Abstract
Objective
To examine risk factors for eczema at age 4 years.
Study design
Beginning at age one, infants of atopic parents (n=636) had annual clinical evaluations and skin prick tests (SPTs) to 15 aeroallergens, and milk and egg. Parents completed validated surveys on eczema and environmental exposures. House dust samples were evaluated for allergens and endotoxin. Eczema was defined as a parental report of scratching, and redness, “raised bumps,” or dry skin/scaling for 6 of the last 12 months.
Results
At age four, 90 children (16%) had eczema. Not having a dog prior to age one and being dog SPT+ at ages one, two, and/or three conferred a four-fold higher risk for eczema at age four (aOR=3.9 [1.6–9.2]; p=0.002). Among dog owners, however, dog SPT+ was not associated with significantly increased risk (aOR 1.3 [0.3–6.8]; p=0.8). Among children with cats prior to age one, cat SPT+ conferred significantly increased risk for eczema (aOR= 13.3 [3.1–57.9]; p<0.001). Among non-cat owners, cat SPT+ was not associated with increased risk (aOR=1.1 [0.5–2.7]; p=0.8).
Conclusion
Dog ownership significantly reduced the risk for eczema at age four among dog sensitized children, and cat ownership combined with cat sensitization significantly increased the risk.
Keywords: Pet keeping, Atopic Dermatitis, Food introduction, Elm, Hygiene hypothesis, CCAAPS
Although time trends in the prevalence of eczema vary greatly by geographic location, the global burden of the disease has increased substantially over the last three decades.1, 2 World-wide, eczema affects 15–30% of children and 2–10% of adults.2 Much attention has recently been focused on genetic variations associated with eczema (e.g. filaggrin).3 Nevertheless, the rapid rise in disease prevalence implies that environmental influences must play a critical role as well.1
Transient sensitization to food allergens is associated with childhood eczema; however, the relative importance of aeroallergen sensitization early in life is not well defined.4, 5 We and others have shown that dog ownership may reduce the risk for childhood eczema.6, 7 Although the protective effect of dog ownership may be mediated by elevated environmental endotoxin levels, evidence to support this conclusion is sparse. In addition, cat ownership has been associated with lower rates of atopic sensitization in some studies, and others have reported an increased risk for eczema among cat owners with filaggrin mutations.8, 9
In this study, we investigated associations between early environmental exposures, including pet ownership patterns, house dust endotoxin levels, sensitization patterns, and the eczema phenotype. Our hypothesis was that early environmental exposures and sensitization patterns would be predictive of eczema at age four. The Cincinnati Childhood Allergy & Air Pollution Study (CCAAPS) population, is a high-risk, atopic birth cohort. Findings regarding eczema at age three and younger have been reported elsewhere.7 In this report, children with eczema at age four were evaluated, as this group may represent a more severe phenotype, at increased risk for persistence of eczema and the development of asthma.10, 11
Methods
CCAAPS is a longitudinal birth cohort study on air pollution and allergy. Newborns in the Cincinnati metropolitan area were identified by public birth records from 2001 to 2003.12, 13 Infants living less than 400 m or greater than 1500 m from the nearest major highway or interstate were eligible for enrollment.13 All 762 infants also had a parent with symptoms of asthma, allergic rhinitis, or eczema, and with at least one positive skin prick test (SPT) to a panel of 15 aeroallergens.12 Parents signed an informed consent, and the study was approved by the Institutional Review Board of the University of Cincinnati.
Children were skin tested by trained clinicians for 15 aeroallergens, plus cow’s milk and hen’s egg, on a yearly basis from ages one through four.12 Parents completed yearly in-person surveys. Physical exams and a clinician’s assessment were performed on the same day as the in-person surveys. A home visit and environmental assessment were conducted prior to age one. House dust samples were collected from a 2 m2 area of floor surface from the infant’s primary living area using a Filter Queen Majestic vacuum cleaner at a rate of 2 min/m2. Monoclonal sandwich ELISA assays were performed for Fel d 1, Can f 1 and Der f 1 concentrations in house dust.14 Endotoxin and (1→3) β-D-glucan concentrations were determined using a Limulus amebocyte lysate assay.15, 16 Glucatell modification of the assay was used for (1→ 3) β-D-glucan and Pyrochrome modification for endotoxin.15, 16
Personal elemental carbon attributable to traffic (ECAT), a marker of exposure to traffic related particles, was determined.17 Briefly, particulate matter less than 2.5 μm in diameter (PM2.5) was collected at ambient monitoring stations.18 Elemental carbon concentrations and absorption coefficients were determined. Multivariable UNMIX and chemical mass balance models were then used to determine ECAT at monitoring stations.18 A land use regression model was applied to estimate ECAT for locations where a child spent greater than eight hours/week between ages six and twelve months.17
Two case definitions of eczema at age four were established a priori. Eczema based on parental report was modified from a validated questionnaire (International Study of Asthma and Allergies in Childhood), and included a parental report of scratching, and redness, “raised bumps,” or dry skin/scaling for at least six of the last 12 months.19 Clinician-diagnosed eczema was a global assessment measure based primarily on physical exam findings from a single office visit with one of several study clinicians. Physical exam findings considered to be consistent with eczema included erythema, papulation, excoriations and/or lichenification. The parental report case definition served as the primary outcome measure as it reflected persistent findings for six of the previous 12 months, and was adapted from a validated survey.26 In addition, in order to avoid potentially misclassifying children as controls who were positive for eczema on physical exam, the comparison group for this analysis had neither a parental report nor a clinician-diagnosis of eczema. The clinician’s diagnosis definition was also used in a sensitivity analysis to validate findings from the parental report. As a secondary outcome measure, we also evaluated a subgroup of children with “atopic eczema,” defined as a parental report of eczema plus a positive SPT at age four.
Statistical Analysis
Potential covariates are listed in Table I (available at www.jpeds.com). A positive skin prick test was defined as a wheal of 3 mm greater than the saline control, along with a flare at least as large as the accompanying wheal. Previous studies have shown that aeroallergen sensitization is less common prior to the age of two, and up to 15–20% of young children may have transient responses on skin testing.20, 21 Although the predictive value of transient aeroallergen sensitization is unclear, it may be a measure of the ability to become sensitized and thus may have clinical relevance in terms of the presence and/or future development of atopic diseases like eczema.20 We therefore considered a child to have a positive skin prick test for an aeroallergen if they had a single positive result for that allergen at ages one, two, and/or three. Because previous studies reported that sensitivity to egg at age one was predictive of atopic manifestations at later ages, and the prevalence and predictive value of egg SPT+ at ages two and three were lower in this study, SPT results for egg at age one only were ultimately included in the multivariable model.22
Table I.
