, 1995]. These differences suggest that some anesthetic drugs (particularly ketamine and chiral local anesthetics) should be administered as single enantiomers. The use of single stereoisomers is gaining popularity. Relatively minor modifications in a drug’s structure can influence its affinity for a specific receptor site and its intrinsic pharmacologic activity. Determination of isomers of intravenous anesthetics in plasma by liquid–liquid extraction, followed by high-performance liquid chromatography made it possible to evaluate the distinct pharmacodynamic and pharmacokinetic profiles of isomers [Jones et al. 1996].
Stereoisomerism and ketamine
Ketamine is a good example to illustrate how subtle changes in stereoisomerism can result in significant changes in structure–activity relationships [Modica et al. 1990]. The pharmacological profile of racemic ketamine is characterized by the so-called dissociative anesthetic state and profound sympathomimetic properties. Ketamine, used clinically as an intravenous analgesic and dissociative anesthetic agent, is a racemate with both pharmacokinetic and pharmacodynamic enantioselectivity [Calvey, 1995]. Until recently, clinically available ketamine was a racemic mixture containing equal amounts of two enantiomers, S and R ketamine (Figure 1).
Figure 1.
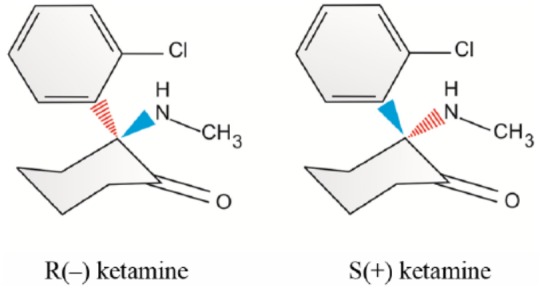
Optical isomers of ketamine.
S ketamine has been found to have a higher clearance and greater potency, as well as a greater therapeutic index than R ketamine. Uptake and metabolism of ketamine into different tissues was found to be enantioselective [Edwards and Mather, 2001]. Ketamine isomer (S+) induces less drowsiness, less lethargy and less impairment in clustered subjective cognitive capacity than equianalgesic small-dose racemic ketamine. In addition, S(+) ketamine causes less decline in concentration capacity and primary memory [Pfenninger et al. 2002]. The S(+) isomer of ketamine has about twice the analgesic potency of the clinically used racemic mixture. Therefore, the known side effects may be reduced when one half of the usual dose is administered. Several prospective, randomized and double-blinded studies have been performed to assess whether the S(+) isomer of ketamine is superior to the racemic mixture with respect to circulatory side effects [Zielmann et al. 1997]. When compared with R ketamine and the racemic mixture, the analgesic and anesthetic potency of S ketamine is at least twofold higher. The pharmacokinetic improvements of S ketamine are characterized by a reduced drug load, along with more rapid recovery. Distinctive effects are observed, when S(+) ketamine is used alone [Adams, 1997]. S(+) ketamine causes fewer psychotic emergent reactions, less agitated behavior, and better intraoperative amnesia and analgesia than its R(–) enantiomer [White et al. 1980, 1985]. S(+) ketamine appears to have distinct differences in pharmaceutical and clinical characteristics compared with its R(–) enantiomer (Table 1).
Table 1.
Distinct characteristics of S(+) ketamine compared with R(–) ketamine.
| Higher affinity for NMDA receptor |
| Higher anesthetic potency |
| Faster clearance |
| Rapid recovery |
| Better analgesia |
| Better intraoperative amnesia |
| Less drowsiness |
| Less lethargy |
| Less impairment in cognition |
| Less decline in capacity to concentrate |
| Less decline in primary memory |
| Few psychotic emergent reactions |
| Less agitated behavior |
NMDA, N-methyl-D-aspartate.
