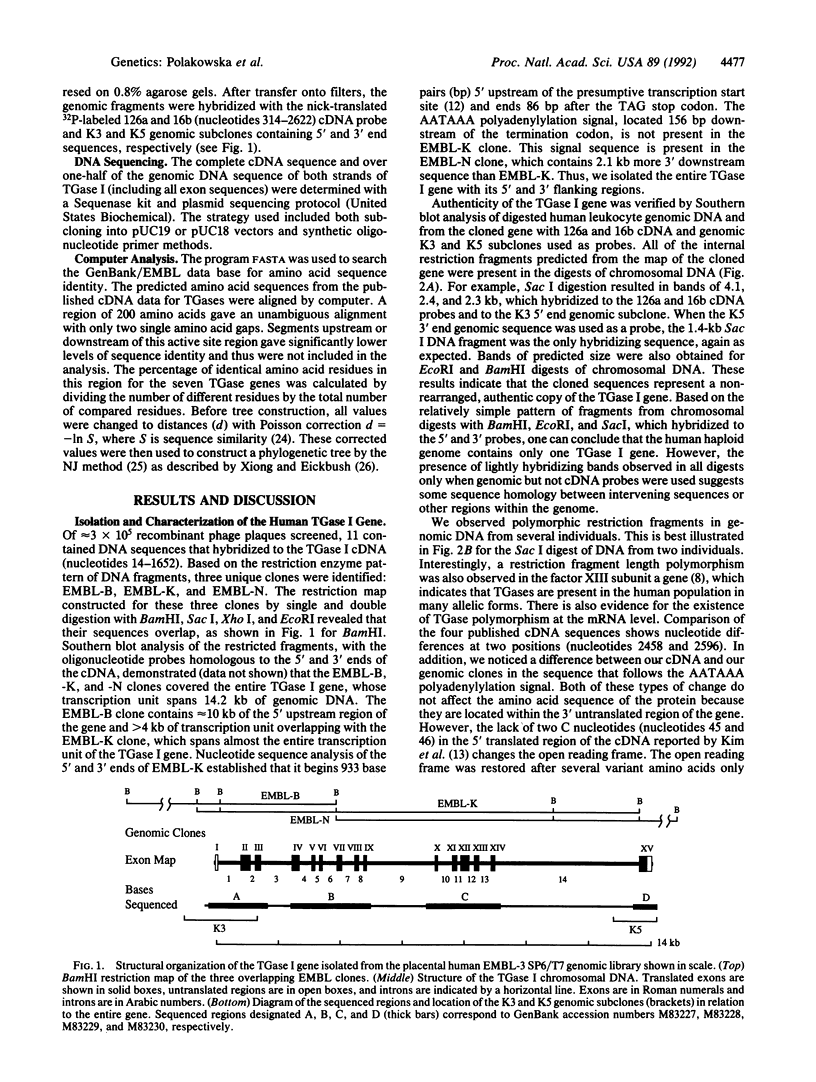
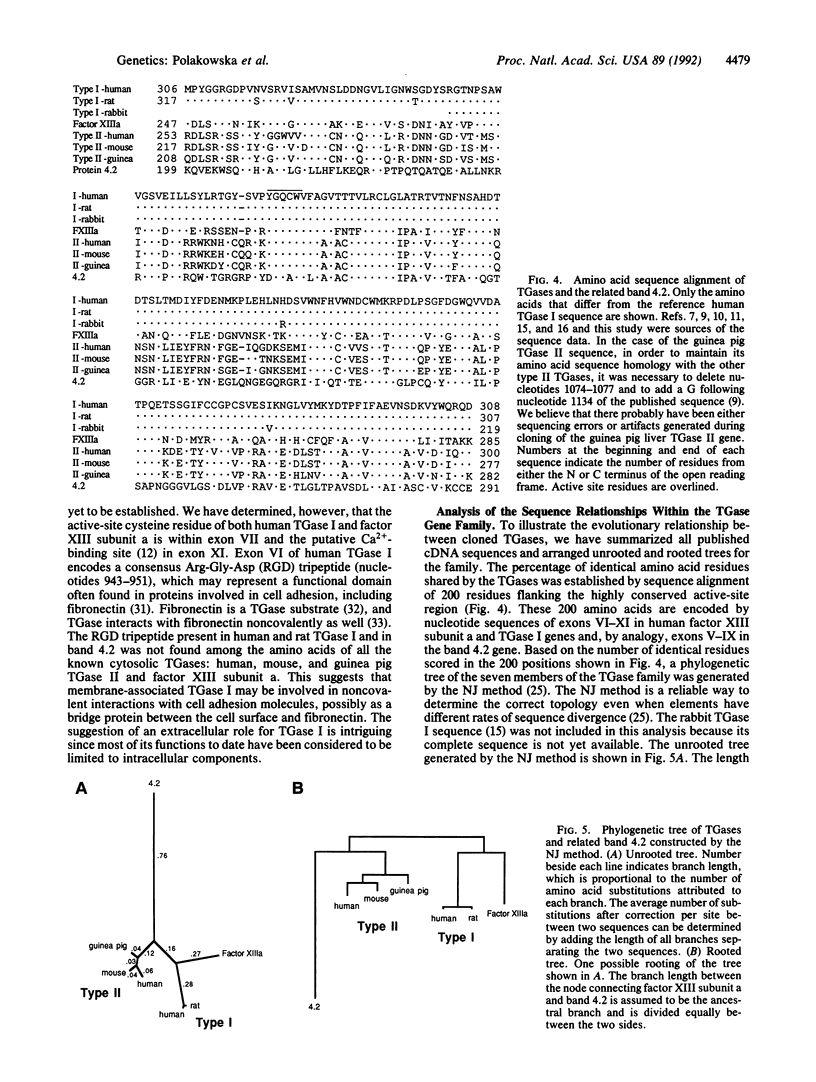
Abstract
Transglutaminases (TGases; protein-glutamine:amine gamma-glutamyltransferase, EC 2.3.2.13) are calcium-dependent crosslinking enzymes that modify proteins posttranslationally. Several distinct types of TGases have been identified, which appear to be encoded by a family of closely related genes. We isolated the gene encoding human keratinocyte-specific type I TGase (TGase I) and characterized its chromosomal organization. The TGase I gene consists of 15 exons separated by 14 introns and exhibits a restriction fragment length polymorphism. Exons appear to encode functional and/or structural domains: exon I and part of exon XV encode untranslated regions, whereas exons VII and XI contain the active site and a presumptive calcium-binding domain, respectively. Interestingly, exon VI of TGase I contains a consensus Arg-Gly-Asp tripeptide sequence whose presence suggests an intriguing extracellular function for the enzyme. We present a likely phylogenetic tree for seven known members of the TGase family based on amino acid sequence similarity. Arguments presented suggest that the active enzyme evolved first and the structural human erythrocyte membrane protein 4.2 (band 4.2) has undergone a rapid change in amino acid sequence. It follows that band 4.2 evolved from the type II TGases, whereas factor XIII subunit a evolved from the type I group.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Floyd E. E., Jetten A. M. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: down regulation by retinoids. Mol Cell Biol. 1989 Nov;9(11):4846–4851. doi: 10.1128/mcb.9.11.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Gentile V., Saydak M., Chiocca E. A., Akande O., Birckbichler P. J., Lee K. N., Stein J. P., Davies P. J. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991 Jan 5;266(1):478–483. [PubMed] [Google Scholar]
- Greenberg C. S., Birckbichler P. J., Rice R. H. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991 Dec;5(15):3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Grundmann U., Amann E., Zettlmeissl G., Küpper H. A. Characterization of cDNA coding for human factor XIIIa. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8024–8028. doi: 10.1073/pnas.83.21.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Bottenus R. E., Davie E. W. Structure of transglutaminases. J Biol Chem. 1990 Aug 15;265(23):13411–13414. [PubMed] [Google Scholar]