Potential Covariates (Estimated Maximum Model)
| Potential covariate | Age of child when data collected | Characteristics of variable |
|---|---|---|
| Sex | Enrollment | |
| Race | Enrollment | African-American versus all others |
| Parental income | Enrollment | 9 categories, lowest= under $9,999, highest= over $110,000; ordinal |
| Highest level of parental education attained | Enrollment | Categorical; analyzed separately by gender |
| History of parental eczema | Enrollment | Yes/No |
| History of parental asthma | Enrollment | Yes/No |
| Dog ownership | Prior to age 1 year (home visit) Dog allergen (Can f 1) levels: home visit |
Yes/No Continuous and in tertiles |
| Cat ownership | Prior to age 1 year (home visit) Cat allergen (Fel d 1) levels: home visit |
Yes/No Continuous and in tertiles |
| Endotoxin | Prior to age 1 year (home visit) | Continuous and in tertiles |
| β-glucan | Prior to age 1 year (home visit) | Continuous and in tertiles |
| Dust-mite (Der p 1) | Prior to age 1 year (home visit) | Continuous and in tertiles |
| Season of birth | Enrollment | Winter: Dec-Feb; Spring: March-May; Summer: June-August; Fall: September-November; Ordinal |
| Breast-feeding | Age 2 years (questionnaire) | Yes/No and Months breastfed |
| Tobacco Smoke Exposure (ETS) | Ages 1–4 years (questionnaire) | 3 variables: (1) Total number of smokers (2) Cigarettes smoked in household (3) Cigarettes smoked by mother Analyzed as None versus Any, and Number of cigarettes smoked |
| ECAT (Elemental carbon attributable to traffic) | Average over first year of life | Measures exposure to traffic related particles; Continuous and in tertiles |
| Daycare attendance | Ages 1–4 years (questionnaire) | Yes/No (each year analyzed separately) |
| Number of siblings | Ages 1 and 2 years (questionnaire) | Analyzed as an ordinal and a continuous variable |
| Skin prick tests (SPTs) | Ages 1–4 years | Positive= >3 mm above saline control, versus Negative |
| Timing of food introduction | Age 6 months, Ages 1–4 years (questionnaire) | Egg & Milk: Before versus after 1, 2, 3, or 4 years of age respectively Nut (peanut or tree nut): Before versus after 2 or 3 years of age, respectively |
| Interaction Terms Dog Ownership * Dog SPT Cat Ownership * Cat SPT |
Ages 1, 2, and/or 3 for SPTs |
Interactions for Dog or Cat respectively & SPTs in multivariable model |
Regardless of univariable findings, all potential covariates listed in Table I were initially included in the maximum estimated logistic regression model and removed by backward elimination from the multivariable model based on an alpha cut-off of 0.15. Variables that remained in the final model included: (1) history of parental eczema; (2) egg ingestion (on a single or multiple occasions) prior to age one year; (3) SPT+ to egg at age one; (4) SPT+ to elm at ages one, two, and/or three; and the interaction terms for (5) dog ownership (yes/no) prior to age one, and dog SPT+ at ages one, two, and/or three; and (6) cat ownership (yes/no) prior to age one and cat SPT+ at ages one, two and/or three. A spline procedure was used to determine the effects of dog and cat allergen concentrations in house dust, with adjustment for other covariates in the multivariable model.23
Results
In total, 636 children completed the annual visit at age four; 14% (n=90) satisfied the definition of eczema based on parental report, 17% (n=107) met criteria for the clinician’s diagnosis, and 75% (n=477) did not have eczema by either case definition. Thirty-nine children (6%) satisfied both the parental report and clinician’s diagnosis of eczema, and 51 met criteria for eczema by parental report but not by clinician’s diagnosis, and 68 met criteria by the clinician’s diagnosis but not by parental report (Table II; available at www.jpeds.com). The prevalence of atopic eczema was 10% (n=55). Data from the questionnaire was complete, and 635 children completed the physical exam. In order to avoid including children in the comparison group who were potentially misclassified (i.e. positive for eczema by clinician’s diagnosis but negative by parental report), 68 children were excluded a priori from the analysis.
Table II.
Number of children with Eczema by Parental Report versus Clinician’s Diagnosis
| No eczema by parental report | Eczema by parental report | Total | |
|---|---|---|---|
| No eczema by clinician’s diagnosis | 477 | 51 | 528 |
| Eczema by clinician’s diagnosis | 68 | 39 | 107 |
| Total | 545† | 90 | 635‡ |
The total number of children with no eczema by parental report was 546 (545 are included in this comparison given that one child did not complete a physical exam)
The total number of children who completed the annual visit at age four was 636 (one child did not complete a physical exam)
Table III presents unadjusted findings for eczema based on the parental report case definition. A history of eczema in either parent was a significant predictor of eczema at age four (p=0.002). High ECAT (elemental carbon attributable to traffic) in the first year of life trended towards a positive association with eczema at age 4 years (p=0.07), and dog ownership prior to age 1 year (p=0.002) also conferred a significant protective effect. Egg ingestion prior to age 1 year demonstrates a trend towards protection (p=0.05). Peanut/tree nut ingestion prior to age 3 years conferred significant protective effects from eczema (p=0.002). Potential covariates that were not significantly predictive included: parental asthma; season of birth; parental income; parental education level; breast-feeding; day-care attendance; number of siblings; tobacco smoke exposure; house dust concentrations of endotoxin, dust-mite allergen, and β-glucan; and a parental report of cow’s milk ingestion prior to age one. Unadjusted findings for SPTs are presented separately (Table IV; available at www.jpeds.com).
Table III.
