Abstract
Background and Aims:
Caudal block (CB) with adjuvants is routinely used in children for anaesthesia. We evaluated the efficacy of the α2 adrenergic agonist, dexmedetomidine at two different doses as an adjuvant to bupivacaine in CB.
Methods:
This study was conducted on ninety children. Control group BD0 received 0.25% bupivacaine 1 ml/kg, whereas, the study groups BD1 and BD2 received 1 μg/kg and 2 μg/kg dexmedetomidine, respectively, with 0.25% bupivacaine 1 ml/kg as a single shot CB. Adequacy of the block, haemodynamic changes, duration of analgesia and side effects were compared. Analysis of Variance was used for between-group comparisons of numerical variables. Student's t-test and Mann–Whitney U-test were used for quantitative data.
Results:
The demography was comparable. Anal sphincter 5 min after administration of the CB was relaxed in 89.3%, 82.1% and 75% of cases in BD0, BD1 and BD2 groups, respectively. The sphincter was relaxed at the end of surgery in all the cases. Comparable haemodynamics was noted with significantly prolonged duration of analgesia in the groups BD1 (964.2 ± 309 min) and BD2 (1152.6 ± 380.4 min) compared to control (444.6 ± 179.4 min). While no complications were encountered in groups BD0 and BD1, bradycardia was observed in four cases of BD2 group with accompanied hypotension in one of them.
Conclusion:
Dexmedetomidine as an adjuvant to bupivacaine improves the quality of CB, provides good operating conditions and increases the duration of post-operative analgesia. We conclude that 1 μg/kg is as effective as 2 μg/kg of dexmedetomidine and with a better safety profile.
Keywords: Adjuvants, bupivacaine, caudal, dexmedetomidine, paediatric regional anaesthesia, post-operative analgesia
INTRODUCTION
Although paediatric regional blocks were introduced in the early 1900s, it was not until the 1980s that interest in them resurfaced. Since then, with the advances in regional techniques, it has gained more popularity. The caudal block (CB) is one of the most commonly used regional techniques in paediatric anaesthesia practice due to its simplicity and safety. The complication rate of 1.5/1000 in the 60% of children who received central neuraxial blocks was reported from the French-Language Society of Pediatric Anesthesiologists after a 1 year study on 24,409 regional blocks in children.[1] CB is used for most surgeries below the umbilicus including herniorrhaphies, orchidopexy, anorectal, urologic and orthopaedic procedures. The block can be accomplished by a single-shot injection or as a continuous infusion through a caudal epidural catheter. The addition of various adjuvants to the local anaesthetics during CB can improve the quality of anaesthesia while providing consistent and sustained analgesia with favourable intraoperative conditions for the surgeon and anaesthesiologist. Along with providing post-operative analgesia, it also reduces requirements of anaesthetic agents intraoperatively thus avoiding excessive sedation.[2]
The use of epidural dexmedetomidine in adults led to its evaluation in paediatric CB. Recent studies suggested that caudal administration of dexmedetomidine could prolong post-operative analgesia in children. Dexmedetomidine is a specific alpha-2 adrenergic receptor agonist with anxiolytic, sedative and analgesic properties acting on the receptors in the brain and spinal cord.[3] Although there are insufficient data available, various studies have demonstrated the dose of dexmedetomidine in CB range from 0.5 µg/kg to 2 µg/kg. A recent meta-analysis comprising six randomised control trials on caudal dexmedetomidine concluded that there are insufficient data regarding the effects of different doses of dexmedetomidine.[4] Hence, this study was undertaken to evaluate the efficient, yet safe dose of dexmedetomidine for CB in children.