Clinical trials for ketamine as an antidepressant
More than 50 clinical trials pertaining to ketamine and depression have been registered to date in the United States (ClinicalTrials.gov). The first placebo-controlled, double-blinded clinical trial of ketamine for major depression was conducted at Yale University in 2000. Seven subjects with major depression completed two test days separated by at least 1 week that involved intravenous treatment with 0.5 mg/kg ketamine hydrochloride or saline solutions [Berman et al. 2000]. These patients also completed the Hamilton Depression Rating Scale (HDRS). The results showed that subjects with depression experienced significant improvement in depressive symptoms within 72 h after ketamine but not placebo infusion. These results suggested a potential role for ketamine in the treatment of depression and have led to more research on the topic.
Diazgranados and colleagues sought to determine if ketamine produces rapid antidepressant effects in subjects with bipolar depression [Diazgranados et al. 2010]. Eighteen subjects with treatment-resistant bipolar depression were involved and maintained at therapeutic levels of lithium or valproate. They received an intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo on two test days two weeks apart. To rate subjects at baseline and post infusion, the Montgomery–Asberg Depression Rating Scale (MADRS) was used. The results showed that within 40 min, depressive symptoms significantly improved in subjects receiving ketamine compared with those receiving placebo. It was also observed that this improvement remained significant through day three and the effect size was largest on day two. Interestingly, the most common adverse effect was dissociative symptoms and this was observed only at the 40-min point.
In the past few years, trials have focused on single intravenous infusions lasting 40 min of racemic ketamine hydrochloride at a dose of 0.5 mg/kg body weight. As mentioned earlier, target populations include those with TRD, bipolar depression and suicidal ideation. Luckenbaugh and colleagues sought to determine whether increased sympathomimetic and hypoglutamatergic effects were related to ketamine’s antidepressant efficacy [Luckenbaugh et al. 2014]. Data were collected from 108 patients with treatment-resistant depression who each received a single subanesthetic ketamine infusion of 0.5 mg/kg. Pearson correlations were performed to examine any associations between rapid changes in dissociation and psychotomimesis with the Clinician-Administered Dissociative States Scale (CADSS) and Brief Psychiatric Rating Scale, respectively. The results presented significant associations between increased CADSS score at 40 min and percentage improvement with ketamine in HDRS at 230 min and on day 7. From this study, one can conclude that only dissociative side effects predict a more robust and sustained antidepressant. This supports the conclusion found by Diazgranados and colleagues, where the most common side effect was dissociative symptoms and there was an improvement in depressive symptoms [Diazgranados et al. 2010).
Hassamal’s group reported the first case of exploring long-term ketamine efficacy as augmentation therapy in TRD [Hassamal et al. 2015]. In this case, the patient went into remission after augmentation with six ketamine intravenous infusions over a 3-week period. The patient’s symptoms reemerged after 4 months, at which time a second series of three intravenous infusions were given. This time, the patient went into remission for 8 months and when the symptoms reemerged, another series of three intravenous infusions were given. The patient received a total of 12 ketamine injections over the course of 18 months with no significant adverse effects.
Mechanism of ketamine action in depression
The antidepressant effects of ketamine are observed at 100 min and are sustained for approximately 7 days after a single dose [Zarate et al. 2006]. This fact suggests that ketamine initiates a cascade of events that result in a rapid response that is sustained even after the drug is metabolized and excreted. Studies have reported enhanced synaptic plasticity and synaptogenesis via numerous molecular and cellular mechanisms [Iadarola et al. 2015]. It is understood that exposure to stress (such as in depression) causes atrophy of neurons in limbic brain regions such as the prefrontal cortex and the hippocampus [McEwen, 2008]. This atrophy includes a decrease in the density of spines and in the number and length of dendrite branches. Li and colleagues found that administration of ketamine (10 mg/kg) intraperitoneally results in the rapid induction of spine number in the PFC [Li et al. 2010]. After 24 h, an increase in the number of mature spines was observed, which indicates that ketamine increases spine stability and function. This was shown to be dependent on stimulation of the mammalian target of rapamycin (mTOR). mTOR is a large serine/threonine kinase that regulates the initiation of protein translation [Naughton et al. 2014]. It is thus suggested that mTOR-mediated pathways may be important for fast-acting antidepressants. Furthermore, it is important to note that other antidepressants do not stimulate mTOR [Li et al. 2010].