Characteristics of Children with Eczema at Age Four Years#
| Characteristic† | N Eczema/N Total (% with Eczema) | Unadjusted Odds ratio [95% CI] | P-value |
|---|---|---|---|
| Male | 52/310 (17%) | 1.2 [0.7–1.8] | 0.5 |
| African-American | 24/131 (18%) | 1.3 [0.8–2.1] | 0.4 |
|
History of parental eczema Either parent |
27/96 (28%) | 2.5 [1.5–4.3] | 0.002 |
| Dog ownership before age 1‡ Cat ownership before age 1‡ |
17/184 (9%) 19/121 (14%) |
0.4 [0.2–0.7] 1.0 [0.6–1.7] |
0.002 0.9 |
|
Diesel (ECAT)§ Highest tertile versus Lowest tertile |
35/177 (20%) 26/181 (14%) |
1.5 [0.8–2.6] | 0.07 |
| Egg ingestion before age 1 Peanut/tree nut before age 3 |
54/383 (14%) 64/447 (14%) |
0.6 [0.4–1.0] 0.4 [0.2–0.7] |
0.05 0.002 |
Total cohort size was 636 children at age four; 90 children (16%) had eczema based on parental report; 477 children were in the comparison group (no eczema by both parental report and clinician’s diagnosis)
Additional covariates that were not significantly predictive are listed in the text.
Based on home visit
Elemental carbon attributable to traffic; average over first year of life
Table IV.
| Skin prick test (SPT) | N Eczema/N Total (% with Eczema) | Unadjusted Odds Ratio [95% CI] | p-value |
|---|---|---|---|
|
| |||
| Any SPT positive at age 4 yrs | 55/273 (20%) | 1.9 [1.2–3.0] | 0.007 |
| Food | |||
| Egg at age 1 yrs | 23/58 (40%) | 4.3 [2.4–7.8] | <0.0001 |
| Milk, ages 1, 2, and/or 3 yrs | 7/24 (29%) | 2.4 [1.0–6.0] | 0.05 |
| Animals | |||
| Dog, ages 1,2, and/or 3 yrs | 20/49 (41%) | 4.8 [2.6–9.1] | <0.0001 |
| Cat, ages 1,2, and/or 3 yrs | 24/67 (36%) | 4.0 [2.4–7.3] | <0.0001 |
| Pollen | |||
| Any tree, ages 1,2, and/or 3 yrs | 37/170 (22%) | 1.9 [1.2–3.0] | 0.01 |
| 18/48 (37.5%) | 4.0 [2.1–7.7] | <0.0001 | |
| Elm, ages 1,2, and/or 3 yrs | 16/55 (29%) | 2.5 [1.3–4.8] | 0.004 |
| Oak, ages 1,2, and/or 3 yrs | 17/75 (23%) | 1.8 [1.0–3.3] | 0.06 |
| Cedar, ages 1,2, and/or 3 yrs | 14/52 (27%) | 2.3 [1.2–4.5] | 0.01 |
| Ragweed, ages 1,2, and/or 3 yrs | 13/46 (28%) | 2.4 [1.2–4.9] | 0.01 |
| Fescue, ages 1,2, and/or 3 yrs | 21/83 (25%) | 2.1 [1.2–3.7] | 0.009 |
| Timothy, ages 1,2, and/or 3 yrs | 28/129 (22%) | 1.7 [1.0–2.9] | 0.04 |
| Mold | 13/48 (27%) | 2.2 [1.1–4.4] | 0.02 |
| Any mold, ages 1, 2, and/or 3 yrs | |||
| Alternaria, ages 1, 2, and/or 3 yrs | |||
Total cohort size was 636 children at 4 years; 90 children (16%) had eczema based on parental report; 477 children were in the comparison group (no eczema by both parental report and clinician’s diagnosis)
Skin prick test results that were not significantly predictive included: Dust-mite (n total=99), Cockroach (n total=76), Aspergillus (n total=59), Penicillium (n total=61), Cladosporium (n total=53), and Maple (n total=75)
Results from a multivariable logistic regression analysis are presented in Table V. After adjusting for the environmental exposures and host characteristics described above, significant predictors of eczema at age four were parental eczema (p=0.03), SPT+ to egg at age 1 year (p<0.001), and SPT+ to elm tree pollen at ages 1, 2, and/or 3 years (p=0.03). Findings for pet ownership and dog/cat SPT are not listed separately in Table V because of the presence of significant interactions involving these covariates. Without adjustment for interactions, however, dog ownership prior to age 1 year was significantly protective from eczema (p=0.009), and dog SPT+ at ages 1, 2, and/or 3 years demonstrated a greater than four-fold significant increased risk of eczema (p=0.003). By themselves neither cat ownership prior to age one, nor cat SPT+ at ages one, two, and/or three were significantly predictive.
Table V.
| N Eczema/N Total (% with Eczema) | Adjusted Odds ratio[95% CI] | p-value | |
|---|---|---|---|
|
| |||
| History of parental eczema | 27/96 (28%) | 2.2 [1.1–4.3] | 0.03 |
|
| |||
| Egg ingestion before age 1 year | 54/383 (14%) | 0.6 [0.3–1.1] | 0.08 |
|
| |||
| Sensitization to egg (age 1 year) | 23/58 (40%) | 3.9 [1.8–8.7] | <0.001 |
|
| |||
| Sensitization to elm (ages 1,2, and/or 3 years) | 18/48 (38%) | 2.8 [1.1–7.2] | 0.03 |
|
| |||
| Interaction Terms | |||
| Dog in home (before age 1; n=184)‡ | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 2/14 (14%) | 1.3§ | 0.8 |
| versus | [0.3–6.8] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 14/160 (9%) | ||
| No Dog in home (before age 1; n=325) | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 17/30 (57%) | 3.9 | 0.002 |
| versus | [1.6–9.2] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 38/253 (15%) | ||
|
| |||
| Cat in home (before age 1; n=121)‡ | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 7/13 (54%) | 13.3§ | <0.001 |
| versus | [3.1–57.9] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 10/95 (11%) | ||
| No Cat in home (before age 1; n=353) | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 16/49 (33%) | 1.1 | 0.8 |
| versus | [0.5–2.7] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 39/304 (13%) | ||
Total cohort size was 636 children at age 4 years; 90 children (16%) met criteria for eczema based parental report
16 (9%) children with eczema owned dogs; 17 (14%) owned cats; based on home visit
Represents adjusted odds ratio for eczema based on the interaction between pet ownership and skin prick test (SPT)
Table II represents covariates that remained in the final model after backward elimination with an alpha cut-off of 0.15.