- Ichinose A., Davie E. W. Characterization of the gene for the a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5829–5833. doi: 10.1073/pnas.85.16.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Hendrickson L. E., Fujikawa K., Davie E. W. Amino acid sequence of the a subunit of human factor XIII. Biochemistry. 1986 Nov 4;25(22):6900–6906. doi: 10.1021/bi00370a025. [DOI] [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Kim H. C., Idler W. W., Kim I. G., Han J. H., Chung S. I., Steinert P. M. The complete amino acid sequence of the human transglutaminase K enzyme deduced from the nucleic acid sequences of cDNA clones. J Biol Chem. 1991 Jan 5;266(1):536–539. [PubMed] [Google Scholar]
- Kim H. C., Lewis M. S., Gorman J. J., Park S. C., Girard J. E., Folk J. E., Chung S. I. Protransglutaminase E from guinea pig skin. Isolation and partial characterization. J Biol Chem. 1990 Dec 15;265(35):21971–21978. [PubMed] [Google Scholar]
- Kopan R., Traska G., Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various stages of keratinization. J Cell Biol. 1987 Jul;105(1):427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgren C., Cohen C. M. Organization of the gene for human erythrocyte membrane protein 4.2: structural similarities with the gene for the a subunit of factor XIII. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4840–4844. doi: 10.1073/pnas.88.11.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgren C., Lawler J., Lambert S., Speicher D., Cohen C. M. Complete amino acid sequence and homologies of human erythrocyte membrane protein band 4.2. Proc Natl Acad Sci U S A. 1990 Jan;87(2):613–617. doi: 10.1073/pnas.87.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Mehta K., Rao U. R., Vickery A. C., Birckbichler P. J. Significance of transglutaminase-catalyzed reactions in growth and development of filarial parasite, Brugia malayi. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1051–1057. doi: 10.1016/s0006-291x(05)80892-7. [DOI] [PubMed] [Google Scholar]
