Abstract
Background and Aims:
Inrathecal opioids like morphine added to local anaesthetic agents have been found to be effective in achieving prolonged post-operative analgesia. Intrathecal dexmedetomidine may be devoid of undesirable side effects related to morphine and hence, this study was designed to evaluate analgesic efficacy, haemodynamic stability and adverse effects of both these adjuvants in patients undergoing gynaecological surgeries.
Methods:
This was a prospective, randomised, double blind study involving 25 patients in each group. Group M received 15 mg of 0.5% hyperbaric bupivacaine with 250 μg of morphine while Group D received 15 mg of 0.5% hyperbaric bupivacaine with 2.5 μg of dexmedetomidine. Characteristics of spinal block, time for first rescue analgesic and total dose of rescue analgesics were noted. Vital parameters and adverse effects were noted perioperatively. Data analysis was done with independent two sample t-test and Mann–Whitney U test.
Results:
Time for first rescue analgesic (P = 0.056) and total analgesic demand were similar in both groups. Duration of sensory (P = 0.001) and motor (P = 000) block was significantly higher in dexmedetomidine group. Itching was noticed in 36% and nausea in 52% of patients in the morphine group, either of which was not seen in dexmedetomidine group.
Conclusion:
Intrathecal dexmedetomidine produces prolonged motor and sensory blockade without undesirable side effects but intraoperative hypotension was more frequent in dexmedetomidine group.
Keywords: Dexmedetomidine, gynaecological surgeries, morphine, post-operative analgesia, spinal anaesthesia
INTRODUCTION
Postoperative pain management is one of the main challenges for anaesthesiologists and even with the help of multimodal analgesia techniques, patients still remain undertreated.[1] Since no single modality for the post-operative pain relief has proven to be effective without side effects, we continue to explore modern strategies with new drug combinations.[2] The addition of different adjuvants intrathecally is an attractive analgesic strategy due to simple and quick technique with low risk of failure and infection. Anaesthesiologists have added multiple adjuvant drugs such as epinephrine, opioids, α2 adrenergic receptor (AR) agonists and many others to local anaesthetic agents. Intrathecal opioids are considered the gold standard in the treatment of post-operative pain with morphine adjudged as the most effective due to its potent and prolonged effect. However, over the years, it is losing popularity due to dose dependent side effects such as pruritus, nausea, vomiting and the most feared risk of delayed respiratory depression.[3] Hence, the search for an agent which can provide potent post-operative analgesia as comparable to morphine without its side effects still continues.
Dexmedetomidine is a highly selective α2 AR agonist which possesses sedative, analgesic and sympatholytic properties and gives prolonged analgesia when used intrathecally without respiratory depression.[4] Intrathecal dexmedetomidine has been found to be ten times more potent analgesic and anaesthetic as compared to intrathecal clonidine and five times more potent than opioids like intrathecal fentanyl.[5,6] Intrathecal morphine when compared to intrathecal α2 AR agonist, clonidine proved to be better post-operative analgesic with significantly less rescue analgesic consumption, but the duration of spinal block was more with clonidine than morphine.[7] The data comparing the efficacy of intrathecal morphine with intrathecal dexmedetomidine is not available. In this study, we decided to compare intrathecal morphine with intrathecal dexmedetomidine as an adjuvant to hyperbaric bupivacaine in gynaecological surgeries.
The primary aim of the study was to compare analgesic efficacy of these two adjuvants while secondary aim was to compare adverse effects, characteristics of spinal block and peri-operative vital parameters.
METHODS
After obtaining institutional ethical committee approval, fifty patients between 18 to 65 years of age, posted for elective gynaecological surgeries under subarachnoid block with hyperbaric 0.5% bupivacaine, were recruited. This was prospective, double blind study conducted between January 2014 to April 2015. Informed consent, written in their own language was obtained from all the patients. Patients with American Society of Anesthesiologists physical status grade III and above, acute or chronic respiratory disease, cognitive or psychiatric disturbances, hypertension or other cardiovascular abnormalities were excluded from the study. Patients were randomly allocated to two groups by computer generated list. Group M received 3 ml of 0.5% hyperbaric bupivacaine with 250 µg of preservative free morphine. Group D received 3 mL of 0.5% hyperbaric bupivacaine with 2.5 µg of dexmedetomidine.
