Abstract
Objectives/Hypothesis
A precise molecular schema for classifying the different cell types of the normal human vocal fold epithelium is lacking. We hypothesize that the true vocal fold epithelium has a cellular architecture and organization similar to that of other stratified squamous epithelia including the skin, cornea, oral mucosa, and esophagus. In analogy to disorders of the skin and gastrointestinal tract, a molecular definition of the normal cell types within the human vocal fold epithelium and a description of their geometric relationships should serve as a foundation for characterizing cellular changes associated with metaplasia, dysplasia, and cancer.
Study Design
Qualitative study with adult human larynges.
Methods
Histologic sections of normal human laryngeal tissue were analyzed for morphology (hematoxylin and eosin) and immunohistochemical protein expression profile, including cytokeratins (CK13 and CK14), cornified envelope proteins (involucrin), basal cells (NGFR/p75), and proliferation markers (Ki67).
Results
We demonstrated that three distinct cell strata with unique marker profiles are present within the stratified squamous epithelium of the true vocal fold. We used these definitions to establish that cell proliferation is restricted to certain cell types and layers within the epithelium. These distinct cell types are reproducible across five normal adult larynges.
Conclusion
We have established that three layers of cells are present within the normal adult stratified squamous epithelium of the true vocal fold. Furthermore, replicating cell populations are largely restricted to the parabasal strata within the epithelium. This delineation of distinct cell populations will facilitate future studies of vocal fold regeneration and cancer.
Level of Evidence
N/A.
Keywords: Vocal fold, true vocal cord, epithelium, stratified squamous, biomarkers, cytokeratin, larynx, involucrin, proliferation, basal cell, differentiation, histology
INTRODUCTION
Epithelia have a characteristic cellular architecture composed of distinct protein expression profiles within different strata of cells.1 For example, the major cell types of the pseudostratified epithelium of the central airways have been well characterized.2 Unlike the normal central airway epithelium, the human true vocal fold epithelium contains regions of stratified squamous epithelium. The expression of various cellular markers has been observed in different cells of this epithelium; however, to date these cells have not been correlated to one another, and the topological arrangement of these cells has not been comprehensively scrutinized.3,4 Such a foundation has proven essential in classifying disorders of the epidermis.
We hypothesize that the true vocal fold epithelium has a cellular architecture and organization similar to the stratified squamous epithelia found in the skin,5 cornea,6 oral mucosa, and esophagus,7 where molecular markers and cellular function of distinct layers of the epithelium have been defined. For example, the cells that directly attach to the basement membrane, basal cells, have been shown to function as stem cells in multiple squamous epithelia.5,8,9 These basal stem cells are thought to produce daughter cells, which move toward the lumen and then differentiate, forming distinct epithelial layers. This classic paradigm for the organization of a stratified epithelium is evident in the early histologic descriptions of the layered cells of the epidermis.10
This report defines distinct layers within the true vocal fold epithelium. Recent attempts to bioengineer laryngeal tissue11,12 and increasing interest in characterizing the early stages of laryngeal cancer13 require a precise definition of distinct layers in the normal state in order to assess whether bioengineered tissues mimic the endogenous organ or similarly to assess how premalignant cells are ordered differently than normal ones. This study defines a molecular nomenclature of the cells of the true vocal fold and thus lays a foundation for the characterization of the physiological and pathological changes that occur within the epithelium during regeneration and disease.
MATERIALS AND METHODS
Human Samples
The Partners Human Research Committee and Massachusetts Eye and Ear Infirmary Human Subjects Committee approved the collection and use of cadaver specimens. Larynges were obtained from autopsy specimens. The specimens were fixed in formalin, embedded in paraffin, and sectioned at 5 μm. Cross sections were taken from the midportion of the membranous vocal folds of three adult male specimens and two adult female specimens.
A serial section was stained with hematoxylin and eosin (H&E) and analyzed by a pathologist to confirm the absence of pathology.
Immunofluorescence
Immunofluorescence was performed on paraffin sections after deparaffinization and rehydration. Phosphate-buffered saline Tween (PBST) was applied prior to antigen retrieval in citrate buffer (pH 6.0). Nonspecific binding was blocked with goat serum (10%) for 10 minutes. Primary antibodies were incubated overnight at 4 °C. Slides were washed with PBST, and secondary fluorescent antibodies were applied. DAPI Fluoromount-G mounting media (SouthernBiotech, Birmingham, AB) was used.