Egg ingestion prior to age one trended towards a protective effect from eczema at age four (p=0.08). The timing of egg introduction was not related to egg SPT+. Early versus delayed introduction of milk and peanuts/tree nuts were not predictive of eczema in the multivariable analysis.
Interaction between Dog Ownership and Skin Prick Test Reactivity to Dog
We next examined the relationship between dog ownership and percutaneous sensitization to dog (Table V and Figure 1). Prior to age one, 184 children owned dogs. Among children who had a dog prior to age one, dog SPT+ at ages one, two, and/or three did not significantly increase the risk for eczema at age four. In contrast, children who did not own dogs (prior to age one), but were dog SPT+ (ages one, two, and/or three), had an almost four-fold increased risk of eczema at age four (p=0.002). Children who owned dogs and were dog SPT- had a slightly lower rate of eczema relative to those who did not own dogs (9% versus 15%); however, this result was not statistically significant. Maternal/paternal dog SPT status did not predict dog ownership. Dog ownership was also independent of dog SPT status in the first three years of life and of endotoxin levels.
Figure 1.
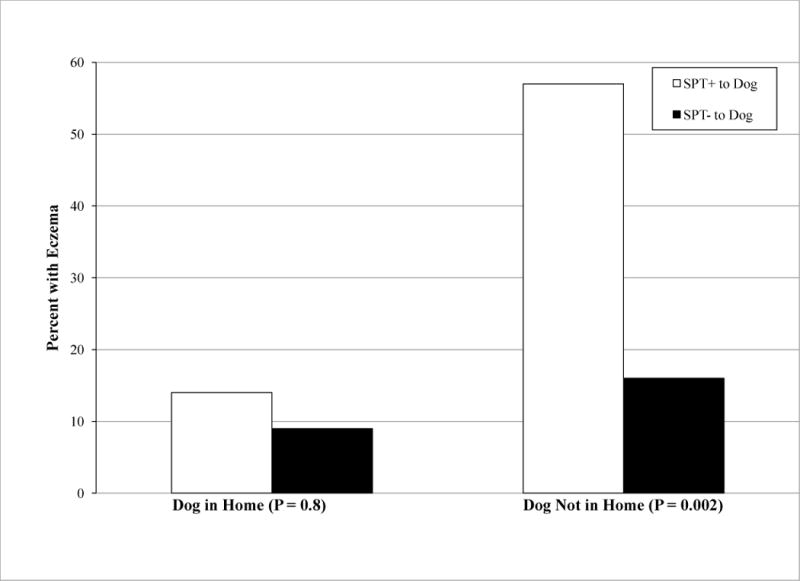
Relationship between Dog Ownership (prior to age 1 year), SPT to Dog (ages 1, 2, and/or 3 years), and Eczema (age 4 years) (p=0.002). Dark bars represent dog SPT positive, and hollow bars represent dog SPT negative.
To verify these findings, we examined the effects of dog allergen levels from house dust samples collected prior to age one on the presence of eczema at age 4 years (Table VI; available at www.jpeds.com). Children exposed to the highest tertile of dog allergen concentrations had a significantly lower risk of eczema (uOR 0.4 [0.2–0.7]; p=0.01).
Table VI.
| Allergen‡ | N Eczema/N Total (% with Eczema) | Unadjusted Odds ratio [95% CI] | p-value |
|---|---|---|---|
| Dog Highest tertile versus Lowest tertile |
12/144 (8%) 59/294 (20%) |
0.4 [0.2–0.7] |
0.01 |
| Cat Highest tertile versus Lowest tertile |
28/177 (16%) 33/230 (14%) |
1.1 [0.7–1.9] |
0.6 |
Total cohort size was 636 children at age 4 years
Eczema based on parental report case definition
From house dust samples collected at home visit (prior to age one)
We next sought to determine if the modifying effect of dog ownership might impact other SPTs as well. With eczema as the outcome, we found no interactions involving dog ownership and SPTs to allergens other than dog. A significant inverse relationship between dog ownership prior to age one and SPT positivity to cat at ages one, two, and/or three (uOR 0.5 [0.3–0.8]; p=0.005) was found, indicating that dog owners were less likely to become sensitized to cats. This relationship was independent of cat ownership or cat allergen levels.
Interaction between Cat Ownership and Skin Prick Test Reactivity to Cat
A similar analysis was performed for cat ownership and cat sensitization (Table V and Figure 2). Prior to age one, 121 children lived with cats. In contrast to children living with dogs, children living with cats prior to age one who were SPT+ to cat at ages one, two, and/or three were 13 times more likely to have eczema at age four than those who were cat SPT negative (p<0.001). Among non-cat owners, no significant relationship was found between SPT positivity to cat and age four eczema (p=0.8). Maternal/paternal cat SPT status were not predictive of cat ownership. Cat SPT+ at ages one through three and cat ownership prior to age one were also independent of one another, and were independent of endotoxin.
Figure 2.

Relationship between Cat Ownership (prior to age 1 year), SPT to Cat (ages 1, 2, and/or 3 years), and Eczema (age 4 years) (p<0.001). Dark bars represent cat SPT positive, and hollow bars represent cat SPT negative.
To verify the above findings, cat allergen levels from house dust samples collected at eight months of age were assessed. Cat allergen levels alone were not predictive of eczema (Table VI). After adjustment for other covariates using a spline procedure, a positive trend between cat allergen exposure and age four eczema was found for levels up to 3.0 μg/g of dust (p=0.10).23
Sensitivity Analysis
A sensitivity analysis was used to compare findings using different eczema definitions, as well as to determine the impact of excluding potentially misclassified children. Findings were similar for most outcomes regardless of the eczema definition employed, with the exception that the protective effect of dog ownership was less pronounced when using the clinician’s diagnosis of eczema (uOR 0.7 [0.5–1.2]; p=0.2). Among the subgroup of children who met both the parental report and clinician’s diagnosis definitions (n=39), major findings were the same as those based on the parental report. Our conclusions were unchanged and precision was increased by including children who were positive for eczema on physical exam but negative by parental report in the comparison (no eczema) group. (Tables VII–IX; available at www.jpeds.com)
Table VII.