- Michel S., Reichert U., Isnard J. L., Shroot B., Schmidt R. Retinoic acid controls expression of epidermal transglutaminase at the pre-translational level. FEBS Lett. 1989 Nov 20;258(1):35–38. doi: 10.1016/0014-5793(89)81609-6. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Stewart B. E., Qin Q., Chakravarty R., Floyd E. E., Jetten A. M., Rice R. H. Primary structure of keratinocyte transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9333–9337. doi: 10.1073/pnas.87.23.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Stewart B. E., Rice R. H. Genomic structure of keratinocyte transglutaminase. Recruitment of new exon for modified function. J Biol Chem. 1992 Feb 5;267(4):2282–2286. [PubMed] [Google Scholar]
- Polakowska R. R., Eddy R. L., Shows T. B., Goldsmith L. A. Epidermal type I transglutaminase (TGM1) is assigned to human chromosome 14. Cytogenet Cell Genet. 1991;56(2):105–107. doi: 10.1159/000133060. [DOI] [PubMed] [Google Scholar]
- Polakowska R., Herting E., Goldsmith L. A. Isolation of cDNA for human epidermal type I transglutaminase. J Invest Dermatol. 1991 Feb;96(2):285–288. doi: 10.1111/1523-1747.ep12464554. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L. A., Chien S., Chang L. S., Lambert K., Bliss S. A., Bouhassira E. E., Nagel R. L., Schwartz R. S., Rybicki A. C. Molecular cloning of human protein 4.2: a major component of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1990 Feb;87(3):955–959. doi: 10.1073/pnas.87.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher S. M., Rice R. H. Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell. 1985 Mar;40(3):685–695. doi: 10.1016/0092-8674(85)90217-x. [DOI] [PubMed] [Google Scholar]
- Turner P. M., Lorand L. Complexation of fibronectin with tissue transglutaminase. Biochemistry. 1989 Jan 24;28(2):628–635. doi: 10.1021/bi00428a032. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. J., Sale W. S., Slife C. W. Fibronectin is a component of the sodium dodecyl sulfate-insoluble transglutaminase substrate. J Biol Chem. 1988 Jun 15;263(17):8464–8469. [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Liew F. M., Konishi K., Yasuno H., Doi H., Hirano J., Fukushima S. Molecular cloning of human epidermal transglutaminase cDNA from keratinocytes in culture. Biochem Biophys Res Commun. 1991 Mar 29;175(3):906–913. doi: 10.1016/0006-291x(91)91651-r. [DOI] [PubMed] [Google Scholar]