Images were visualized using an Olympus IX81 inverted fluorescence microscope or an Olympus FV10i Confocal Laser Microscope (Olympus, Tokyo, Japan). The primary antibodies used are summarized in Table I. The secondary antibodies were purchased from Invitrogen (conjugated to Alexa Fluor 488, Alexa Fluor 549, Alexa Fluor 647). Images were collected at 4×, 40×, and 60× and merged with cellSens digital imaging software (Olympus) and Fiji .14
TABLE I.
Antibodies for Paraffin Sections.
| Antigen | Host | Source | Catalogue No. | Dilution |
|---|---|---|---|---|
| CK13 | mouse | Thermo | 2D7 | 1:10 |
| CK8 | rat | DSHB | Troma-1 | 1:10 |
| NGFR/p75 | rabbit | Abcam | ab52987 | 1:25 |
| Involucrin | mouse | Sigma | SY5 | 1:100 |
| CK 14 | mouse | Millipore | LL022 | 1:50 |
| NGFR/p75 | rabbit | Abcam | ab52987 | 1:10 |
| Ki67 | rat | eBioscience | 14–5698 | 1:100 |
CK = cytokeratin; Ki67 = proliferation marker; NGFR = nerve growth factor receptor.
The trachea was used as the positive control for respiratory markers and the negative control for squamous markers. The esophagus was the positive control for squamous markers and negative control for respiratory markers. Stains performed without primary antibodies were also used as negative controls.
RESULTS
Histology and Morphology
All H&E sections of true vocal fold tissue were analyzed by a head and neck pathologist (p.m.s.) to ensure that the samples were free of dysplasia or keratosis.
Molecularly Defining the Stratified Squamous Region of the Vocal Fold
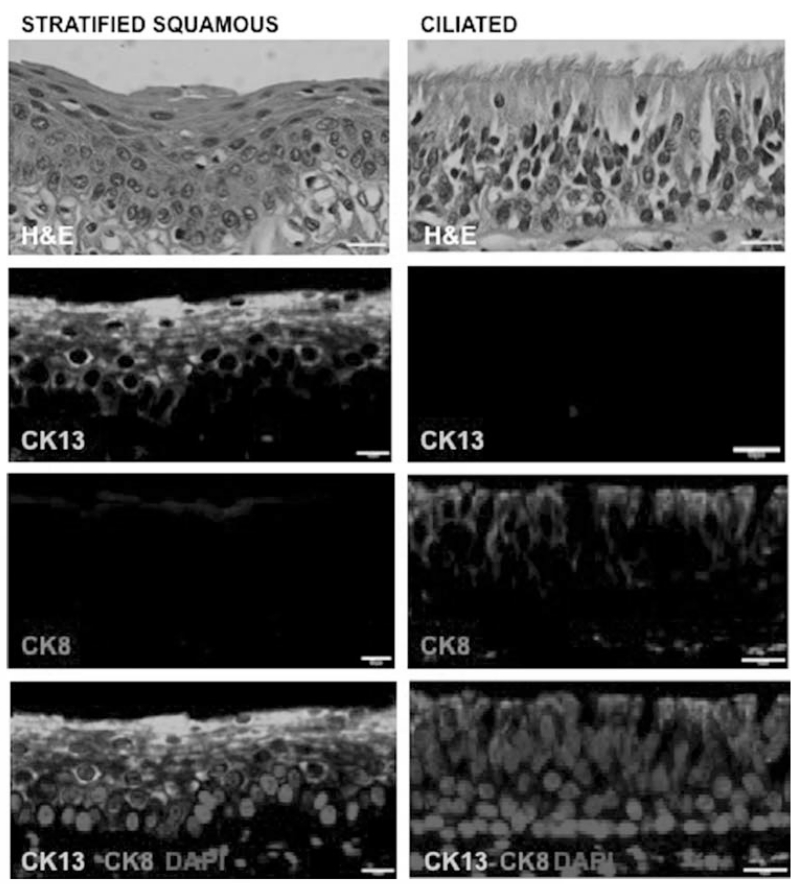
In our samples, the stratified squamous epithelium of the human true vocal fold expresses cytokeratin 13 (CK13) in the absence of cytokeratin 8 (CK8) expression. In contrast, CK8 is expressed in the ciliated epithelium of the larynx in the laryngeal ventricle, supraglottis, and subglottis. Indeed, CK8 and CK13 staining are mutually exclusive and clearly delineate the region of stratified squamous epithelium in the human vocal fold (Fig. 1, left panels) and ciliated epithelium (Fig. 1, right panels). Of note, CK13 expression extends throughout the entire stratified epithelium, excepting the majority of basal cells that directly attach the basement membrane (Fig. 1, bottom left panel). Cytokeratin 13 is also expressed in intermediate epithelial cells flanking the supraglottic and infraglottic transition regions of the true vocal fold and regions of the false vocal fold (data not shown).