Part I of Sensitivity Analysis. Multivariable Analysis to determine predictors of Eczema Based on Clinician’s Diagnosis at Age 4 Years†±
| N Eczema/N Total (% with Eczema) | Adjusted Odds ratio [95% CI] | p-value | |
|---|---|---|---|
|
| |||
| History of parental eczema | 32/115 (28%) | 2.4 [1.4–4.4] | 0.003 |
|
| |||
| Egg ingestion before age 1 year | 71/426 (17%) | 0.9 [0.5–1.6] | 0.7 |
|
| |||
| Sensitization to egg (age 1 year) | 27/72 (38%) | 4.3 [2.2–8.5] | <0.001 |
|
| |||
| Sensitization to elm (ages 1,2, and/or 3 years) | 16/55 (29%) | 2.1 [0.9–5.1] | 0.1 |
|
| |||
| Interaction Terms | |||
| Dog in home (before age 1; n=197)‡ | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 4/17 (24%) | 2.6§ | 0.2 |
| versus | [0.7–9.9] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 24/180 (13%) | ||
| No Dog in home (before age 1; n=319) | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 14/33 (42%) | 1.8 | 0.2 |
| versus | [0.7–4.8] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 51/286 (18%) | ||
|
| |||
| Cat in home (before age 1; n=120) ‡ | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 9/18 (50%) | 7.4§ | 0.002 |
| versus | [2.2–25.3] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 14/102 (14%) | ||
| No Cat in home (before age 1; n=402) | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 17/58 (29%) | 0.6 | 0.4 |
| versus | [0.3–1.6] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 55/344 (16%) | ||
Total cohort size was 636 children at age four; 107 children (18%) met criteria for eczema based on the clinician’s diagnosis
28 (26%) children with eczema based on the clinician’s diagnosis owned dogs; 23 (21%) owned cats; based on the home visit
Represents adjusted odds ratio for eczema based on the interaction between pet ownership and skin prick test (SPT)
Table E3 represents covariates that remained in the final model after backward elimination with an alpha cut-off of 0.15.
Table IX.
Part III of Sensitivity Analysis. Multivariable Analysis to determine predictors of Eczema Based on Parental Report at Age 4 Years†± WITHOUT EXCLUSION OF 68 CHILDREN WHO WERE POSITIVE BY CLINICIAN’S DIAGNOSIS BUT NEGATIVE BY PARENTAL REPORT
| N Eczema/N Total (% with Eczema) | Adjusted Odds ratio [95% CI] | p-value | |
|---|---|---|---|
|
| |||
| History of parental eczema | 27/115 (23%) | 2.0 [1.1–3.7] | 0.03 |
|
| |||
| Egg ingestion before age 1 year | 54/426 (13%) | 0.6[0.3–1.1] | 0.07 |
|
| |||
| Sensitization to egg (age 1 year) | 23/72 (32%) | 3.1[1.5–6.5] | 0.002 |
|
| |||
| Sensitization to elm (ages 1,2, and/or 3 years) | 18/55 (33%) | 2.7[1.2–6.1] | 0.01 |
|
| |||
| Interaction Terms | |||
| Dog in home (before age 1; n=197)‡ | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 2/17 (12%) | 1.3§ | 0.7 |
| versus | [0.3–6.5] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 14/180 (8%) | ||
| No Dog in home (before age 1; n=319) | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 17/33 (52%) | 4.8 | <0.001 |
| versus | [2.1–10.8] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 38/286 (13%) | ||
|
| |||
| Cat in home (before age 1; n=120)‡ | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 7/18 (39%) | 7.3§ | 0.002 |
| versus | [2.1–25.0] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 10/102 (10%) | ||
| No Cat in home (before age 1; n=402) | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 16/58 (28%) | 1.1 | 0.8 |
| versus | [0.5–2.5] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 39/344 (11%) | ||
Total cohort size was 636 children at age four; 90 children (15%) met criteria for eczema based on the parental report definition WITHOUT removal from the analysis of children who were negative for eczema by parental report but positive based on the clinician’s diagnosis. Children who were negative by parental report were included in the comparison (no eczema) group for this analysis regardless of findings from the clinician’s diagnosis.
16 (9%) children with eczema based on both the parental report and the clinician’s diagnosis owned dogs; 17(14%) owned cats; based on the home visit
Represents adjusted odds ratio for eczema based on the interaction between pet ownership and skin prick test (SPT)
Table E5 represents covariates that remained in the final model after backward elimination with an alpha cut-off of 0.15.
Analysis in African-Americans
Interactions between pet ownership and sensitization were not found in the African-American subpopulation, possibly because very few African-Americans owned pets (Table X; available at www.jpeds.com). Nevertheless, African-Americans who owned cats prior to age one (n=6; 3%) were twelve times more likely to have eczema at age four than those who did not (aOR 12.4 [1.2–133.7]; p=0.04). In contrast, none of the African-Americans who owned dogs (n=17; 9%) prior to age one had eczema at age four.
Table X.
Multivariable Analysis of Predictors of Eczema Based on Parental Report at Age 4 Years in African-Americans#
| Adjusted Odds ratio [95% CI] | p-value | |
|---|---|---|
| History of parental eczema | 2.2 [0.4–10.9] | 0.3 |
| Egg ingestion before age 1 year | 0.3 [0.07–1.4] | 0.1 |
| Sensitization to egg (age 1 year) | 1.8 [0.3–9.2] | 0.5 |
| Sensitization to elm (ages 1,2, and/or 3 years) | 1.8 [0.3–10.2] | 0.5 |
| Dog ownership before age 1 year | Cannot estimate* | |
| Sensitization to dog (ages 1,2, and/or 3 years) | 4.5 [1.1–18.2] | 0.04 |
| Cat ownership in year 1 year† | 12.4 [1.2–133.7] | 0.04 |
| Sensitization to cat (ages 1,2, and/or 3 years) | 1.4 [0.4–5.5] | 0.7 |
The African-American subpopulation included 131 children at age four; 24 had eczema based on the parental report
Unable to accurately estimate the effect of dog ownership or assess for an interaction with sensitization due to the low prevalence of dog ownership in the African-American subpopulation (17 African-Americans owned dogs prior to age one; none of the dog owners had eczema).
6 African-American children owned cats prior to age one; three of these had eczema at age 4 years. The interaction between cat and cat-ownership was not calculated due to collinearity between these variables in the African-American subpopulation.