One such mechanism of action begins by the blockage of the NMDA receptor by ketamine, which leads to less activation of eukaryotic elongation factor 2 (eEF2) kinase, which gradually results in loss of eEF2 phosphorylation and desuppression of brain-derived neurotrophic factor (BDNF) translation [Autry et al. 2001]. BDNF translation triggers tyrosine-related kinase B (TrkB) signaling which leads to transphosphorylation and downstream activation of extracellular signaling related kinases (ERKs) and protein kinase B, as well as suppression of glycogen synthase kinase 3. The combination of these events activates mTOR, which in turn leads to increased synaptic proteins and spine densities resulting in synaptogenesis [Li et al. 2010]. The ultimate result of this pathway leads to positive behavioral effects. Further supported by Haile et al. (2014), this pathway suggests that plasma BDNF acts as a peripheral biomarker and is relevant to ketamine antidepressant response. In this study, BDNF levels were associated with clinical outcomes for patients receiving ketamine therapy for TRD, observed using the MADRS.
Psychotomimetic effects of R(–) ketamine and S(+) ketamine
Ketamine is a racemic mixture containing equal parts of R(–) ketamine and S(+) ketamine. S ketamine has about a fourfold greater affinity for the NMDA receptor and greater anesthetic potency [Domino, 2010]. Vollenweider and colleagues observed through a positron emission tomography study in healthy volunteers that psychotomimetic doses of S ketamine increase cerebral metabolic rates of glucose (CMRglu) in the frontal cortex and thalamus, suggesting that the psychotomimetic and hyperfrontal metabolic actions of ketamine are probably induced by its S isomer [Vollenweider et al. 1997]. They also found that equimolar doses of R ketamine tended to decrease CMRglu across the brain, producing no psychotic symptoms but rather a state of relaxation and elation. Zhang’s group explored this phenomenon by testing juvenile mice in the tail suspension test and forced swimming test after neonatal dexamethasone exposure [Zhang et al. 2014]. The results showed that R ketamine appears to be a potent and safe antidepressant relative to S ketamine in animals. Although both R and S ketamine induce antidepressant effects, the authors observed that R ketamine has a longer lasting action. Robson and colleagues found that R ketamine not only interacts with NMDA receptors but also has affinity for σ1 receptor chaperones [Robson et al. 2012]. Therefore, it is unlikely that the NMDA receptor plays a large role in the long-term antidepressant effects of R ketamine, and there are possible interactions with other systems.
Yang and his coworkers reported one of the most novel and noteworthy studies with regard to ketamine isomerization and its effects on depression. This study compared the two stereoisomers of ketamine with regard to their potential antidepressant efficacies. To explore this, the effects of each stereoisomer on BDNF TrkB signaling and synaptogenesis in selected brain regions were examined. In order to observe and quantify depression, the social defeat stress and learned helplessness mice models of depression were used. It was found that R ketamine shows a greater potency and longer-lasting antidepressant effect than S ketamine. R ketamine also induces a more potent beneficial effect and decreased dendritic spine density, BDNF TrkB signaling and synaptogenesis in the prefrontal cortex, Cornus Ammonis Region 3 (CA3) and dentate gyrus. It was also found that S ketamine generated side effects such as hyper locomotion, whereas R ketamine did not. In conclusion, R ketamine was found to be a potent, long-lasting and safe antidepressant free of psychotomimetic side effects in animal studies. This effect is mediated by increased BDNF TrkB signaling and synaptogensis in the PFC, Dentate Gyrus (DG) and CA3, as mentioned earlier [Yang et al. 2015]. Further studies are needed to explore these systems.