Fig. 1.
Stratified squamous epithelium of the adult human vocal fold (left panels) can be defined by the expression of CK13 (green) and the absence of CK8 (red). The ciliated laryngeal epithelium (right panels) displays the converse expression pattern. The bottom panels show markers overlaid on nuclei stained by DAPI. CK13 expression extends throughout the entire stratified epithelium, sparing some rare basal cells that directly attach the basement membrane. Scale bars 10 μm. CK = cytokeratin; H&E = hematoxylin and eosin. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Defining Distinct Layers Within the CK13-Positive Stratified Squamous Epithelium
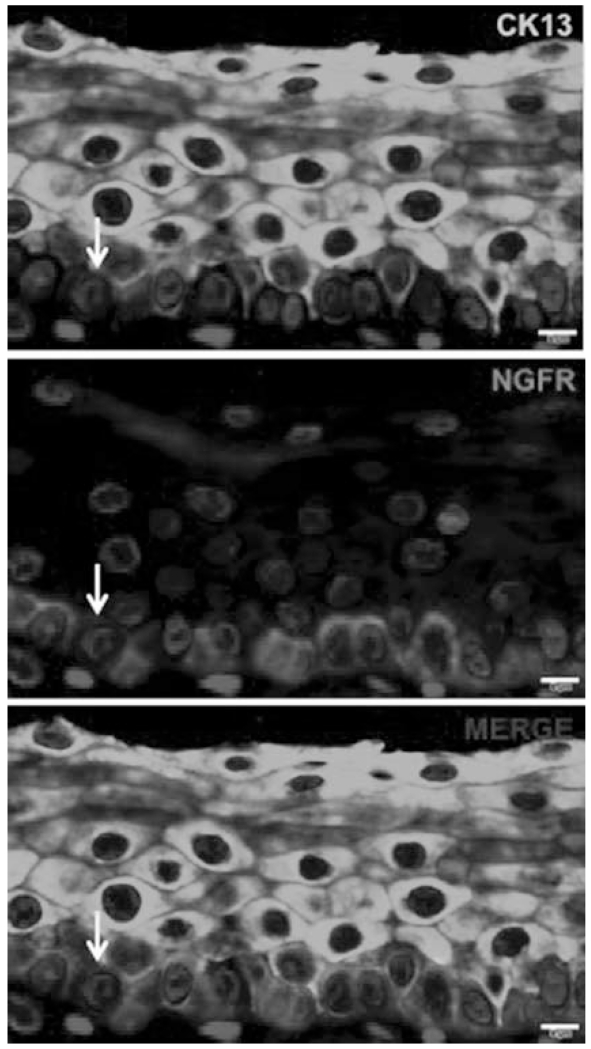
As noted above, the broad pattern of CK13 expression within the stratified squamous vocal fold epithelium includes all of the cell layers extending downward from the luminal surface, with the exception of the majority of the single cell layer thick basal cell stratum (Fig. 1, bottom left panel and Fig. 2). Basal cells are defined by their close apposition to the basement membrane and the specific expression of nerve growth factor receptor (NGFR/p75). There are occasional cells that are double-positive for both CK13 and NGFR (Fig. 2, arrow), which may represent transitional cells exiting from the basal cell layer and beginning to differentiate into a more superficial parabasal cell type. However, the vast majority of CK13 expressing cells do not express NGFR/p75 (Fig. 2).
Fig. 2.
