Abstract
Objective:
Therapeutic drug monitoring (TDM) enables individualization in the treatment to optimize clinical benefit and minimize drugs' side effects. Critically ill septic patients represent a challenge for antimicrobial treatment because of pathophysiological impact of sepsis on pharmacokinetics of drugs. The aim of this study was to assess the appropriateness of gentamicin and amikacin dosing in critically and noncritically ill patients, as well as to estimate the need for its regular therapeutic monitoring.
Subjects and Methods:
It was a prospective study which included 31 patients on gentamicin and 16 patients on amikacin from four different units who met the inclusion criteria. Trough concentrations of drugs were measured in serum just before third or fourth dose of antibiotic, whereas peak concentrations were measured in serum 1 h after the completion of drug administration (steady state). Relevant data on patients' clinical course of disease, comorbidities, and concomitant medication were collected from medical charts in order to identify their possible influence on drugs' concentrations.
Results:
Peak concentrations of amikacin were in reference range in 81.8% critically ill and in 80% of noncritically ill patients (P = 0.931). Peak concentrations of gentamicin were in reference range in 88.9% critically ill and in 77.3% of noncritically ill patients (P = 0.457).
Conclusion:
Serum concentrations of aminoglycosides (amikacin and gentamicin) were in reference range in most of the patients in our study, suggesting that dosing of these drugs in the University Hospital Clinical Center, Banja Luka, was adequate. In patients without kidney or liver disease, regular TDM of aminoglycosides is not necessary.
Keywords: Aminoglycosides, infection, therapeutic drug monitoring
Introduction
Therapeutic drug monitoring (TDM) is defined as the laboratory measurement of drug serum concentration along with adequate medical interpretation influencing the management of drug therapy in patients.[1,2] On the other hand, TDM represents individualization of drug dosing maintaining its serum concentration within set range enabling the assessment of safety and efficacy of certain drug in various clinical conditions.[3] The purpose of TDM can be found in individualization of therapeutic regimen to gain optimal therapeutic benefits for the patient when treated with certain drugs.[4,5] TDM has significance in improving patient outcome and is utmost important in drugs with narrow therapeutic index, drugs with high pharmacokinetic variability, or in patients with gastrointestinal, hepatic, or renal insufficiency.[6,7]
Studies on aminoglycosides have demonstrated shorter hospitalization in patients who underwent TDM.[8] In TDM, therapeutic range is used as a guide for optimal drug concentration in serum, but this range should not be considered as an absolute one. In certain patients, drug effect will be evident even in case of too low serum concentration, whereas other patients will experience toxic effects, when drug serum concentration is even in therapeutic range. Hence, dosing of drugs should not be led only by serum drug concentration, but by its clinical response as well.[9]
In TDM, concentration of drugs is mostly measured in serum, whole blood, or in saliva. Time of sampling plays an important role since it is only performed after drug has reached steady state.
Antibiotics are drugs with wide therapeutic index and are administered in usual doses in majority of the patients, with exceptions of aminoglycosides, (especially gentamicin) where toxicity is closely associated with increased serum concentration and glycopeptides (especially vancomycin), where therapeutic failure is closely associated with too low serum concentration.[10]
Data on TDM of gentamicin and amikacin in critically and noncritically ill patients in hospitals of Republic of Srpska and Bosnia and Herzegovina are very scarce. Hence, this study was conducted to investigate (a) if serum concentrations of gentamicin and amikacin are within therapeutic range in mechanically-ventilated patients (critically ill) in the Medical Intensive Care Units (MICUs) as well as in noncritically ill patients in medical units (b) appropriateness of gentamicin and amikacin dosing in these units, and (c) the if there is any necessity of introducing TDM of aminoglycosides in our institution.
