Abstract
Objective:
To evaluate studies of pharmacist-led interventions on potentially inappropriate prescribing among community-dwelling older adults receiving primary care to identify the components of a successful intervention.
Data sources:
An electronic search of the literature was conducted using the following databases from inception to December 2015: PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature, MEDLINE (through Ovid), Trip, Centre for Reviews and Dissemination databases, Cochrane Database of Systematic Reviews, ISI Web of Science, ScienceDirect, ClinicalTrials.gov, metaRegister of Controlled Trials, ProQuest Dissertations & Theses Database (Theses in Great Britain, Ireland and North America).
Review methods:
Studies were included if they were randomised controlled trials or quasi-randomised studies involving a pharmacist-led intervention compared to usual/routine care which aimed to reduce potentially inappropriate prescribing in older adults in primary care. Methodological quality of the included studies was independently assessed.
Results:
A comprehensive literature search was conducted which identified 2193 studies following removal of duplicates. Five studies met the inclusion criteria. Four studies involved a pharmacist conducting a medication review and providing feedback to patients or their family physician. One randomised controlled trial evaluated the effect of a computerised tool that alerted pharmacists when elderly patients were newly prescribed potentially inappropriate medications. Four studies were associated with an improvement in prescribing appropriateness.
Conclusion:
Overall, this review demonstrates that pharmacist-led interventions may improve prescribing appropriateness in community-dwelling older adults. However, the quality of evidence is low. The role of a pharmacist working as part of a multidisciplinary primary care team requires further investigation to optimise prescribing in this group of patients.
Keywords: Pharmacist interventions, prescribing, older people, primary care, systematic review
Introduction
Medication-related problems are common in older adults and are associated with increased morbidity, adverse drug events (ADEs), extended hospital stays and increased mortality.1–3 Potentially inappropriate prescribing (PIP) can introduce the risk of an ADE which has the potential to outweigh the drug’s clinical benefit, particularly when a safer or more effective alternative treatment option is available.4 The term ‘potentially’ is used, as the physician may have considered the potential negative consequences of prescribing the drug as well as alternative treatment options for that patient but chose to proceed with a given approach.5 Recent evidence indicates that the prevalence of PIP in older adults in primary care is high, with nationally representative estimates in Ireland, Northern Ireland and the United Kingdom at 36%, 34% and 29%, respectively, using an explicit measure of inappropriate prescribing.6–8 Curtis et al. conducted a retrospective cohort study using a national sample of prescription drug claims for patients aged over 65 years enrolled with a pharmaceutical benefit manager in the United States. The study highlighted that more than one in five patients filled a prescription for one or more drugs of concern based on the Beers’ revised list of drugs to be avoided in elderly populations.9
A number of screening tools have been developed to assess the appropriateness of prescribing, which use an explicit (criterion-based) or implicit (judgement-based) approach.3 Explicit tools are usually developed from published literature, multidisciplinary expert panels and consensus validation methods. The potential drawbacks of using explicit criteria include a lack of transparency of the literature used, reliability of the consensus techniques and conflicts of interest of the expert panels.10 Explicit criteria include the Beers and STOPP/START (Screening Tool of Older Persons Prescriptions/Screening Tool to Alert doctors to Right Treatment) criteria.4,11 Beers criteria contain several medicines that are either not prescribed or not available in most European drug formularies, thus its application in an European Union (EU) setting is limited.12 The STOPP criteria comprise a physiological system–based screening tool designed for use in Europe. It aims to identify potentially inappropriate medicines (PIMs) by listing explicit rules for avoidance of particular medicines in older people. In addition, potential prescribing omissions (PPOs) have been identified by an accompanying screening tool known as START.13
In implicit approaches, healthcare professionals use information from the patient and published reviews to assess the appropriateness of medicines.3,14 The Medication Appropriateness Index (MAI) is an example of a validated implicit tool.15 It consists of 10 criteria that relate to a number of different prescribing domains, for example, indication, effectiveness, dose, duration, correct directions, practical directions, drug–drug interactions, drug–disease interactions, duplication and cost. The tool generates a weighted score (ranging from 0 to 18) per drug that serves as a measure of medication appropriateness. A higher score indicates an increased level of inappropriateness.15 The application of implicit tools is time-consuming, depends on the users knowledge and in addition, the MAI does not address under-prescribing.3
Across transitions of care, evidence indicates that pharmacists play a significant role in gate-keeping medication appropriateness, with respect to quality and safety of prescribing.16 Research suggests that pharmacists can reduce PIP and adverse health outcomes in patients across a range of healthcare settings by utilising explicit and implicit screening tools systematically.17–20
To date, evidence has been collated on various pharmacist-led interventions to reduce PIP across healthcare settings. However, no review has summarised the totality of evidence regarding the impact of pharmacist-led interventions to reduce PIP in older adults specifically in primary care.