Basal cells express NGFR/p75 in a single layer of cells sitting atop the basement membrane. In contrast, nonbasal cells express CK13. A rare cell expresses both NGFR and CK13 (arrow) and may represent a differentiating transitional cell. Scale bars 5 μm. CK = cytokeratin; NGFR = nerve growth factor receptor. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
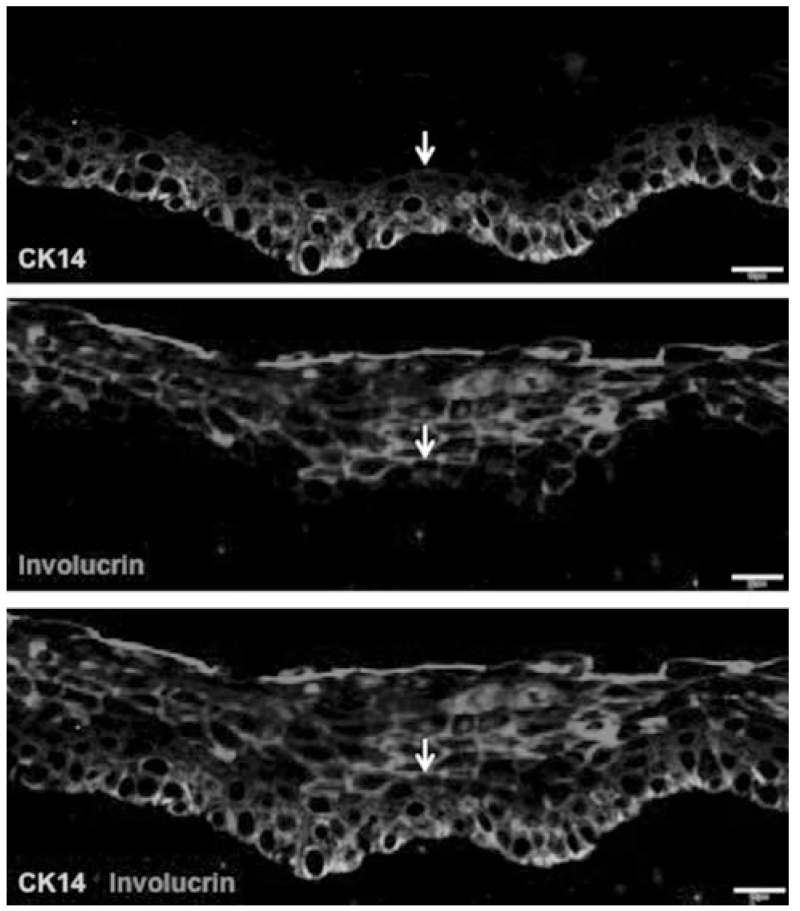
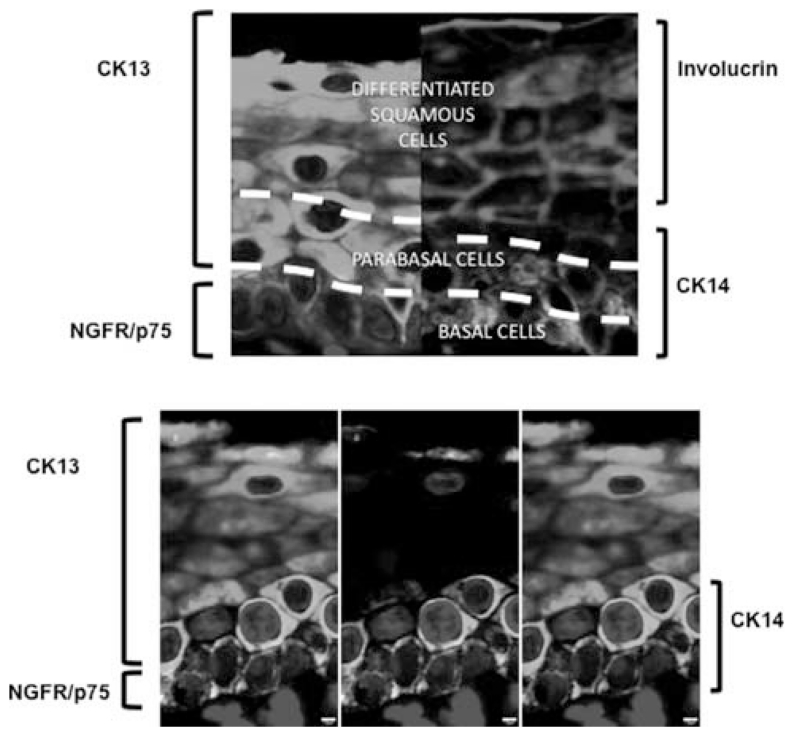
Further strata of cells can be identified by using the expression pattern of involucrin and cytokeratin 14 (CK14) to distinguish cell types. Cytokeratin 14 is expressed in the bottom three to four layers of epithelial cells, including the single cell layer thick basal cell stratum, whereas involucrin is expressed in the upper layers of the epithelium. Interestingly, most cells expressing CK14 do not express involucrin, but together they account for nearly every cell type in the epithelium (Fig. 3). Thus, these two markers broadly distinguish cells in an involucrin+ upper strata (4–6 cell layers thick) and a CK14+ lower strata (3–4 cell layers thick). However, occasional cells double positive for CK14 and involucrin are observed (arrow) and again are hypothesized to represent transitional cells (Fig. 3). Of the multiple layers of CK14+ cells, the lowest layer represents the NGFR+ single-cell thick basal cell stratum, whereas the CK14+NGFR− cells above the basal cell layer are arranged in a two to three cell layers thick region, which we refer to as a parabasal strata. In aggregate, three distinct layers of cells are described by correlating the expression NGFR, CK14, and involucrin (Fig. 4). We refer to these layers as a basal layer, a parabasal layer, and a differentiated squamous cell layer.