Subjects and Methods
This was a prospective study conducted in patients hospitalized in the MICU, Surgical ICU (SICU), infectious disease unit and general surgical unit as well as other units of University Hospital Clinical Center, Banja Luka. This study included 31 patients who were treated with gentamicin and 16 patients treated with amikacin who met the inclusion criteria. Study inclusion criteria were patients (1) diagnosed with infection (2) treated with amikacin or gentamicin (3) older than 18 years of age (4) not on renal replacement therapy (hemodialysis, peritoneal dialysis, continuous hemodiafiltration, etc.,) (5) with no chronic renal or liver failure, and (6) nonpregnant. Patient data were collected on diagnosis, comorbidities, inflammation parameters, thrombocytes, serum creatinine, urea, bilirubin, serum glucose, aspartate aminotransferase, alanine aminotransferase, detected microorganism, antibiogram, concomitant treatment, time of sampling, time of antibiotic administration, and adverse drug effects (nephrotoxicity, neurotoxicity, ototoxicity) by reviewing patients' charts, laboratory reports, and other medical documentation. Patients who had one or more organ's failure due to infection and progressed to hemodynamic instability were considered as suffering from severe life-threatening infection (critically ill).
Protocol of blood sampling
Blood sampling was done by nurses who were part of medical teams in two ICUs and two medical units using following protocol:
First blood sample (3-5 mL) was taken half an hour before the second dose of drug (when antibiotic was administered once daily) and third or fourth dose of drug (when antibiotic was administered twice or three times daily)
Second blood sample (3-5 mL) was taken 1 h after the administration of the fourth dose of drug
Both blood samples were sent to Institute of Laboratory Diagnostics within 2 h
Samples were stored at −20°C in the Institute of Laboratory Diagnostics.
Following ranges were considered normal with standard dosing of gentamicin and amikacin:
Gentamicin, Cmin : <1 mg/L, Cmax : 5-10 mg/L (8-10 mg/L for severe infections)
Amikacin, Cmin : 5-10 mg/L, Cmax : 20-30 mg/L (40 mg/L in life-threatening infections).
Analytical method
In vitro quantitative measurements of gentamicin and amikacin serum concentrations were performed using Cobas® (Roche/Hitachi cobas c systems, cobas c 501), with measurement range of 1.7-80 μg/L (1.2-55.2 μmol/L). The test is based on homogenous enzymatic technique for quantitative analysis of gentamicin and amikacin in human serum or plasma. Principle of the test implies competition between drug in the sample and the drug marked with enzyme glucose-6-phosphate dehydrogenase (G6PDH) for binding to receptors on antibodies. Enzymatic activity is decreased by binding to antibodies, which enables the determination of serum drug concentration by measurement of enzymatic activity. Active enzyme converts oxidized nicotine amide dinucleotide (NAD) into NADH, which results in change of absorption, which is measured using spectrophotometry. The process is not distracted by endogenic G6PDH since coenzyme reacts only with bacterial (Leuconostoc mesenteroides) enzyme in the test.
Statistical analysis
Data were analyzed for descriptive statistics and significant differences in amikacin and gentamicin serum concentration among critically and noncritically ill patients using the Chi-square, Fisher exact, one-way ANOVA, and Student's t-test using SPSS version 20 (IBM).[11] One-way ANOVA and Student's t-test were used to compare means, whereas the Chi-square and Fisher exact test were used for data cross-tabulation. A P < 0.05 was considered statistically significant.