METHODS
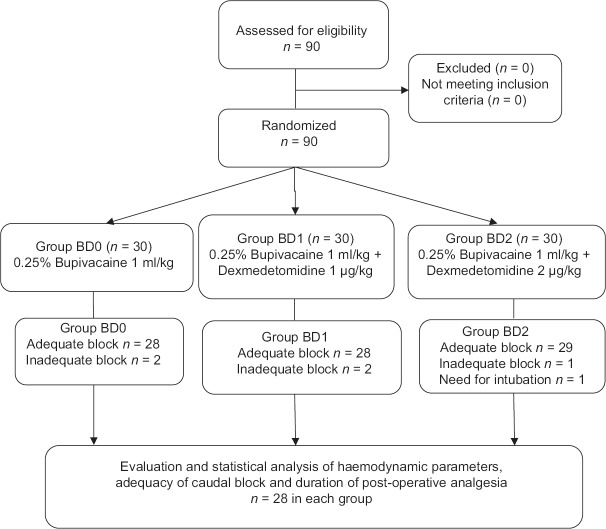
After the Institutional Ethics Committee approval, written informed consent was obtained from the parents of all subjects. Ninety children belonging to American Society of Anesthesiologists Physical Status I and II, aged between 6 months and 8 years, undergoing elective surgeries were randomly assigned into three groups using a computer generated randomised numbers [Figure 1]. Exclusion criteria included parent/guardian refusal, infection at the site of injection, coagulopathies or on anticoagulation therapy, congenital abnormalities of lower spine and meninges, anticipated difficult airway, requiring endotracheal intubation and intermittent positive pressure ventilation, surgeries in prone position and history of allergy to study drugs. The patient and the observer were blinded to the study drugs.
Figure 1.
The study design
The sample size was calculated using 95% confidence interval and power of the study being 80%. We expected an odds ratio of four to detect the average duration of analgesia and a significance level of 0.05. The sample size was 28 in each group with allocation ratio being 1:1:1. The number was increased to thirty in each group.
After a detailed history taking, complete physical examination and routine investigations were undertaken for all patients. The patients were kept fasting as per the standard NPO guidelines. Patients were pre-medicated with syrup triclofos 100 mg/kg and oral atropine 0.03 mg/kg, 45 min before the surgery. Patients were shifted to the operation theatre and pre-induction monitors connected (electrocardiography, precordial stethoscope, pulse oximeter and non-invasive blood pressure). Patients were induced with oxygen and nitrous oxide in 1:1 ratio and halothane 1–3% using Jackson-Rees breathing system. Intravenous (IV) access was secured with appropriate size cannula and lactated Ringers' solution was started as per the calculated fluid requirements. Appropriate size Proseal Laryngeal Mask Airway® (PLMA) was inserted after deepening the plane of anaesthesia with injection propofol 3 mg/kg. Anaesthesia was maintained with oxygen and nitrous oxide in 1:1 ratio and 0.4% halothane with the patient breathing spontaneously via Jackson-Rees breathing system.
The CB was performed under all aseptic precautions in lateral decubitus position by a trained anaesthesiologist. After negative aspiration for cerebrospinal fluid or blood, one of the following drug combinations was injected into the caudal epidural space – Group BD0 received 1 ml/kg of 0.25% bupivacaine with 1 ml normal saline, group BD1 received 1 ml/kg of 0.25% bupivacaine with dexmedetomidine 1 µg/kg diluted to 1 ml with normal saline and group BD2 received 1 ml/kg of 0.25% bupivacaine with dexmedetomidine 2 µg/kg diluted to 1 ml with normal saline.
Dexmedetomidine 100 µg/ml preparation was used. The dosage was calculated according to the patient's weight, loaded using an insulin syringe rounded off to the closest unit and diluted to one ml with normal saline.
Patient's heart rate and blood pressure were monitored after administration of CB every 5 min for the first 30 min and every 15 min subsequently up to 90 min by an observer who was blinded to the study drug. The laxity of the anal sphincter was checked 5 min after the administration of the CB and at the end of the surgery.
No narcotics, analgesics or sedatives were administered intraoperatively. If anal sphincter was not relaxed or there was movement on incision or tachycardia intraoperatively, indicating that the CB was inadequate, analgesia was supplemented with injection fentanyl 2 µg/kg and the plane of anaesthesia was deepened by increasing halothane to 0.4–1% and/or by injection propofol 1 mg/kg. The cases would be excluded from the study as it implied that the block itself had failed.