Fig. 3.
CK14 labels the bottom three to four cell layer thick strata of epithelial cells, whereas involucrin labels the upper five to six layers of cells. Most CK14 expressing cells do not express involucrin. Very occasional cells coexpress CK14 and involucrin (arrow). Scale bars 10 μm. CK = cytokeratin. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Fig. 4.
Three distinct layers of cells can be distinguished within the stratified squamous epithelium of the true vocal fold, as indicated by the expression of CK13 (green) and NGFR (red) on the top left panel and involucrin (magenta) and CK14 (cyan) on the top right panel. In the lower panels, CK13, CK14, and NGFR-staining demonstrate a population of NGFR−, CK13+, and CK14+ parabasal cells. The lower panels include CK13, CK14, and NFGR (left); NGFR and CK13 (center); and CK13 and CK14 (right). Scale bars 2 μm. CK = cytokeratin; NGFR = nerve growth factor receptor. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
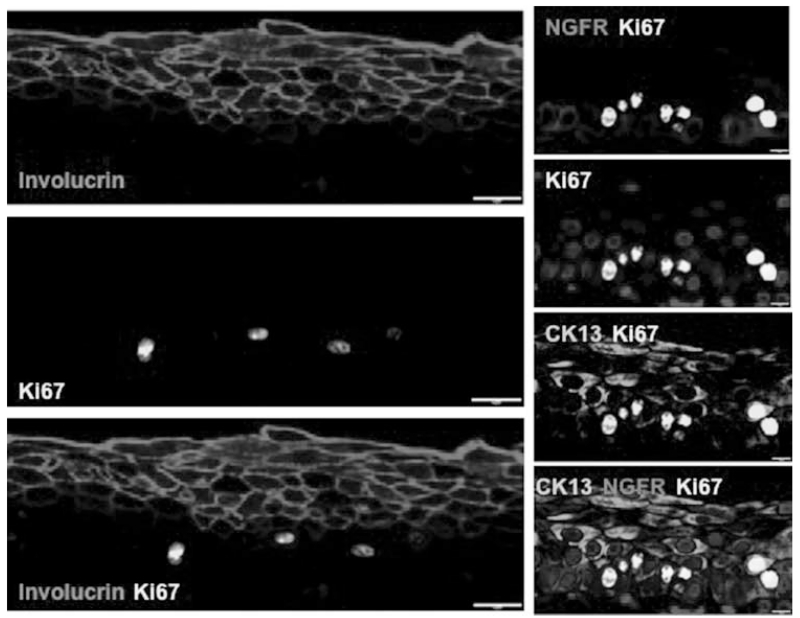
We then attempted to categorize the proliferative behavior of the three strata of cells that we identified by staining for the proliferation marker Ki67. The Ki67+ cells were rare in the involucrin-positive uppermost layer of cells, consistent with the notion that these cells are postmitotic differentiated cells. Surprisingly, Ki67 is predominantly expressed in the NGFR−CK13+ parabasal cell layers (Fig. 5). Thus, the parabasal CK13+NGFR− cell population is the most replicative cell layer, whereas the NGFR+ putative stem cell population is largely quiescent, and the differentiated involucrin+ cell population appears postmitotic.
Fig. 5.