Patients did not receive any premedication. All the patients were preloaded with 10 mL/kg of lactated Ringer's solution through 18 gauge intravenous (IV) cannula and monitored with electrocardiography, pulse oximetry (SpO2) and non invasive blood pressure (NIBP).
Under all aseptic precautions, subarachnoid block was administered in right lateral position with 26 gauge Quincke needle at L3-L4/L2-L3 space by midline approach. Both the groups received 3 ml 0.5% hyperbaric bupivacaine in 5 ml syringes. For Group M, 1 unit of morphine from 40 units insulin syringe (250 µg) was added to syringe containing bupivacaine. For Group D, 1 unit of dexmedetomidine (2.5 µg) from 40 units insulin syringe was added to syringe containing bupivacaine. Both the operator and the investigator were blinded about the drug, which was prepared by an independent investigator. Immediately after giving injection patients were turned to supine position. The completion of injection was taken as time zero of induction of anaesthesia.
Sensory level was monitored every 2 min for initial 30 min using pin prick method with 24 gauge hypodermic needle. Time for onset of analgesia, time to achieve sensory level of T10 was noted. The highest sensory level achieved and times taken to achieve that were noted. After initial 30 min, sensory level was monitored every 15 min till end of surgery. Motor blockade was assessed using modified Bromage scale [8]; Grade 0 – No weakness, full power, Grade 1 – Can flex knees but cannot raise legs, Grade 2 – Only foot movements, Grade 3 – Complete paralysis. Time taken for onset of motor block and to achieve Bromage 3 was noted.
Heart rate (HR), systolic blood pressure, diastolic blood pressure, oxygen saturation (SpO2) and sedation score were monitored every 5 min for initial 30 min and later on every 15 min till the end of surgery. Hypotension was defined as fall in BP of more than 30% from baseline value and was treated with incremental doses of injection ephedrine and IV crystalloids. Bradycardia was defined as HR <60/min and was treated with injection atropine. Sedation score was assessed as per modified Ramsay sedation score;[9] 1 – agitated, 2 – anxious but alert, 3 – drowsy but responding to commands, 4 – responds on glabellar tap, 5 – responds to deep, painful stimuli.
Duration of surgery was noted. Post-operatively, time for regression to S2 dermatome was noted by pin prick with 24 gauge hypodermic needle on posteromedial aspect of thigh. Time to reach Bromage grade 0 was noted. All the patients were monitored for presence of nausea/vomiting, pruritus, sedation, signs of respiratory depression (respiratory rate/min and SpO2), HR and NIBP every 2 hourly for 12 h and then every 4 hourly for next 12 h. Injection diclofenac 1.5 mg/kg was given intramuscularly as rescue analgesic. Time for first rescue analgesic and total number of analgesic doses used were noted.
Nausea/vomiting was treated with injection ondansetron 4 mg IV. Pruritus was treated with injection promethazine 25 mg IM which was repeated after 1 h if needed. Oxygen by Hudson mask was provided if SpO2 decreased to <94%. Injection naloxone (in dose of 0.1–0.2 mg IV bolus, to be repeated as needed every 3–4 min) was reserved for patients with respiratory rate of <8/min.
All the patients were catheterised with Foley's catheter no. 14, before start of surgery. Study ended at 24 h after induction of anaesthesia.
All data were assessed using IBM Statistical Package for Social Sciences (SPSS) version 21 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA). The sample size was calculated based on previous studies (difference of 155 min and standard deviation [SD] 153 min) according to time for first analgesic demand.[10,11] A sample size of 21 patients in each group were needed for 90% power of study with 5% significance level. Twenty five patients were included in each group to allow possible drop outs. Data are expressed as either Mean ± SD or numbers and percentages. Demographic data, type and duration of surgery, characteristics of spinal block were analysed using independent two sample t-test. The time for first analgesic demand and total analgesic requirements, regression of sensory and motor blockade were compared using Mann–Whitney U-test. Intraoperative bradycardia and hypotension were analysed as number of episodes and compared with independent t-test. Rest of the side effects were compared using Chi-square or Fisher's exact test, whichever was applicable. A Kaplan–Meier survival curve was obtained for time for first analgesic demand (Figure 1). P < 0.05 was considered statistically significant at two-sided 95% of confidence interval.
Figure 1.