An analysis of atopic eczema (eczema plus a positive SPT) for the entire cohort can be found in Table XI (available at www.jpeds.com).
Table XI.
Multivariable Analysis to determine predictors of Atopic Eczema at Age 4 Years†
| Adjusted Odds ratio [95% CI] | p-value | |
|---|---|---|
|
| ||
| History of parental eczema | 2.0 [0.8–4.8] | 0.14 |
|
| ||
| Egg ingestion before age 1 year | 0.4 [0.2–0.9] | 0.03 |
|
| ||
| Sensitization to egg (age 1 year) | 6.5 [2.5–16.7] | <0.001 |
|
| ||
| Sensitization to elm (ages 1,2, and/or 3 years) | 4.4 [1.6–12.0] | 0.004 |
|
| ||
| Interaction Terms | ||
| Dog in home (before age 1)‡ | ||
| SPT+ versus − to Dog (ages 1,2, and/or 3) | 2.4 [0.3–17.0]§ | 0.4 |
| No Dog in home (before age 1) | ||
| SPT+ versus - to Dog (ages 1,2, and/or 3) | 5.0 [1.8–13.9] | 0.002 |
|
| ||
| Cat in home (before age 1)‡ | ||
| SPT+ versus - to Cat (ages 1,2, and/or 3) | 10.3 [2.5–43.2]§ | 0.001 |
| No Cat in home (before age 1) | ||
| SPT+ versus - to Cat (ages 1,2, and/or 3) | 1.4 [0.5–4.2] | 0.6 |
Total cohort size was 636 children at age four; Atopic eczema was defined as parental report of eczema plus a positive skin prick test (SPT) at age four (n=55 children)
Based on home visit
Represents adjusted odds ratio for atopic eczema based on the interaction between pet ownership and SPT
Discussion
Consistent with previous studies, children in the CCAAPS cohort with a history of parental eczema and skin prick test positivity to egg at age one were at significantly increased risk for eczema at age four.4, 24 As previously reported, early dog ownership conferred a significant protective effect from eczema at age four.7, 24 Also, consistent with recent reports, cat ownership by itself did not confer a significantly increased risk for eczema.9 After adjustment for these and other known risk factors, several novel findings became apparent.
We report significant interactions between pet ownership and sensitization to pets, with the outcome of eczema. We found that dog owners with early sensitization to dog were almost four times less likely to have eczema at age four than non-dog owners. Thus, early dog ownership significantly reduced the risk of eczema associated with dog sensitization. Even though there was also a trend towards a protective effect of dog ownership among children who were not sensitized to dog, this finding was not statistically significant. In contrast to these findings, cat ownership significantly increased the risk for eczema among cat sensitized children. The magnitude of this effect was pronounced, as cat owners with early sensitization to cat were 13 times more likely to have eczema at age four than non-cat owners. Sensitization to dogs or cats was independent of dog or cat ownership, respectively, presumably because of the ubiquity of dog and cat allergens in a variety of indoor environments. The aforementioned relationships were also independent of endotoxin levels.
It is tempting to attribute the protective effect of dog ownership to a natural form of immunotherapy, whereby constant exposure to high levels of dog antigen over time might induce tolerance to dog allergen in sensitized children. Consistent with the literature, early dog ownership was also protective from the development of skin prick test positivity to cat.8 This suggests that dog ownership may have broader tolerogenic effects. T cell lines of dog allergic subjects produce high levels of IL-10 and IFN-gamma, cytokines associated with the induction of tolerance, after stimulation with certain Can f 1 epitopes.25 In another study involving children of atopic parents, dog ownership in infancy was associated with increased secretion of the anti-inflammatory cytokine IL-10, along with a reduced risk for childhood eczema and decreased sensitization to foods and aeroallergens.6 These effects were later found to be independent of endotoxin levels.26 In this study, dog ownership was independent of endotoxin levels, and endotoxin levels were unrelated to the presence of eczema. In addition, although we previously reported an interaction involving dog ownership and endotoxin levels with asthma at age one, we did not find evidence for an interaction involving endotoxin and dog ownership with eczema at age four.16 It is therefore possible that dog antigens exert immune modulating effects distinct from those attributed to endotoxin.26
Why cat ownership accentuated the positive association between sensitization to cat and eczema, rather than promoting tolerance is not clear. The novel finding that interactions between pet ownership and sensitization appear to be specific for dog or cat allergens suggests that animal exposures may exert antigen specific effects, the mechanisms of which are poorly understood. Recent studies have highlighted the positive association between exposure to cats and eczema among children with filaggrin mutations.9 In contrast, dog ownership does not appear to increase the risk for eczema associated with filaggrin variants.24 Further research regarding gene-environment interactions between genetic variants in skin barrier proteins and pet ownership may be particularly germane.
Recent studies indicate that delayed introduction of allergenic foods is unlikely to prevent eczema and other atopic diseases, and that earlier introduction of these foods might be beneficial.27 In this study, delaying egg introduction until after age one did not decrease the risk for eczema at age four, and earlier introduction may have been protective. This finding was statistically significant for children with atopic eczema, arguably the group at highest risk to develop asthma (Table XI). Although the possibility of reverse causation due to food restriction based on parental food allergies or the presence of eczema in the first few months of life cannot be absolutely ruled out, our results do support recently modified guidelines not to delay egg introduction in high-risk children.28 Findings regarding the timing of nut ingestion should be interpreted with caution, given that effects were not significant after adjustment for other covariates in the multivariable model.
Even though the role of food introduction and sensitization to foods in the development of eczema has received much attention, the association between aeroallergen sensitization and eczema early in life may be underappreciated.5 One of the most important predictors of eczema at age four was sensitization to American elm, a tree pollen, at ages one, two, and/or three. We previously reported that elm pollen was a less common sensitizer in infancy relative to other trees.12 Elm remained a less common sensitizer through age three (Table IV). Among children sensitized to elm, however, almost 40% had eczema, compared with 29% for oak, 23% for red cedar, and 21% for maple. Our findings may therefore indicate that children with eczema are prone to early sensitization to aeroallergens that are less frequent sensitizers in other children.