Ketamine and ECT
ECT is known as a highly beneficial treatment of unipolar and bipolar depression and it generally has a more rapid onset of action than conventional antidepressants [Kellner et al. 2012]. However, the limited data on the onset of ECT’s antidepressant action suggests that a range of five to seven treatments or about 2 weeks is required to cause significant reductions in depressive symptoms. Ghasemi and colleagues sought to investigate the antidepressant effects of ketamine in comparison with ECT in hospitalized patients with depression experiencing a major depressive episode [Ghasemi et al. 2014]. In this study, 18 patients experiencing a major depressive episode were randomly assigned either to the ECT group or the ketamine group. The ECT group who were given 0.5 mg atropine and 2–3 mg/kg thiopental intravenously followed by succinylcholine (0.5 mg/kg) as muscle relaxant underwent three ECT sessions every 48 h, while the ketamine group received three infusions of ketamine hydrochloride (0.5 mg/kg) over 45 min on three test days (every 48 h). Clinical assessment was performed using the HDRS and the Beck Depression Inventory (BDI). The results suggest that a single infusion of ketamine reduced scores of the BDI and the HDRS more compared with that of ECT. In fact, the first infusion of ketamine led to a 42% reduction of more than half of depressive symptoms in the case of 44% of participants. However, the first ECT showed a 12% reduction of depressive symptoms. These data suggest that the antidepressant effects of ketamine are more rapid and effective than ECT in the early stages of treatment.
Studies showed that ketamine may protect against the cognitive side effects of ECT [Rasmussen et al. 1996]. One study compared anesthesia during ECT with ketamine versus methohexital [Rasmussen et al. 2014]. The results showed no significant differences in depression or cognitive outcomes between the two drugs. It was also found that ketamine anesthesia does not accelerate the antidepressant effect of ECT or diminish the cognitive side effects. Finally, this study explained that there is no apparent benefit of ketamine for speed or quality of post-ECT recovery.
Okamoto and colleagues determined whether ketamine as the anesthetic during ECT would provide a greater antidepressant effect than the antidepressant effect obtained with propofol [Okamoto et al. 2010]. The results showed that the HDRS scores improved earlier in the ketamine group than the propofol group. Also, decreases in HDRS scores were significantly greater in the ketamine group and it was suggested that it is possible to improve symptoms of depression earlier by using ketamine anesthesia. This seems to contradict Rasmussen and colleagues’ findings [Rasmussen et al. 2014]. However, methohexital and propofol are two different drugs that may cause different effects and interactions. Further work is necessary to clarify this.
Future outlook
The idea of using ketamine as an antidepressant is relatively new and has recently been receiving a lot of attention. Some recent studies are exploring other routes of ketamine administration such as intranasal treatment or oral treatment [Ghasemi et al. 2014]. Many questions remain to be answered, such as what is the optimal dose and route of administration, what are the long-term side effects of this treatment, and how would it be possible to sustain the response? For instance, a recently published article examines the advantages and applications of using intranasal drug delivery (INDD) in neuropsychiatry [Andrade, 2015]. The advantage of using INDD administration is that this system offers a route directly to the brain and bypasses issues relating to gastrointestinal absorption, first-pass metabolism and the blood brain barrier, among other things. Using INDD, frequent injections of ketamine could be bypassed which would allow for a more practical strategy for maintenance therapy. The question still remains as to whether maintenance treatment with intranasal ketamine could be a viable strategy to extend treatment gains which has not been investigated to date in the context of depression.
Also, ketamine has been suggested to induce near-death-experience-like phenomenology and could preserve or possibly enhance end-of-life brain activity [Jansen, 2000]. This presents the possibility that end-of-life brain activity may correspond with near-death experiences and out-of-body experiences, which are also induced through the use of ketamine. Perhaps this suggests that conscious awareness otherwise known as the ‘soul’ can exist after death. Based on this, it would be interesting to know whether ketamine be acumen to the human soul.