Proliferation is largely confined to suprabasal layers of the vocal fold epithelium. Left panels: The majority of Ki67 expression (yellow) is seen in involucrin− cells. Coexpression of Ki67 (yellow) and Involucrin (magenta) is rare. Right panels: Within the proliferative compartment, Ki67 positive cells (yellow) are predominantly found in cytokeratin 13+NGFR− parabasal cells (arrows). NGFR (red) is expressed in basal cells. Scale bars left panels 10 μm; scale bars right panels 5 μm. CK = cytokeratin; Ki67 = proliferation marker; NGFR = nerve growth factor receptor. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
DISCUSSION
In this study, we aimed to present a molecular signature of newly identified distinct cell layers within the adult human stratified squamous epithelium of the true vocal fold. We first defined the region of stratified true vocal fold epithelium as an epithelium expressing CK13 in order to distinguish it from CK8-expressing ciliated epithelium. This definition eliminates the possibility that we assess laryngeal intermediate epithelium, which has features of both squamous and ciliated epithelium and which frequently expresses both CK8 and CK13.15,16 Intermediate epithelia, at various stages between columnar and squamous epithelium, have been described in the nasopharynx, the epiglottis, and in other parts of the larynx.17,18 Interestingly, in addition to its expression in normal stratified squamous epithelia, CK13 is also expressed in tissue undergoing squamous metaplasia, whereas reduced CK13 expression is associated with dysplasia and squamous cell cancer.19
Because several studies have examined the expression patterns of CKs by immunofluorescence and by polymerase chain reaction in normal and precancerous laryngeal tissues,3,4,20,21 and have correlated changes in CK expression with malignant progression, we characterized a battery of CK antibodies to define strata in normal laryngeal tissue. Indeed, we find that CK13 and CK14 together reliably and reproducibly mark every one of the cells of the epithelium, but they overlap considerably. Within the CK13+ cell layers, however, the involucrin+ subset of layers occurs at the luminal surface of the epithelium and likely represents the most mature differentiated squamous cells.22,23 Indeed, involucrin is known to be expressed at the surface of stratified squamous epithelia of the epidermis, hard palate, and buccal mucosa.24 Of note, an association between involucrin expression and well-differentiated tumors has been demonstrated in several studies.25,26
Basal cells, on the other hand, are morphologically defined as those cells closest to the basement membrane and are often thought to represent the germinative stem cells of their respective tissues. Basal cells are generally known to express NGFR, CK14, and p63, in addition to other markers.13 After testing a battery of known basal cell markers within the true vocal fold stratified epithelium, we found that NGFR is the only reliable antibody that marks basal cells exclusively. Surprisingly, markers associated with the basal cells of other tissues (including p63) are normally detected in parabasal cells.
Several studies of various epithelia have identified stem cells within the basal cell population, suggesting that the basal cell layer contains the least mature germinative cells of the epithelium.2,8,13 However, no definitive proof of a stem cell can be proffered without lineage tracing, and this has yet to be performed within the vocal fold epithelium. In many respiratory and epidermal epithelia, basal stem cells are the replicating workhorses of the epithelium. In contrast, we find that the vast majority of Ki67+ cells lie within the NGFR-CK13+CK14+ parabasal layer of cells. Because basal cells are often thought to represent stem cells, we speculate that the stem cells of the vocal fold epithelium are quiescent in analogy to the stem cells of the hematopoietic system.27 Instead, we suggest that the vocal fold epithelium maintains itself largely through a transit-amplifying set of parabasal progenitor cells. Perhaps the stem cell compartment is necessary to regenerate the epithelium after injury or infection, but normally it may represent a reserve population of protected cells. Because we find only very rare Ki67+ cells within the uppermost involucrin+ layers, this lends credence to the notion that involucrin defines the most mature differentiated cells within the epithelium of the true vocal fold, which presumably subsume an epithelial barrier function and provide physical integrity to the epithelial surface.