It has recently been suggested that exposure to traffic-related air pollutants may increase the risk for eczema.29 In this study, elemental carbon attributable to traffic, a marker of diesel exposure, trended towards a positive association with eczema in the univariable analysis. This finding was not replicated in the multivariable analysis, however, suggesting that confounding factors may explain the previously reported positive association between air pollution and eczema.
Although bias is a potential concern for studies involving pet keeping, there are several ways that bias was minimized in this study. First, dog and cat ownership and early sensitization to dogs and cats, respectively, were independent of one another. Pet keeping was unrelated to parental sensitization patterns. In addition, the prevalence of pet keeping in this high-risk birth cohort was very similar to the general population.16 We adjusted for numerous other possible risk factors in the logistic regression model. Even though maternal smoking and socioeconomic status in particular have been cited as having an association with pet keeping, these factors did not impact our results. Furthermore, the sensitivity analysis conducted to validate our eczema definitions revealed similar findings regardless of the case definition employed (Tables VII–IX).
Finally, it is difficult to speculate regarding the impact of our findings on non-atopic eczema, given that our cohort was enriched for atopy. Findings from the small subgroup of children with non-atopic eczema suggest that parental eczema may be the most important determinant of eczema among non-atopic children, and that other predictors of atopic eczema may not be as relevant to this group (Appendix; available at www.jpeds.com). In addition, findings regarding pet ownership and sensitization may not be applicable to African-Americans, given the small number of African-Americans who owned pets.
In conclusion, in this prospective birth cohort involving children of atopic parents, an interaction between pet ownership and pet sensitization was discovered that has not been previously reported. Early dog ownership attenuated the risk for eczema among dog-sensitized children, and early cat ownership accentuated the association between cat sensitization and eczema. In addition, we found that early sensitization to elm, a relatively uncommon sensitizer in young children, was strongly associated with the presence of eczema at age four. Delayed introduction of egg beyond one year was not found to be protective from atopic eczema.
Acknowledgments
We would like to thank Heidi Sucharew, PhD, and Enas Al-shaikh, MS, at the University of Cincinnati Department of Environmental Health for their assistance with SAS programming involving several variables used for this study. In addition, we would like to thank Jeff Burkle, BS, at the University of Cincinnati Department of Environmental Health (NIEHS ES11170) for his assistance with the CCAAPS dataset. We would also like to acknowledge Rolanda Olds, BS, and Zana Lummus, PhD, at the University of Cincinnati Immunology laboratory for their work with the house dust samples. Finally, we thank the children and families of the CCAAPS birth cohort for their continued involvement with this project.
Supported by NIEHS ES11170 & NIAID T32 AI60515.
Abbreviations
- CCAAPS
Cincinnati Childhood Allergy and Air Pollution Study
- SPT
skin prick test
- uOR
unadjusted odds ratio
- aOR
adjusted odds ratio
- ECAT
elemental carbon attributable to traffic
- PM2.5
particulate matter less than 2.5 μm in diameter
Appendix
Sensitivity Analysis of Eczema Definition
Despite low agreement between the parental report and clinician’s diagnosis (kappa=0.3), most results were the same using either outcome definition (Table I). Using the clinician’s diagnosis, a history of parental eczema and egg SPT+ at age one were still predictive of eczema at age four (aOR 2.4 [1.4–4.4]; p=0.003 and 4.3 [2.2–8.5]; p<0.001, respectively). The significant interaction between cat ownership and positive cat SPT (ages 1–3) was also present using the clinician’s diagnosis eczema definition (aOR 7.4[2.2–25.3]; p=0.002 among cat owners with cat SPT+ versus aOR 1.1[0.4–3.0]; p=0.9 among non-cat owners with cat SPT+).
Perhaps of greatest relevance, all of the findings from the parental report case definition were replicated in the subgroup of children who had eczema by both parental report and the clinician’s diagnosis (Table VIII). The significant association between egg SPT+ at age one and eczema at age four (aOR 5.6 [2.2–14.4]; p<0.001), and the significant interaction involving cat and cat SPT were present in this subgroup (aOR 7.7[1.5–38.6]; p=0.02 among cat owners with cat SPT+ versus 1.1[0.4–3.0]; p=0.9 among non-cat owners with cat SPT+). The same was true for dog and dog SPT (aOR 3.8 [0.4–36.4]; p=0.3 among dog owners with dog SPT+ versus 5.1[1.9–14.0]; p=0.002 among non-dog owners with dog SPT+).
Table VIII.
Part II of Sensitivity Analysis. Multivariable Analysis to determine predictors of Eczema Based on Both Parental Report and Clinician’s Diagnosis at Age 4 Years†±
| N Eczema/N Total (% with Eczema) | Adjusted Odds ratio [95% CI] | p-value | |
|---|---|---|---|
|
| |||
| History of parental eczema | 13/82 (16%) | 1.8 [0.7–4.9] | 0.2 |
|
| |||
| Egg ingestion before age 1 year | 28/357 (8%) | 1.2 [0.5–2.8] | 0.8 |
|
| |||
| Sensitization to egg (age 1 year) | 13/48 (27%) | 5.6 [2.2–14.4] | <0.001 |
|
| |||
| Sensitization to elm (ages 1,2, and/or 3 years) | 9/39 (23%) | 2.9 [1.0–8.5] | 0.06 |
|
| |||
| Interaction Terms | |||
| Dog in home (before age 1; n=163)‡ | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 1/13 (8%) | 3.8§ | 0.3 |
| versus | [0.4–36.4] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 4/150 (3%) | ||
| No Dog in home (before age 1; n=257) | |||
| SPT+ to Dog (ages 1,2, and/or 3) | 11/24 (46%) | 5.1 | 0.002 |
| versus | [1.9–14.0] | ||
| SPT− to Dog (ages 1,2, and/or 3) | 18/233 (8%) | ||
|
| |||
| Cat in home (before age 1; n=102)‡ | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 4/10 (40%) | 7.7§ | 0.02 |
| versus | [1.5–38.6] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 7/92 (8%) | ||
| No Cat in home (before age 1; n=321) | |||
| SPT+ to Cat (ages 1,2, and/or 3) | 8/41 (20%) | 1.1 | 0.9 |
| versus | [0.4–3.0] | ||
| SPT− to Cat (ages 1,2, and/or 3) | 15/280 (5%) | ||
Total cohort size was 636 children at age four; 39 children (8%) met criteria for eczema based on both the parental report and the clinician’s diagnosis
5 (13%) children with eczema based on both the parental report and the clinician’s diagnosis owned dogs; 11(28%) owned cats; based on the home visit
Represents adjusted odds ratio for eczema based on the interaction between pet ownership and skin prick test (SPT)
Table E4 represents covariates that remained in the final model after backward elimination with an alpha cut-off of 0.15.