Conclusion
The NMDA receptor antagonist ketamine has recently been found to exhibit rapid antidepressant effects in major depression and bipolar disorder. This effect is especially important in TRD and depression with suicidal ideation. It is suggested that ketamine has an effect on the mTOR-mediated pathways, which work to increase synaptogenesis and decrease depressive symptoms. The individual effects of R(–) ketamine and S(+) ketamine suggest that R ketamine appears to be a potent and safe antidepressant relative to S ketamine. The antidepressant properties of ketamine have gained considerable attention and it seems that this is the direction of future treatment with regard to major depression.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
John Muller, Department of Anesthesiology, Stony Brook Medical Center, Stony Brook, NY, USA.
Sahana Pentyala, Department of Anesthesiology, Stony Brook Medical Center, Stony Brook, NY, USA.
James Dilger, Department of Anesthesiology, Stony Brook Medical Center, Stony Brook, NY, USA.
Srinivas Pentyala, Department of Anesthesiology, Stony Brook Medical Center, Stony Brook, NY 11794, USA.
References
- Adams H. (1997) S-(+)-ketamine. Circulatory interactions during total intravenous anesthesia and analgesia-sedation. Anaesthesist 46: 1081. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2015) Intranasal drug delivery in neuropsychiatry: focus on intranasal ketamine for refractory depression. J Clin Psychiatry 76: 628–631. [DOI] [PubMed] [Google Scholar]
- Autry A., Adachi M., Nosyreva E., et al. (2011) NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature 475: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R., Cappiello A., Anand A., Oren D., Heninger G., Charney D., et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Calvey T. (1995) Isomerism and anaesthetic drugs. Acta Anaesthesiol Scand Suppl 106: 83. [DOI] [PubMed] [Google Scholar]
- Clemens J., Nimmo W., Grant I. (1982) Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. Pharm Sci 71: 539–542. [DOI] [PubMed] [Google Scholar]
- Diazgranados N., Ibrahim L., Brutsche N., Newberg A., Kronstein P., Khalife S., et al. (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino E. (2010) Taming the ketamine tiger. Anesthesiology 113: 678–686. [DOI] [PubMed] [Google Scholar]
- Edwards S., Mather L. (2001) Tissue uptake of ketamine and norketamine enantiomers in the rat: indirect evidence for extrahepatic metabolic inversion. Life Sci 69: 2051. [DOI] [PubMed] [Google Scholar]
- Ghasemi M., Kazemi M., Yoosefi A., Ghasemi A., Paragomi P., Amini H., et al. (2014) Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 215: 355–361. [DOI] [PubMed] [Google Scholar]
- Haile C., Murrough J., Iosifescu D., Chang L., Al Jurdi R., Foulkes A., et al. (2014) Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassamal S., Spivey M., Pandurangi A. (2015) Augmentation therapy with serial intravenous ketamine over 18 months in a patient with treatment resistant depression. Clin Neuropharmacol 38: 212–216. [DOI] [PubMed] [Google Scholar]
- Hevers W., Hadley S., Lüddens H., Amin J. (2008) Ketamine, but not phencyclidine, selectivity modulates cerebellar GABAA receptors containing α6 and δ subunits. J Neurosci 28: 5383–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola N., Niciu M., Richards E., Vande Voort J., Ballard E., Lundin N., et al. (2015) Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis 6: 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen K. (2000) A review of the nonmedical use of ketamine: use, users and consequences. J Psychoactive Drug 32: 419–433. [DOI] [PubMed] [Google Scholar]
- Jones D., Nguyen K., McLeish M., Crankshaw D., Morgan D. (1996) Determination of (R)-(+)- and (S)-(–)-isomers of thiopentone in plasma by chiral high-performance liquid chromatography,J Chromatogr B Biomed Appl 675: 174. [DOI] [PubMed] [Google Scholar]