At the end of the surgery, PLMA was removed in the deeper planes of anaesthesia and patient was administered 100% oxygen via facemask till the patient was shifted to the post-anaesthesia care unit (PACU). Patients were monitored by the PACU staff for vitals, and FLACC pain scale assessment was carried out at 1, 2, 3, 4, 6, 8, 12 and 24 h after CB.[5]
Duration of post-operative analgesia was defined as the time interval between the administration of CB and the first requirement of supplementary analgesia for the patient.
In this study, we defined respiratory depression as a decrease in SpO2 of <95% and was treated with oxygen supplementation or positive pressure ventilation if required. Hypotension was defined as a decrease of systolic pressure to <70 plus twice the age in years (70 + 2 [age in years]) and associated with poor peripheral perfusion. Bradycardia, defined as heart rate <80 beats/min for ages below 1 year and <60 beats/min for ages above 1 year.
When the FLACC pain scale score was more than 4, analgesia was supplemented with diclofenac sodium suppository (1–2 mg/kg) or syrup ibuprofen (4–8 mg/kg). The study concluded when the first supplementary analgesic was administered or at the end of 24 hours, whichever was earlier.
Side effects such as nausea, vomiting, urinary retention, shivering and agitation were also noted and recorded.
Data were analysed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. Numerical variables were presented as mean and standard deviation (SD) and categorical variables were presented as percentages. One-way analysis of variance was used for between-group comparisons of numerical variables. Post hoc analysis was performed using Tukey's test for between-group comparisons of categorical variables. Student's t-test and the Mann–Whitney U-test were also used for analysis of difference of means for the quantitative data. P < 0.05 was considered statistically significant.
RESULTS
All the three groups were homogeneous with respect to age, sex, body weight and duration of surgeries [Table 1].
Table 1.
Demographic profile
The CB was adequate in 85 out of 90 cases and did not require any further supplementation of analgesics intra-operatively for the surgery to proceed. The anal sphincter was relaxed in 89.3%, 82.1% and 75% of the cases in groups BD0, BD1 and BD2, respectively, 5 min after the administration of the CB. This difference between the three groups was not statistically significant (P = 0.378). At the end of surgery, however, the anal sphincter was lax in all the patients.
There were no significant differences in the heart rates and mean blood pressures within the groups over time or between the groups at any time interval [Figures 2 and 3].
Figure 2.
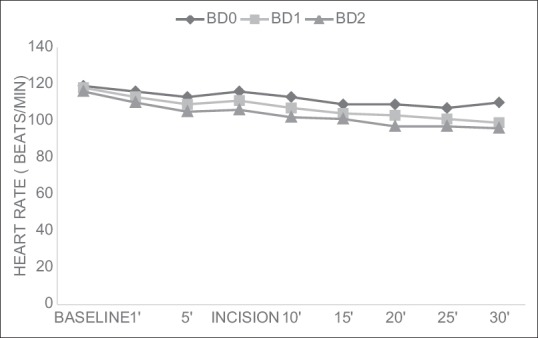
Changes in heart rate (mean±SD)
Figure 3.
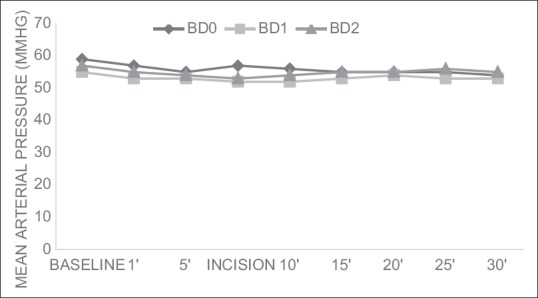
Changes in mean arterial pressure(mean±SD)
The time to first analgesic requirement (total duration of post-operative analgesia) in the BD0 group was 444.6 ± 179.4 min (range of 4–13 h); whereas in the BD1 group, it was 964.2 ± 39 min (range of 4–24 h) and in the BD2 group, it was 1152.6 ± 380.4 min (range of 4–24 h) [Table 2]. The differences in the mean duration of analgesia were highly significant (P < 0.001) between BD0 and BD1 and between BD0 and BD2 groups. The difference between BD1 and BD2 was insignificant (P = 0.056). About 25% of cases in the BD1 group did not require any rescue analgesic in the first 24 h versus 58% of the cases in the BD2 group. All the cases in the group BD0 received rescue analgesics within 24 h.