Results
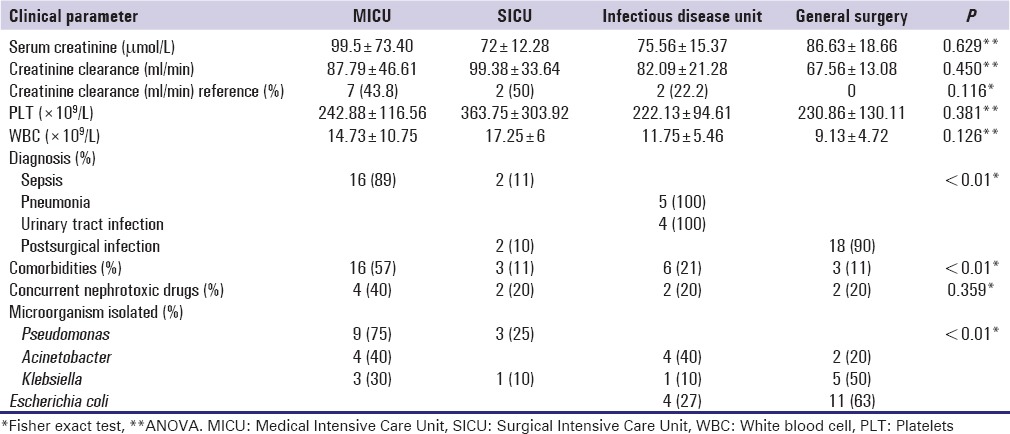
This study included 47 patients treated in four different units with gentamicin (31) or amikacin (16) whose demographic characteristics are shown in Table 1. Significant differences were found in aminoglycosides use between different units [Table 1]. We also found significant differences in patients' diagnosis, presences of comorbidities, and difference in isolated microorganisms between different units on Fisher exact tests [Table 2]. Postsurgical infections in MICU were significantly low compared to other units, whereas significantly higher postsurgical infections in general surgery unit; urinary tract infections, and pneumonia in infectious disease unit, and sepsis in MICU (P = 0.000). Similarly, comorbidities were significantly higher in MICU and lower in general surgery unit. Presence of Pseudomonas was low and higher Escherichia coli were observed in general surgery unit, whereas higher Pseudomonas and low E. coli were observed in MICU (P = 0.0005 and P = 0.0007 respectively) [Table 2].
Table 1.
Demographic characteristics of patients
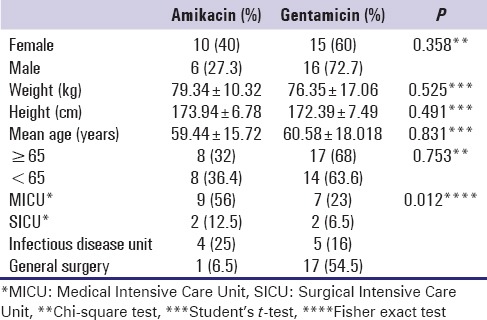
Table 2.
Clinical data of patients
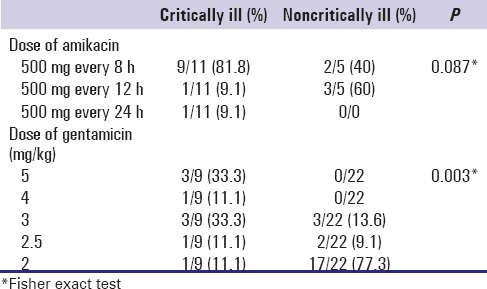
Dosing of amikacin and gentamicin in critically and noncritically ill patients are presented in Table 3. We found statistically significant difference in gentamicin doses between two groups of patients (P = 0.003). Use of gentamicin dose of 5 mg/kg was significantly lower, whereas use of dose of 2 mg/kg was higher in noncritically ill patients.
Table 3.
Amikacin and gentamicin dosing in critically and noncritically ill patients
Comparing amikacin Cmax mean values between groups of patients with different dosage regimens using one-way ANOVA, we found no statistical difference (F 2 =0.061; P = 1). Comparing number of patients with amikacin Cmax in reference range between different dosage regimens, we found that more patients had amikacin Cmax in reference range with the highest dose than in lower dose groups (58% vs. 33% vs. 9%, respectively), but this difference was not statistically significant (χ 22 =2.424; P = 0.298). Gentamicin was administered in one daily dose in 19 (61.3%) patients, whereas total daily dose of gentamicin was divided in two single doses in 12 (38.7%) patients. Comparing gentamicin Cmax mean values between groups of patients with different dosage regimens using one-way ANOVA, we found significant difference (F 4 =117.45; P = 0.037). Mean values of gentamicin Cmin were significantly higher in 5 mg/kg group compared to 3 mg/kg group (P = 0.05) compared to 2.5 mg/kg group (P = 0.005) and compared to 2 mg/kg group (P = 0.012). Mean values of gentamicin Cmin were higher in 5 mg/kg group compared to 4 mg/kg group but without statistical significance (P = 0.636). Comparing number of patients with gentamicin Cmax in reference range between different dosage regimens, we did not find significant difference between groups (χ 24 =6.308; P = 0.177). Analyzing minimal serum concentrations (Cmin ) and maximun serum concentrations (Cmax ) of aminoglycosides used in the study, we found out that most patients had Cmin inside reference range (40/47 [85%]). Similarly, most studied patients had Cmax in therapeutic range (28/47 [65%]). The highest percentage of reference Cmin was noted in SICU and infectious disease unit (100%), whereas the highest percentage of reference Cmax was noted in infectious disease unit (78%) as presented in Figure 1.