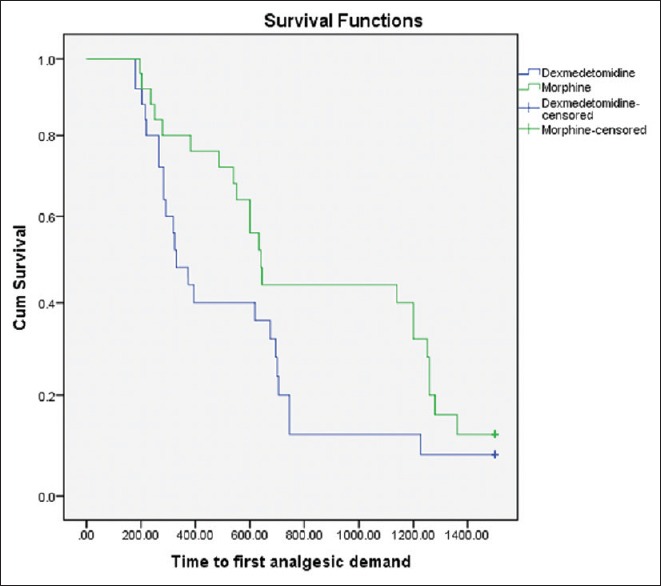
Kaplan–Meier curve for first analgesic demand as function of time (P = 0.083, by Log rank test)
RESULTS
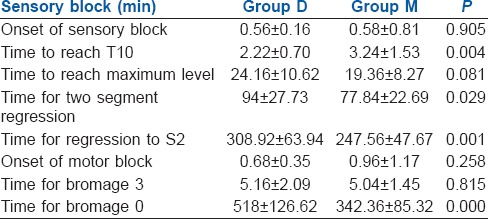
The mean age, height, weight of the patients and type of surgery were similar in both the study groups [Table 1]. The time for first rescue analgesic was similar in both groups with no statistical significance (P = 0.056) considering 95% of confidence interval (0.000–0.058). (Figure 1) Total number and amount of analgesics needed in 24 h was also same in both groups [Table 2]. Two patients in group D and three patients in Group M did not require any analgesic in 24 h. There was no significant difference between the onset of sensory and motor block in both groups [Table 3]. Time taken to reach T10 was significantly less in Group D (P = 0.004). Thirty-six per cent of patients in Group D achieved T2 level, whereas in Group M 44% of patients reached T4 level and none to T1, though the difference was not statistically significant. The time for two segment regression was more in Group D (P = 0.029). Time for regression to S2 was significantly higher in Group D (P = 0.001). Time for a return to Bromage grade 0, was significantly higher in Group D (P = 000).
Table 1.
Demographic characteristics
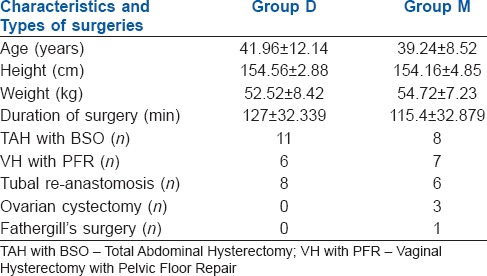
Table 2.
Post-operative analgesia
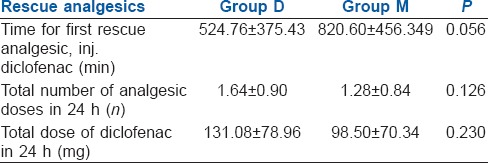
Table 3.
Characteristics of spinal block
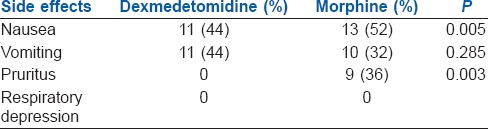
The intra and post-operative side effects are summarised in Tables 4 and 5 respectively. Intraoperative hypotension was more frequent in Group D than Group M (44%). However, there were no episodes of hypotension or bradycardia in both groups during post-operative period. Sedation scores were similar in both groups. Pruritus was seen in nine patients and nausea in 13 patients in group M. No case in either group developed respiratory depression. Shivering was noticed in three patients in Group D.
Table 4.
Intraoperative side effects

Table 5.