Therefore, the aim of this review is to evaluate studies of pharmacist-led interventions on medication prescribing among community-dwelling older adults receiving primary care to identify the components of a successful intervention.
Methods
Standardised reporting guidelines
This systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) standardised reporting guidelines to ensure the standardised conduct and reporting of the research.21 The PRISMA checklist is provided in Appendix 3.
Study identification
An electronic search of the literature was conducted using the following databases from inception to December 2015: PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), MEDLINE (through Ovid), Trip, Centre for Reviews and Dissemination databases, Cochrane Database of Systematic Reviews, ISI Web of Science, ScienceDirect, ClinicalTrials.gov, metaRegister of Controlled Trials (mRCT), ProQuest Dissertations & Theses Database (theses in Great Britain, Ireland and North America). A combination of the following keywords and MeSH terms were used: ‘primary care’ or ‘primary health care’ or ‘outpatient care’ AND ‘prescribing’ or ‘prescription’ and ‘aged’ or ‘middle aged’ or ‘elderly’ AND ‘pharmacist’ or ‘pharmaceutical care’. There were no date or language restrictions on the searches. A list of the search strategies for each database is provided in Appendix 1. The references of final search results were handsearched along with handsearching the references of some already published reviews and the authors’ own records.17,22
Study selection
Studies were included if they met the following inclusion criteria:
Study design. All randomised (cluster) controlled trials (RCTs), quasi-RCTs, controlled before and after studies and interrupted time series (ITS) designs were included.
Population. Community-dwelling older adults aged ≥65 years. Studies based on nursing home populations were excluded. A recent Cochrane Review published by Alldred et al (2016).19 focused on interventions to optimise prescribing for older patients in care homes. In all 8 out of the 12 studies retrieved were pharmacist-led.19 Therefore, it was decided that this question had already been answered for patients in this particular setting.
Intervention. Pharmacist-led interventions were defined as any intervention where the pharmacist had the lead role in an intervention designed to reduce PIP or improve medication appropriateness in primary care. The comparison group was usual care or other active interventions not focused on medication appropriateness.
Outcome. The primary outcome measure was the change in prescribing appropriateness using a validated explicit or implicit screening tool for the detection of PIP, that is, Beers criteria, STOPP/START, MAI. Secondary outcomes included any clinical or patient self-reported outcomes (e.g. quality of life, patient satisfaction).
Studies were excluded if they were currently ongoing, if there was a lack of reply from the author for supplementary information and if they only carried out an economic analysis. A list of the excluded studies reviewed with reasons for exclusion is provided in Appendix 2.
Study selection and data extraction
Two reviewers (D.O.R. and K.W.) independently read the titles and/or abstracts of the identified papers and eliminated irrelevant studies. Studies considered to be eligible for inclusion were read in full and their suitability for inclusion was determined independently by two reviewers (D.O.R. and K.W.). Disagreements were managed by consensus. However, if this was not successful, consensus was sought by a third reviewer (S.B.).