Figure 1.
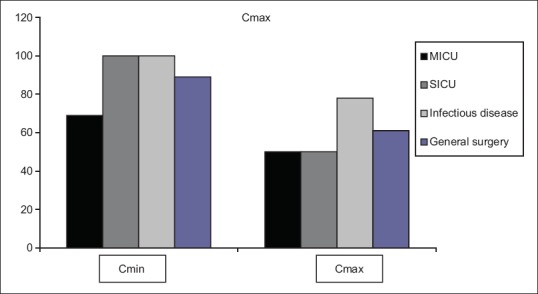
Frequency of Cmin and Cmax of aminoglycosides in reference range on four units
Comparing Cmin mean values of amikacin and gentamicin between patients treated in four different units using one-way ANOVA, we have not found significant difference (P = 0.653 and P = 0.676, respectively). Similarly, comparing Cmax mean values of amikacin between patients treated in four different units, we did not find any significant difference (P = 0.890), while difference between units in gentamicin Cmax mean values was borderline significant (P = 0.049). Chi-square test was used to compare the percentage of Cmin of Cmax of amikacin and gentamicin in four different units and statistically significant difference was not detected.
Table 4 represent the results of comparing amikacin and gentamicin Cmin and Cmax between critically and noncritically ill patients and the only significant difference was noted in gentamicin Cmax ; we found significantly higher gentamicin Cmax mean value in critically ill patients (P = 0.007).
Table 4.
Comparison of amikacin and gentamicin Cmax and Cmin between critically and noncritically ill patients
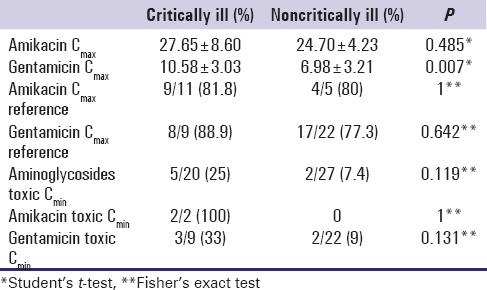
Comparing the number of patients who had Cmin of aminoglycosides higher than recommended (gentamicin Cmin >1 mg/L; amikacin Cmin >10 mg/L) with the number of patients who had creatinine clearance (CrCL) lower then reference value using Chi-square test, we found that higher number of patients with toxic Cmin had decreased CrCL (60% vs. 40%), but the difference was not significant (χ 2[2] =1.1; P = 0.577). Comparing the number of patients who had Cmax of aminoglycosides higher than recommended (gentamicin Cmax >10 mg/L; amikacin Cmax >40 mg/L) with the number of patients who had CrCL lower then reference value using Chi-square test, we found that higher number of patients with toxic Cmax had decreased CrCL (80% vs. 20%), but the difference was not significant (χ 2[1] =0.621; P = 0.431).