Table 2.
Duration of analgesia

Four patients in the BD2 group developed bradycardia (heart rate <60 bpm) which was corrected with injection atropine 0.3 mg IV and did not require any further interventions. Three of these patients developed bradycardia in the post-operative period, whereas one patient had bradycardia intraoperatively at the 23rd min after the administration of the block. There were no episodes of bradycardia in the other two groups.
There was associated hypotension in one of the cases with bradycardia and was corrected with fluid bolus and injection atropine IV. No other side effects were noted in any of the cases.
DISCUSSION
Regional anaesthesia techniques are now well established in the practice of paediatric anaesthesia. Regional blocks, in addition to minimising the potential exposure of the developing brain to general anaesthetics, may also improve the post-operative outcomes by reducing respiratory complications, attenuation of the stress responses, cardiac stability and reduction in hospital stay.[6] CB remains the most popular and frequently performed block in infants and children undergoing surgical procedures, due to its long-standing familiarity amongst anaesthesiologists, a high success rate and a good safety profile.[7] The main disadvantage is that the effectiveness of this technique is limited by the duration of action of the local anaesthetics. Various additives to local anaesthetics have been used as adjuncts to improve the quality and the duration of the block. Dexmedetomidine is a potent and a highly selective alpha-2 adrenergic agonist having sedative, sympatholytic and analgesic effects and has been described as a safe and effective additive in many anaesthetic applications and analgesic techniques. It is available as a preservative-free solution and contains no stabilisers or additives and hence, safely used in central neuraxial blocks. The main interest of our study was to evaluate the efficacy and safety of two doses of caudally administered dexmedetomidine in providing adequate intraoperative anaesthesia and post-operative analgesia along with post-operative prolongation of the duration of the CB.
A study on 178 patients correlated the laxity of patient's anal sphincter with the effectiveness of CB. The authors found that the sensitivity and specificity were highest with the sphincter tone test (sensitivity 95.22%, specificity 92.86%), followed by the heart rate response (sensitivity 92.82%, specificity 78.57%) and the swoosh test (sensitivity 66.51%, specificity 35.71%).[8] The anal sphincter tone test is a sign of working CB.[9]
We recorded five failed CBs: Two each in BD0 and BD1 groups and one in BD2 group. In these cases, there were movements on incision and anaesthesia was supplemented with injection fentanyl IV and injection propofol IV to deepen the plane of anaesthesia. Although 3 out of the 5 cases had a relaxed anal sphincter 5 min after the administration of the block, the block was judged to be inadequate as there were movements and hyperventilation at the time of incision. This could be due to an inadequate level of CB or a patchy block. In 2 out of the 5 cases, the anal sphincter tone was intact, both at 5 min after the block was administered and at the end of surgery. Anaesthesia was supplemented due to increases in heart rate, blood pressure and limb movements at the time of incision.
There were no haemodynamic variations noted in our study between the three groups
Similar haemodynamic profiles were observed in previous studies with doses of 2 µg/kg and 1 µg/kg of dexmedetomidine added to 0.25% bupivacaine as adjuvants to CB.[10,11]
A wide range (13.9–21.3 h) in the duration of analgesia with the addition of dexmedetomidine to CB has been reported in several studies.[10,11] This wide variation could be due to a number of factors such as doses of dexmedetomidine, differences in premedication and volatile anaesthetics, type of surgeries, indications for rescue analgesia, assessment of pain and statistical analysis. In this study, the duration of analgesia was significantly prolonged in the groups with dexmedetomidine as an adjuvant, BD1 (964.2 ± 39 min) and BD2 (1152.6 ± 380.4 min) compared to control group BD0 (444.6 ± 179.4 min). This difference between the three groups was highly significant, both clinically and statistically. The duration of analgesia was longer and the number of cases requiring rescue analgesics within 24 h was fewer in the BD2 group compared to BD1 group.