Post-operative side effects
DISCUSSION
The antinociceptive properties of intrathecal α2 AR agonists are produced by inhibiting the release of c fibre transmitters, by inhibition of release of substance P and by hyperpolarising post synaptic dorsal horn neurons. Dexmedetomidine is highly specific, potent and selective α2 AR agonist. Hence, intrathecal dexmedetomidine results in potent analgesia as compared to clonidine with lesser side effects such as bradycardia, hypotension and sedation. Animal studies have been conducted with intrathecal dexmedetomidine in dose range of 2.5–100 µg, however, risk of neurotoxicity cannot be denied at dosage more than three µg.[12,13,14] Human studies have shown that 3–15 µg of dexmedetomidine co-administered with local anaesthetics has a dose-dependent effect on anaesthesia, analgesia and haemodyanamic stability.[5,15,16,17,18] Similar results were seen with other α2 AR agonist, clonidine, in doses of 15–45 µg when added to intrathecal local anaesthetic.[19]
Morphine injected intrathecally results in analgesia by same mechanisms as that of α2 AR agonists. Analgesia is adequate and long lasting due to its hydrophilicity, decreased systemic absorption, cephalad spread in the cerebrospinal fluid and slow rate of clearance from the opioid receptors.[20] Till date no consensus has been reached regarding the optimal dose of single shot intrathecal morphine but appears to be 100–250 µg.[21] Considering the ceiling dose for analgesic efficacy of intrathecal morphine, we decided to study 250 µg of morphine.[22,23] The authors comparing intrathecal morphine with intrathecal α2 AR agonist compared 100 µg of intrathecal clonidine with 1 mg of intrathecal morphine.[7] Dexmedetomidine is said to be ten times more potent than clonidine.[9,24] Based on this data, we decided to compare 2.5 µg of dexmedetomidine with 250 µg of morphine.
When compared to intrathecal clonidine, intrathecal morphine was found to be better post-operative analgesic (P < 0.001) with far less rescue analgesic consumption in 24 h.[7] In our study, both intrathecal morphine and dexmedetomidine were similar in first analgesic demand time and total analgesic requirement. The difference between total analgesic dose required between two groups was not significant. The study comparing 3 µg of intrathecal dexmedetomidine with 30 µg of intrathecal clonidine as an adjuvant to 12 mg of hyperbaric bupivacaine, did not find any difference between sensory and motor block duration between two groups (P = 0.3).[6] Study comparing intrathecal morphine with clonidine found prolonged sensory duration with intrathecal clonidine.[7] In the current study, we found faster onset with prolonged sensory and motor blockade with intrathecal dexmedetomidine as compared to intrathecal morphine. α2 ARs are seen in dorsal horn laminae I, II, V with specific mRNA in ventral horn more than dorsal horn.[25] This could be the reason for more potent anaesthetic action of dexmedetomidine.
All previous human studies have shown prolonged sensory and motor blockade with intrathecal dexmedetomidine. One study found intrathecal dexmedetomidine provides pronged motor and sensory block with haemodyanamic stability and reduced demand of rescue analgesic as compared to intrathecal clonidine and fentanyl.[5] Another study comparing intrathecal fentanyl and dexmedetomidine confirmed same findings with significant difference in time for two segment regression.[15] In our study, we got similar results regarding sensory and motor block duration. In other studies, dose of dexmedetomidine was 3 and 5 μg, respectively. Different doses of intrathecal dexmedetomidine used as adjuvant to hyperbaric bupivacaine have shown that higher dose of dexmedetomidine was associated with faster onset and slower regression of both motor and sensory block with reduced analgesic requirement in the post-operative period.[18,19,20] In the current study, mean time to reach T10 level was significantly less in dexmedetomidine group, which is acceptable considering delayed onset of action of morphine.
Intrathecal morphine and dexmedetomidine both are known to cause hypotension by action on ARs.[26] In our study, hypotension was seen more frequently in dexmedetomidine group than morphine. We found incidence of pruritus in morphine group was 36% which is comparable to previous studies. Incidence of nausea in Group M was 52% in our study, which is much higher than earlier documentation.[27] Chances of respiratory depression at low doses of intrathecal morphine are negligible which is confirmed by our study.[3,27]
Limitation of our study was the speed of injection while giving spinal anaesthesia was not decided which could have played role in onset of analgesia and reaching T10 level. Another limitation of the study was rescue analgesic was given on demand by patient and pain score was not monitored.