Discussion
From patients demographic characteristics, it can be concluded that the use of aminoglycosides did not differ between male and female patients and older or younger patients in our study. However, use of gentamicin was significantly higher in the patients treated on general surgery unit compared to other wards, whereas amikacin was most frequently used in MICU. Measured clinical parameters were similar between critically and noncritically ill patients. Serum concentrations of gentamycin and amikacin were within the reference range in most patients included in this study. In majority of the patients treated with amikacin (n = 15), total daily amount of drug was divided in two or three single doses which is in opposite to widely accepted once daily dosing.[12,13] However, in most of the patients treated with gentamycin, drug was administered once daily, which is consistent with most recommendations.[13]
In critically ill patients, it is of high importance to take into account the influence of pathophysiological changes on pharmacokinetic and pharmacodynamics parameters of administered antibiotic, caused by septic state.[14] Besides this, it is important to continuously evaluate treatment in relation to changes in disease severity and to modify doses of antibiotics accordingly.[15] Pharmacokinetic parameters which needs to be taken into account and which demonstrate if adequate serum drug concentrations are present in site of action are volume of distribution, CL, half-life (t1/2 ), Cmax, Cmin, and area under the plasma drug concentration-time curve (AUC), whereas pharmacodynamics parameters include time over minimal inhibitory concentration (T > MIC), Cmax /MIC, and AUC24 /MIC.[16]
Duszynska et al. showed that it is necessary to administer loading doses of amikacin in treatment of critically ill patients in order to rapidly achieve therapeutic drug serum concentration.[17] In our study, most patients were administered maximal dose of amikacin (500 mg every 8 h) regardless of body weight which resulted in achieving therapeutic drug serum concentrations in most of the patients. On the other hand, these doses of amikacin did not result in toxicity since Cmin was higher than 10 mg/L in only 2 of 16 (12.5%) patients. Certain studies demonstrated the benefit of gentamycin TDM where it was shown that patients treated with gentamycin which serum concentration was regularly monitored had shorter hospital stay[8] while another study[18] demonstrated that regular gentamycin TDM does not lead to better clinical outcomes in patients treated in ICUs. In our study, despite the fact that most patients were treated with gentamicin dose 2 mg/kg despite recommendations,[19] 25 (80.6%) patients had gentamicin serum concentration within therapeutic range. Besides that, only five out of 31 patients (16.1%) had Cmin over 1 mg/L, a threshold for nephrotoxicity. Comparing therapeutic serum concentrations of amikacin and gentamicin between critically and noncritically ill patients, we found that in critically ill patients gentamicin Cmax were significantly higher than in noncritically ill (P = 0.007) and amikacin Cmax were higher in critically ill patients but without statistical significance (P = 0.485). Reason for this can be attributed to the administration of significantly higher doses of gentamicin and nonsignificantly higher doses of amikacin in critically ill compared to noncritically ill patients. On the other side, we noted a similar number of patients in both groups who had therapeutic serum concentrations of antibiotics which were around 80% for both aminoglycosides. Potentially toxic serum concentrations of amikacin and gentamicin were similar between two groups of patients (P = 0.308 and P = 0.096, respectively).
Our study had few limitations; we included relatively small number of patients and measured drugs' serum concentration only at one point in time and did not follow the trend. We did not follow the course of infection or assessed the clinical outcome and compared it with drugs' serum concentrations. Besides this, we were not able to perform measurements of serum drugs' concentrations immediately, but samples were stored until the end of the study, where all blood samples were analyzed once. This was done due to economic savings associated with testing and reflects on the reason why we could not advice on antibiotics dose change.
Finally, influence of other co-administered medications on patients' renal functions eventually impacting aminoglycosides concentration was not considered in this study.
The strength of our study lays in the fact that it had a prospective design; patients were observed prospectively in four different units in a large university hospital without making any impact on their treatment because we wanted to assess objectively the appropriateness of established treatment policies. This is the first study on TDM of aminoglycosides in our country which is developing country with limited funds.