Previous studies indicate a prolongation of duration of post operative analgesia; in a study on eighty children undergoing lower abdominal and perineal surgeries, duration of post-operative analgesia was significantly prolonged in all cases at three different doses of dexmedetomidine (0.5 µg/kg, 1 µg/kg and 1.5 µg/kg) added to 0.2% ropivacaine.[12]; another study using 0.25% ropivacaine 1 ml/kg with and without dexmedetomidine 2 µg/kg caudally showed that the mean duration of post-operative analgesia in the ropivacaine group was 5.5 h and in the ropivacaine-dexmedetomidine group, 14.5 h, with a P < 0.001.[13]
Bradycardia and hypotension, the most common adverse effects of IV alpha-2 adrenoreceptor agonists appear to be less pronounced in children than in adults. These effects can be readily managed with volume expansion or sympathomimetic drugs or both.
One of the drawbacks of this study was not having planned for assessment of sedation post-operatively at regular intervals. Thus, sedation, one of the main side effects of dexmedetomidine, was not assessed.
There was a delay in the relaxation of the anal sphincter tone in group BD2 compared to the other two groups though not statistically significant. A larger sample size would be required to assess the significance of the same. The time of onset of relaxation of the anal sphincter also needs to be evaluated when dexmedetomidine is used as an adjuvant in CBs.
The study terminated at the time of first rescue analgesic or at the end of 24 h. As a result, the maximum duration of post-operative analgesia provided by the addition of dexmedetomidine as an adjuvant to caudal bupivacaine was not evaluated.
CONCLUSION
Caudal administration of 0.25% bupivacaine 1 ml/kg with both doses of dexmedetomidine (1 μg/kg or 2 μg/kg) resulted in the prolongation of the duration of analgesia. Although both the doses proved to be good choices as adjuvants to bupivacaine CB, it is advisable to use dexmedetomidine 1 μg/kg, as it gives a wider safety margin and better haemodynamic stability.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Giaufré E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children: A one-year prospective survey of the French-Language Society of Pediatric Anesthesiologists. Anesth Analg. 1996;83:904–12. doi: 10.1097/00000539-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Markakis DA. Regional anesthesia in pediatrics. Anesthesiol Clin North America. 2000;18:355–81, vii. doi: 10.1016/s0889-8537(05)70168-1. [DOI] [PubMed] [Google Scholar]
- 3.Bajwa S, Kulshrestha A. Dexmedetomidine: An adjuvant making large inroads into clinical practice. Ann Med Health Sci Res. 2013;3:475–83. doi: 10.4103/2141-9248.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong Y, Ren H, Ding X, Jin S, Chen Z, Li Q. Analgesic effect and adverse events of dexmedetomidine as additive for pediatric caudal anesthesia: A meta-analysis. Paediatr Anaesth. 2014;24:1224–30. doi: 10.1111/pan.12519. [DOI] [PubMed] [Google Scholar]
- 5.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 6.Walker SM, Yaksh TL. Neuraxial analgesia in neonates and infants: A review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115:638–62. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moores A, Fairgrieve R. Regional anaesthesia in paediatric practice. Curr Anaesth Crit Care. 2004;15:284–93. [Google Scholar]
- 8.Verghese ST, Mostello LA, Patel RI, Kaplan RF, Patel KM. Testing anal sphincter tone predicts the effectiveness of caudal analgesia in children. Anesth Analg. 2002;94:1161–4. doi: 10.1097/00000539-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Dave NM, Garasia M. A comparison of the effectiveness of predictors of caudal block in children-swoosh test, anal sphincter tone, and heart rate response. J Anaesthesiol Clin Pharmacol. 2012;28:17–20. doi: 10.4103/0970-9185.92428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 11.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 12.Bharti N, Praveen R, Bala I. A dose-response study of caudal dexmedetomidine with ropivacaine in pediatric day care patients undergoing lower abdominal and perineal surgeries: A randomized controlled trial. Paediatr Anaesth. 2014;24:1158–63. doi: 10.1111/pan.12478. [DOI] [PubMed] [Google Scholar]
- 13.Anand VG, Kannan M, Thavamani A, Bridgit MJ. Effects of dexmedetomidine added to caudal ropivacaine in paediatric lower abdominal surgeries. Indian J Anaesth. 2011;55:340–6. doi: 10.4103/0019-5049.84835. [DOI] [PMC free article] [PubMed] [Google Scholar]