Data were extracted based on study design and setting, patient demographics and inclusion criteria, details of the intervention and comparison, length of follow-up and outcome measures used. Authors were contacted to provide supplementary information when insufficient data were provided in the study. The authors of five studies were contacted for further information having read their titles and abstracts. Three replied; however, none of these studies fulfilled the inclusion criteria. Despite emailing the authors of the other two studies on two different occasions, we received no reply. Therefore, these studies were not included.
Assessment of risk of bias
Two reviewers (D.O.R. and R.G.) independently assessed the risk of bias for ITS using the Effective Practice and Organisation of Care (EPOC) risk of bias criteria and for RCTs using the Cochrane Collaboration’s tool for assessing risk of bias.23,24 In any case of disagreement, consensus was reached with a third reviewer (K.W.).
Results
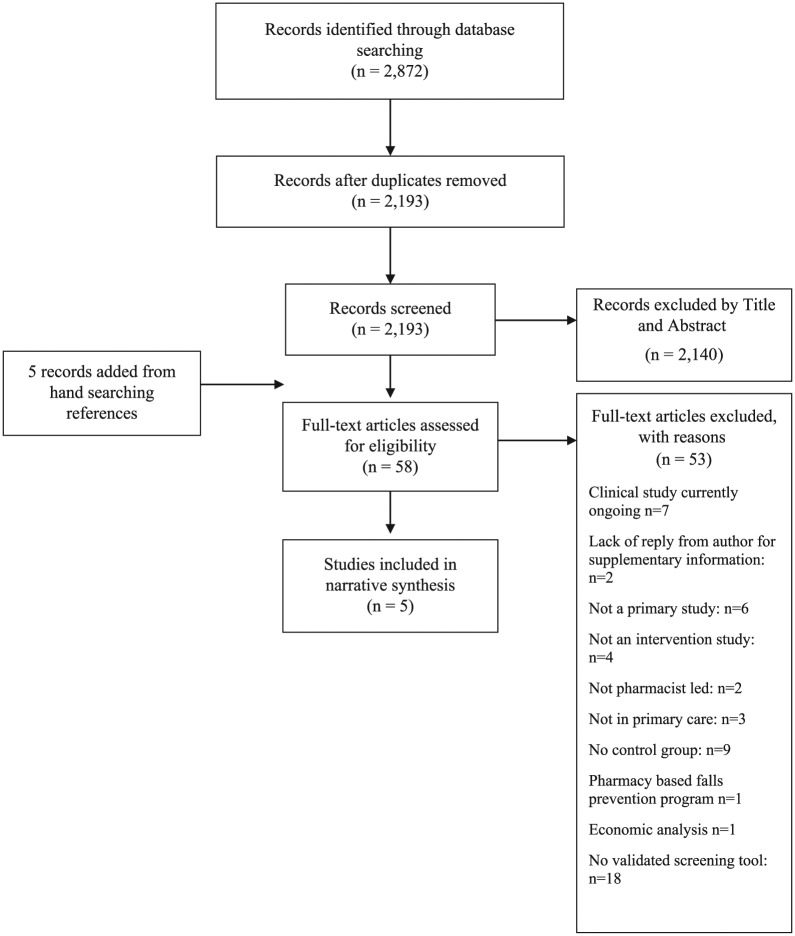
A total of 2193 studies were identified following removal of duplicates, and five additional studies were located from handsearching references. A PRISMA flowchart (Figure 1) describes the flow of studies in the review. A total of 58 full-text studies were assessed for eligibility. At the end of the process, five studies were eligible for inclusion in the systematic review. Of the five included studies, three were conducted in the United States, one in Europe and one in New Zealand.25–29
Figure 1.
A PRISMA flowchart outlining the procurement of five included studies.
The characteristics of the five included studies are summarised in Table 1.
Table 1.