CONCLUSION
Intrathecal dexmedetomidine provides post-operative analgesia comparable to intrathecal morphine without undesirable side effects such as pruritus and nausea. Further studies are needed to compare higher doses of dexmedetomidine with morphine as an intrathecal adjuvant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.White PF, Kehlet H. Improving postoperative pain management: What are the unresolved issues? Anesthesiology. 2010;112:220–5. doi: 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]
- 2.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–93. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 3.Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: A meta-analysis. Anaesthesia. 2009;64:643–51. doi: 10.1111/j.1365-2044.2008.05817.x. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: A double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013;29:496–502. doi: 10.4103/0970-9185.119151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty DJ, Carabine UA, Milligan KR. Comparison of the analgesic effects of intrathecal clonidine and intrathecal morphine after spinal anaesthesia in patients undergoing total hip replacement. Br J Anaesth. 1993;71:661–4. doi: 10.1093/bja/71.5.661. [DOI] [PubMed] [Google Scholar]
- 8.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SN, Subedi A, Prasad JN, Regmi MC. A comparative study to assess the effect of intrathecal bupivacaine with morphine or butorphanol on post operative pain relief following abdominal and vaginal hysterectomy. Health Renaissance. 2013;11:246–9. [Google Scholar]
- 11.Raval DL, Chaudhary M. A clinical comparative study between dexmedetomidine v/s clonidine with bupivacaine intrathecally in major orthopaedic lower limb surgery. J Res Med Dent Sci. 2014;2:77–82. [Google Scholar]
- 12.Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27:3182–90. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbe MB, Penning JP, Ozaki GT, Yaksh TL. Spinal and systemic action of the alpha 2 receptor agonist dexmedetomidine in dogs. Antinociception and carbon dioxide response. Anesthesiology. 1994;80:1057–72. doi: 10.1097/00000542-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Isgüzar O, Baris S, Bozkurt A, Can B, Bilge S, Türe H. Evaluation of antinociceptive and neurotoxic effects of intrathecal dexmedetomidine in rats. Balkan Med J. 2012;29:354–7. doi: 10.5152/balkanmedj.2012.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55:347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 17.Yektas A, Belli E. The effects of 2 mcg and 4 mcg doses of dexmedetomidine in combination with intrathecal hyperbaric bupivacaine on spinal anesthesia and its post operative analgesic characteristics. Pain Res Manag. 2014;19:75–81. doi: 10.1155/2014/956825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eid HE, Mohamed SA, Hend Y. Dose related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4:83–95. [Google Scholar]
- 19.De Kock M, Gautier P, Fanard L, Hody JL, Lavand'homme P. Intrathecal ropivacaine and clonidine for ambulatory knee arthroscopy: A dose-response study. Anesthesiology. 2001;94:574–8. doi: 10.1097/00000542-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Ummenhofer WC, Arends RH, Shen DD, Bernards CM. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92:739–53. doi: 10.1097/00000542-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Cohen E. Intrathecal morphine: The forgotten child. J Cardiothorac Vasc Anesth. 2013;27:413–6. doi: 10.1053/j.jvca.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: Finding the right balance. Drugs. 2011;71:1807–19. doi: 10.2165/11596250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Rathmell JP, Lair TR, Nauman B. The role of intrathecal drugs in the treatment of acute pain. Anesth Analg. 2005;101(5 Suppl):S30–43. doi: 10.1213/01.ANE.0000177101.99398.22. [DOI] [PubMed] [Google Scholar]
- 24.Kalso EA, Pöyhiä R, Rosenberg PH. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol Toxicol. 1991;68:140–3. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith MS, Schambra UB, Wilson KH, Page SO, Hulette C, Light AR, et al. Alpha 2-adrenergic receptors in human spinal cord: Specific localized expression of mRNA encoding alpha 2-adrenergic receptor subtypes at four distinct levels. Brain Res Mol Brain Res. 1995;34:109–17. doi: 10.1016/0169-328x(95)00148-l. [DOI] [PubMed] [Google Scholar]
- 26.Solomon RE, Gebhart GF. Intrathecal morphine and clonidine: Antinociceptive tolerance and cross-tolerance and effects on blood pressure. J Pharmacol Exp Ther. 1988;245:444–54. [PubMed] [Google Scholar]
- 27.Meylan N, Elia N, Lysakowski C, Tramèr MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: Meta-analysis of randomized trials. Br J Anaesth. 2009;102:156–67. doi: 10.1093/bja/aen368. [DOI] [PubMed] [Google Scholar]