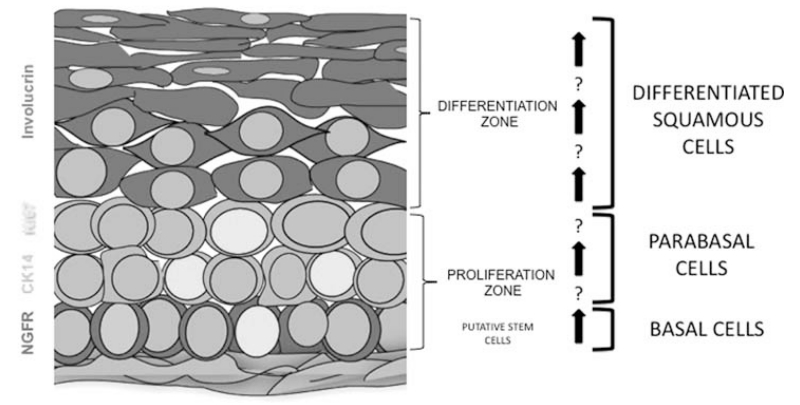
In all, we have attempted to provide a molecular roadmap for distinct populations of cells that exist within strata within the adult stratified squamous epithelium of the human true vocal fold. The distribution of markers suggests a functional segregation of different cell types, as well as a gradient of differentiation from basal stem cells to the most mature differentiated luminal squamous cells (Fig. 6). Additional studies on pure distinct cell populations will be required to test their cellular and molecular function. Interestingly, the most replicative cells are often considered as cellular sources of malignancy. In this case, those cells are not putative basal stem cells, but are most likely transit-amplifying parabasal progenitors. We note, for example, that in the case of leukemia, although mutations can arise in stem cells, the leukemogenic action of many oncogenes and tumor suppressors occurs at the level of transit-amplifying progenitors. Indeed, in a murine model, Leydon et. al demonstrated BrdU label-retaining cells that turn over slowly are largely quiescent stem/cell progenitors,28 whereas parabasal cells are rapidly replicating, which suggests that they act as transit-amplifying progenitor cells. However, determining which cell is the true cell of origin of laryngeal cancer requires some form of lineage tracing, or a computational reconstruction of the clonal evolution of cells within a cancer based on single-cell genomic or transcriptional data.29 That said, our newly presented molecular architecture offers a preliminary method for characterizing the cells types and their geometric aberrations in disorders of the laryngeal epithelium ranging from metaplasia to premalignancy to frank laryngeal cancer.
Fig. 6.
Distinct layers within the stratified squamous epithelium of the true vocal fold. The cartoon depicts layers within the stratified squamous epithelium. Basal cells are characterized by NGFR/p75 expression (red). Ki67 expression (yellow) is noted to occur largely in the involucrin−CK14+ parabasal cell layers. Differentiated luminally biased involucrin-positive cells (magenta) are the most differentiated cells of the epithelium and are postmitotic functional barrier cells. CK = cytokeratin; Ki67 = proliferation marker; NGFR = nerve growth factor receptor. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
CONCLUSION
We established that three specific layers of cells exist within the normal adult stratified squamous true vocal fold epithelium and are defined by the expression patterns of NGFR, CK14, CK13, and involucrin. Infrequent double-positive cells were observed at the boundaries of strata and may represent transitional cell types. Furthermore, despite noting proliferating basal cells, we placed most of the proliferating cells within a transit-amplifying parabasal cell layer and demonstrated that Ki67 is excluded from the involucrin-positive differentiated luminal cell layers.
Acknowledgments
This work was completed at the Center for Regenerative Medicine, Massachusetts General Hospital.
We thank Chris Nims and Louise Collins from the Howe Library at the Massachusetts Eye and Ear Infirmary. We also wish to extend our thanks to Fariba Houman and Denise McCauley for institutional review board support. Finally, we thank all of the members of the Rajagopal Laboratory, Massachusetts General Hospital, for their reading of the article, valuable discussion, and support.
The Massachusetts Life Sciences Center and the Massachusetts Eye and Ear Infirmary supported this work. This study was also supported by the following: grant from the Massachusetts Life Sciences (r.a.f); Massachusetts Eye and Ear Infirmary, NIH-NIDCD Loan Repayment Grant (j.r.d); grant from NHLBI Progenitor Cell Biology Consortium (5U01HL099997-04), R01 (1R01HL116756-01a) from NIH/NHLBI; NIH-NHLBI Early Career Research New Faculty (P30) award (5P30HL101287-02); Harvard Stem Cell Institute Junior Investigator Grant, and New York Stem Cell Foundation-Robertson Investigator Grant (j.r.).