Conclusion
Based on our data, we can conclude that dosing of gentamicin and amikacin was adequate in most patients treated in MICUs and in general medical units with only few patients in whom Cmin were higher than recommended, which could have resulted in adverse events because of these antibiotics. No undesirable effects of gentamycin and amikacin were noted in any patient included in this study. Therefore, regular TDM of amikacin and gentamicin is not necessary to be performed in patients treated in our institution without renal or hepatic failure, since usual dosing of these antibiotics provides safe and efficient treatment of infections caused by susceptible microorganisms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Touw DJ, Neef C, Thomson AH, Vinks AA. Cost-Effectiveness of Therapeutic Drug Monitoring Committee of the International Association for Therapeutic Drug Monitoring and Clinical Toxicology. Cost-effectiveness of therapeutic drug monitoring: A systematic review. Ther Drug Monit. 2005;27:10–7. doi: 10.1097/00007691-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24:1–10. doi: 10.3904/kjim.2009.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkett DJ. Pharmacokinetics made easy: Therapeutic drug monitoring. Aust Prescr. 1997;20:9–11. [Google Scholar]
- 4.Atkinson AJ, Jr, Nordstrom K. The challenge of in-hospital medication use: An opportunity for clinical pharmacology. Clin Pharmacol Ther. 1996;60:363–7. doi: 10.1016/S0009-9236(96)90192-7. [DOI] [PubMed] [Google Scholar]
- 5.Shenfield GM. Therapeutic drug monitoring beyond 2000. Br J Clin Pharmacol. 2001;52(Suppl 1):3S–4S. doi: 10.1046/j.1365-2125.2001.0520s1003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick R. Clinical Pharmacy and Therapeutics. 4th ed. London, UK: Churchill Livingstone; 2007. Practical pharmacokinetics; pp. 24–38. [Google Scholar]
- 7.Milena P. Farmakokinetika. 4th ed. Belgrade, Serbia: School of Pharmacy, Belgrade University; 2012. [Google Scholar]
- 8.Crist KD, Nahata MC, Ety J. Positive impact of a therapeutic drug-monitoring program on total aminoglycoside dose and cost of hospitalization. Ther Drug Monit. 1987;9:306–10. doi: 10.1097/00007691-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Birkett DJ. Therapeutic drug monitoring. Aust Prescr. 1997;20:9–11. [Google Scholar]
- 10.Thompson A. Why do therapeutic drug monitoring. Pharm J. 2004;273:153. [Google Scholar]
- 11.IBM SPSS Statistics for Windows. Version 20.0. Armonk, NY: IBM Corp.; 2011. IBM Corp. [Google Scholar]
- 12.Craig WA. Optimizing aminoglycoside use. Crit Care Clin. 2011;27:107–21. doi: 10.1016/j.ccc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Beaucaire G, Leroy O, Beuscart C, Karp P, Chidiac C, Caillaux M. Clinical and bacteriological efficacy, and practical aspects of amikacin given once daily for severe infections. J Antimicrob Chemother. 1991;27(Suppl C):91–103. doi: 10.1093/jac/27.suppl_c.91. [DOI] [PubMed] [Google Scholar]
- 14.Pichala PT, Kumar BM, Zachariah S, Thomas D, Saunchez L, Gerardo AU. An interventional study on intensive care unit drug therapy assessment in a rural district hospital in India. J Basic Clin Pharm. 2013;4:64–7. doi: 10.4103/0976-0105.118801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolau DP. Optimizing antimicrobial therapy and emerging pathogens. Am J Manag Care. 1998;4(10 Suppl 2):S525–30. [Google Scholar]
- 16.Nicolau DP. Optimizing outcomes with antimicrobial therapy through pharmacodynamic profiling. J Infect Chemother. 2003;9:292–6. doi: 10.1007/s10156-003-0279-x. [DOI] [PubMed] [Google Scholar]
- 17.Duszynska W, Taccone FS, Hurkacz M, Kowalska-Krochmal B, Wiela-Hojenska A, Kübler A. Therapeutic drug monitoring of amikacin in septic patients. Crit Care. 2013;17:R165. doi: 10.1186/cc12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed Abdelrahim HE, Ab Rahman AF, Mohamed Ibrahim MI. Therapeutic drug monitoring of gentamicin in patients with bronchopneumonia: Cost considerations and patient outcomes. Eurasian J Med. 2012;44:1–5. doi: 10.5152/eajm.2012.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg EJ, Barclay ML, Duffull SB. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol. 1995;39:605–9. [PMC free article] [PubMed] [Google Scholar]