- Kellner C., Greenberg R., Murrough J., Bryson E., Briggs M., Pasculli R. (2012) ECT in treatment-resistant depression. Am J Psychiatry 169: 1238–1244. [DOI] [PubMed] [Google Scholar]
- Kessler R., Berglund P., Demler O. (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R., Banasr M., Dwyer J., Iwata M., et al. (2010) mTOR dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh D., Niciu M., Ionescu D., Nolan N., Richards E., Brutsche N., et al. (2014) Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Dis 159: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R., Baghdoyan H. (2002) Ketamine and MK-801 decrease acetylcholine release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep 25: 617–622. [PubMed] [Google Scholar]
- McEwen B. (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica P., Tempelhoff R., White P. (1990) Pro- and anticonvulsant effects of anesthetics (part II). Anesth Analg 70: 433. [DOI] [PubMed] [Google Scholar]
- Naughton M., Clarke G., O’Leary O., Cryan J., Dinan T. (2014) A review of ketamine in affective disorders: Current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanism of action. J Affect Dis 156: 24–35. [DOI] [PubMed] [Google Scholar]
- Okamoto N., Nakai T., Sakamoto K., Nagafusa Y., Higuchi T., Nishikawa T. (2010) Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT 26: 223–227. [DOI] [PubMed] [Google Scholar]
- Pfenninger E., Durieux M., Himmelsehe S. (2002) Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology 96: 357. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Jarvis M., Zorumski C. (1996) Ketamine anesthesia in ECT. Convuls Ther 12: 217–223. [PubMed] [Google Scholar]
- Rasmussen K., Kung S., Lapid M., Oesterle T., Geske J., Nuttall G., et al. (2014) A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Res 215: 362–365. [DOI] [PubMed] [Google Scholar]
- Rezvani A. (2006) Involvement of the NMDA system in learning and memory. In: Levin E., Buccafusco J. (eds), Animal Models of Cognitive Impairment. Boca Raton, FL: CRC Press, pp. 37–48. [PubMed] [Google Scholar]
- Robson M., Elliott M., Seminerio M., Matsumoto R. (2012) Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo. Eur Neuropsychopharmacol 22: 308–317. [DOI] [PubMed] [Google Scholar]
- Sleigh J., Harvey M., Voss L., Denny B. (2014) Ketamine – more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care 4: 76–81. [Google Scholar]
- Tan S., Lam W., Wai M., Yu W., Yew D. (2012) Chronic ketamine administration modulates midbrain dopamine system in mice. PLOS One 7: e43947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry A., Gioanni Y., Dégénétais E., Glowinski J. (2000) Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10: 411–419. [DOI] [PubMed] [Google Scholar]
- Vollenweider F., Leenders K., Oye I., Hell D., Angst J. (1997) Differential psychopathology and patterns of cerebral glucose utilization produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7: 25–38. [DOI] [PubMed] [Google Scholar]
- Vuyk J., Sitsen E., Reekers M. (2015) Intravenous anesthetics. In: Miller R. (ed.), Miller’s Anesthesia, 8th ed. Philadelphia, PA: Elsevier Saunders, pp. 821–828. [Google Scholar]
- White P., Ham J., Way W., Trevor A. (1980) Pharmacology of ketamine isomers in surgical patients. Anesthesiology 52: 231. [DOI] [PubMed] [Google Scholar]
- White P., Schuttler J., Shafer A., Stanski D., Horai Y., Trevor A. (1985) Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth 57: 197. [DOI] [PubMed] [Google Scholar]
- Yang C., Shirayama Y., Zhang J., Ren Q., Yao W., Ma M., et al. (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C., Jr, Singh J., Carlson P. (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li S., Hashimoto K. (2014) R (–)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116: 137–141. [DOI] [PubMed] [Google Scholar]
- Zielmann S., Kazmaier S., Schnull S., Weyland A. (1997) S-(+)-ketamine and circulation. Anaesthesist 46(Suppl. 1): S43. [DOI] [PubMed] [Google Scholar]