As a separate sensitivity analysis, we evaluated the impact of including children in the comparison (no eczema) group who were negative for eczema on parental report but positive by the clinician’s diagnosis (Table IX). Conclusions for all outcomes in the multivariable analysis were unchanged and confidence intervals were smaller (precision was increased) when we added back these 68 children who had been excluded a priori because of concerns for misclassification.
Predictors of Atopic Eczema at Age Four Years
Atopic eczema (n=55) was analyzed with adjustment for covariates described in the results section (Tables I and III). Results were similar to those with eczema alone (Table X). The positive associations between sensitization to egg and atopic eczema, and between elm and atopic eczema were stronger (aOR 6.5 [2.5–16.7]; p<0.001 and aOR 4.4 [1.5–12.7]; p=0.006, respectively) than for eczema alone. The protective effect of early egg introduction was statistically significant in this subgroup (aOR 0.4 [0.2–0.9]; p=0.03). Statistically significant effect modification by dog ownership on dog SPT was also present. As before, children with no dog who were dog SPT+ were at significantly increased risk for atopic eczema (aOR 5.0 [1.8–13.9]; p=0.002). The significant interaction between cat ownership and cat SPT was also replicated. The effect of parental eczema was no longer statistically significant. In a separate analysis, we found that parental eczema was the only predictor that trended towards significance among children with non-atopic eczema at age four (n=45; aOR 2.1 [0.9–5.0]; p=0.09).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118:209–13. doi: 10.1016/j.jaci.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 3.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 4.Hon KL, Leung TF, Ching G, et al. Patterns of food and aeroallergen sensitization in childhood eczema. Acta Paediatr. 2008;97:1734–7. doi: 10.1111/j.1651-2227.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 5.Grabbe J, Zuberbier T, Wagenpfeil S, Czarnetzki BM. Skin prick tests to common allergens in adult atopic eczema and rhinitis patients: reproducibility on duplicate and repeated testing. Dermatology. 1993;186:113–7. doi: 10.1159/000247320. [DOI] [PubMed] [Google Scholar]
- 6.Gern JE, Reardon CL, Hoffjan S, et al. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004;113:307–14. doi: 10.1016/j.jaci.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Biagini Myers JM, Wang N, Lemasters GK, et al. Genetic and Environmental Risk Factors for Childhood Eczema Development and Allergic Sensitization in the CCAAPS Cohort. J Invest Dermatol. 2009;130:430–437. doi: 10.1038/jid.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 9.Bisgaard H, Simpson A, Palmer CN, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5:e131. doi: 10.1371/journal.pmed.0050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127:502–8. doi: 10.1378/chest.127.2.502. [DOI] [PubMed] [Google Scholar]
- 11.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 12.LeMasters GK, Wilson K, Levin L, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–11. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Chapman MD, Aalberse RC, Brown MJ, Platts-Mills TA. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988;140:812–8. [PubMed] [Google Scholar]
- 15.Iossifova YY, Reponen T, Bernstein DI, et al. House dust (1–3)-beta-D-glucan and wheezing in infants. Allergy. 2007;62:504–13. doi: 10.1111/j.1398-9995.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campo P, Kalra HK, Levin L, et al. Influence of dog ownership and high endotoxin on wheezing and atopy during infancy. J Allergy Clin Immunol. 2006;118:1271–8. doi: 10.1016/j.jaci.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan PH, Lemasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115:278–84. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, McDonald R, Martuzevicius D, Biswas P, Grinshpun SA, Kelly A, et al. UNMIX modeling of ambient PM2.5 near an interstate highway in Cincinnati, OH, USA. Atmos Environ. 2006;40(S2):378–95. doi: 10.1016/j.atmosenv.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 20.de Bilderling G, Mathot M, Agustsson S, Tuerlinckx D, Jamart J, Bodart E. Early skin sensitization to aeroallergens. Clin Exp Allergy. 2008;38:643–8. doi: 10.1111/j.1365-2222.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 21.Ryan PH, Lemasters GK, Lockey JE, Khurana Hershey GK, Villareal M, Bernstein DI. Persistent Aeroallergen Sensitization at Ages One and Two in the Cincinnati Childhood Allergy and Air Pollution Study [Abstract] J Allergy Clin Immunol. 2006;117:S150. [Google Scholar]
- 22.Rhodes HL, Sporik R, Thomas P, Holgate ST, Cogswell JJ. Early life risk factors for adult asthma: a birth cohort study of subjects at risk. J Allergy Clin Immunol. 2001;108:720–5. doi: 10.1067/mai.2001.119151. [DOI] [PubMed] [Google Scholar]
- 23.Boucher KM, Slattery ML, Berry TD, Quesenberry C, Anderson K. Statistical methods in epidemiology: a comparison of statistical methods to analyze dose-response and trend analysis in epidemiologic studies. J Clin Epidemiol. 1998;51:1223–33. doi: 10.1016/s0895-4356(98)00129-2. [DOI] [PubMed] [Google Scholar]
- 24.Bisgaard H, Halkjaer LB, Hinge R, et al. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009;123:1355–60 e5. doi: 10.1016/j.jaci.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Immonen A, Farci S, Taivainen A, et al. T cell epitope-containing peptides of the major dog allergen Can f 1 as candidates for allergen immunotherapy. J Immunol. 2005;175:3614–20. doi: 10.4049/jimmunol.175.6.3614. [DOI] [PubMed] [Google Scholar]
- 26.Bufford JD, Reardon CL, Li Z, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 27.Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics. 2008;121:e44–52. doi: 10.1542/peds.2006-3553. [DOI] [PubMed] [Google Scholar]
- 28.Thygarajan A, Burks AW. American Academy of Pediatrics recommendations on the effects of early nutritional interventions on the development of atopic disease. Curr Opin Pediatr. 2008;20:698–702. doi: 10.1097/MOP.0b013e3283154f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgenstern V, Zutavern A, Cyrys J, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–7. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]