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Dai X, Segre JA. Transcriptional control of epidermal specification and differentiation. Curr Opin Genet Dev. 2004;14:485–491. doi: 10.1016/j.gde.2004.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Velden LA, et al. Cytokeratin and vimentin expression in normal epithelium and benign lesions of the vocal cords. Acta Otolaryngol (Stockh) 1996;116:325–331. doi: 10.3109/00016489609137851. [DOI] [PubMed] [Google Scholar]
- 4.Van der Velden LA, et al. Cytokeratin and vimentin expression in normal epithelium and squamous cell carcinomas of the larynx. Eur Arch Otorhinolaryngol. 1997;254:376–383. doi: 10.1007/BF01642554. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzeszczynska J, et al. Differentiation and molecular profiling of human embryonic stem cell-derived corneal epithelial cells. Int J Mol Med. 2014;33:1597–1606. doi: 10.3892/ijmm.2014.1714. doi:10.3892/ijmm.2014.1714. [DOI] [PubMed] [Google Scholar]
- 7.Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 8.Rock JR, Randell SH, Hogan BLM. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanpain C, Horsley V, Fuchs E. Epithelial stem Cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 11.Long JL, Zuk P, Berke GS, Chhetri DK. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope. 2010;120:125–131. doi: 10.1002/lary.20719. [DOI] [PubMed] [Google Scholar]
- 12.Leydon C, Selekman J, Palecek SP, Thibeault S. Human embryonic stem cell-derived epithelial cells in a novel in vitro model of vocal mucosa. Tissue Eng Part A. 2013;19:2233–2241. doi: 10.1089/ten.tea.2012.0744. doi: 10.1089/ten.TEA.2012.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, et al. Biological and clinical significance of p75 NTR expression in laryngeal squamous epithelia and laryngocarcinoma. Acta Otolaryngol (Stockh) 2012;132:314–324. doi: 10.3109/00016489.2011.639086. [DOI] [PubMed] [Google Scholar]
- 14.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flint PW, Cummings CW. Otolaryngology: Head & Neck Surgery. Mosby Elsivier; Philadelphia, PA: 2010. [Google Scholar]
- 16.Janfaza P, Jr, Nadol JB, Galla RJ, Fabian RL, Montgomery WW. Surgical Anatomy of the Head and Neck. Harvard University Press; Cambridge, MA: 2011. [Google Scholar]
- 17.Nakano T. Intermediate epithelium. Kaibogaku Zasshi. 1998;73:87–92. [PubMed] [Google Scholar]
- 18.Nakano T, Muto H. The transitional zone in the epithelium lining the mouse epiglottis. Acta Anat (Basel) 1987;130:285–290. doi: 10.1159/000146459. [DOI] [PubMed] [Google Scholar]
- 19.Malecha MJ, Miettinen M. Expression of keratin 13 in human epithelial neoplasms. Virchows Arch A Pathol Anat Histopathol. 1991;418:249–254. doi: 10.1007/BF01606063. [DOI] [PubMed] [Google Scholar]
- 20.Van der Velden LA, Schaafsma HE, Manni JJ, Ramaekers FC, Kuijpers W. Cytokeratin expression in normal and (pre)malignant head and neck epithelia: an overview. Head Neck. 1993;15:33–146. doi: 10.1002/hed.2880150209. [DOI] [PubMed] [Google Scholar]
- 21.Cohen-Kerem R, et al. Detection of cytokeratins in normal and malignant laryngeal epithelia by means of reverse transcriptase-polymerase chain reaction. Ann Otol Rhinol Laryngol. 2002;111:149–154. doi: 10.1177/000348940211100207. [DOI] [PubMed] [Google Scholar]
- 22.Katou F, et al. Differential expression of cornified cell envelope precursors in normal skin, intraorally transplanted skin and normal oral mucosa. Br J Dermatol. 2003;148:898–905. doi: 10.1046/j.1365-2133.2003.05288.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan MJ, Mills SE, Rice RH, Johns ME. Involucrin in laryngeal dysplasia. A marker for differentiation. Arch Otolaryngol Chic Ill 1960. 1984;110:713–716. doi: 10.1001/archotol.1984.00800370015003. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs S, Ponec M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch Oral Biol. 2000;45:149–158. doi: 10.1016/s0003-9969(99)00116-8. [DOI] [PubMed] [Google Scholar]
- 25.Nozoe T, et al. Significance of immunohistochemical expression of p27 and involucrin as the marker of cellular differentiation of squamous cell carcinoma of the esophagus. Oncology. 2006;71:402–410. doi: 10.1159/000108611. [DOI] [PubMed] [Google Scholar]
- 26.Roland NJ, Rowley H, Scraggs M, Johnson P, Jones AS. MIB-1 and involucrin expression in laryngeal squamous carcinoma: the relationship to host and tumour factors and survival. Clin Otolaryngol Allied Sci. 1996;21:429–438. doi: 10.1046/j.1365-2273.1996.00821.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson A, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann NY Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 28.Leydon C, Bartlett RS, Roenneburg DA, Thibeault SL. Localization of label-retaining cells in Murine vocal fold epithelium. J Speech Lang Hear Res. 2011;54:1060–1066. doi: 10.1044/1092-4388(2010/10-0267). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treutlein B, et al. Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature. 2014;509:371–375. doi: 10.1038/nature13173. [DOI] [PMC free article] [PubMed] [Google Scholar]