Study design, characteristics and outcomes of the included studies.
| Author | Country | Setting | Study design | Aim of the study | No. of patients | Mean age (years) ± SD | Female (%) | Mean no. of Rx meds per patient at baseline ± SD | Mean summated MAI score per patient at baseline ± SD | Mean summated MAI score per patient post-intervention ± SD | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bryant et al. 25 | New Zealand | General practitioner (GP) practices in a primary health care. | Randomised controlled trial (RCT) | The objective was to determine whether involvement of community pharmacists undertaking clinical medication reviews, working with general practitioners, improved medicine-related therapeutic outcomes for patients. | I: 269 C: 229 |
I: 75.9 (range 64–92) C: 74.9 (range 60–91) |
I: 64.7% C: 52.4% |
N/A | I: 5.1 C: 4.5 |
I: 3.1 C: 4.2 |
Change in the number of medicines used: more meds were started in the control group than in the intervention group (p < 0.0001). More dosage reductions and medicine switches in the intervention group than in the control group (p = 0.037). Recommendations implemented: 46% of recommendations were implemented, 16% partially implemented. Quality of life (SF-36): improvement in emotional role (13.4-unit difference, p = 0.024) and social functioning (7.7-unit difference, p = 0.019) for the control group. No other domains changed significantly. |
| Hanlon et al. 26 | United States | A general medicine clinic of a Veterans Affairs (VA) medical centre. | RCT | To evaluate the effect of sustained clinical pharmacist interventions involving elderly outpatients with polypharmacy and their primary physicians. | I: 105 C: 103 |
I: 69.7 ± 3.5 C: 69.9 ± 4.1 |
I: 1.9% C: 0.0% |
I: 7.6 ± 2.8 C: 8.2 ± 2.7 |
I: 17.7 ± 6.2 C: 17.6 ± 6.1 |
I: 12.8 ± 7.2 C: 16.7 ± 7.1 (At 12 months) |
Quality of life (SF-36): No significant difference between groups (p = 0.99). Adverse drug event (ADE) (%): no significant difference between groups (p = 0.19). Medication compliance (%): no significant difference between groups (p = 0.88). Medication knowledge (%): no significant difference between groups (p = 0.29). VA prescribed meds: no significant difference between groups (p = 0.83). General healthcare satisfaction: no significant difference between groups (p = 0.70). Pharmacy-related healthcare satisfaction: no significant difference between groups (p = 0.52). |
| Raebel et al. 29 | United States | Kaiser Permanente Colorado (KPCO) Medical offices and pharmacies. |
RCT | To determine whether a computerised tool that alerted pharmacists when patients aged ≥65 years were newly prescribed potentially inappropriate medicines, which was effective in decreasing the proportion of patients dispensed these medications. | I: 29,840 C: 29,840 |
Median age (5th, 95th percentiles) I: 74 (66, 88) C: 74 (66, 88) |
I: 57% C: 57% |
Median (5th, 95th percentiles) I: 7 (1, 17) C: 7 (2, 16) |
N/A | N/A | 1.8% of intervention versus 2.2% of control had newly dispensed PIP (p = 0.002). RRR = 16%, ARR = 0.3%. Dispensing rates differed between groups for amitriptyline (p < 0.001; 37% RRR) and diazepam (p = 0.02; 21% RRR). |
| Richmond et al.28 | England | All general practices in five primary care trusts (PCTs). | Interrupted time series (ITS) and repeated measures studies | To estimate the effectiveness of pharmaceutical care for older people, shared between GPs and community pharmacists in the United Kingdom, relative to usual care. Usual care: patients within each of the five PCTs on a waiting list before they received pharmaceutical care. |
A total of 551 were followed through pharmaceutical care. | 80.4 ± 4.1 | 43.2% | 8.1 ± 3.1 | 23.6 ± 19.5 | N/A | Quality of life (SF-36): Mental score: no. = 742, mean = 47.8, SD = 12.2. Physical score: no. = 742, mean = 33.0, SD =10.4. No. of items on repeat prescription: no. = 760, mean = 7.29, SD = 2.23. Serious adverse events: pharmaceutical care model was not associated with any of the reported serious adverse events. |
| Taylor et al. 27 | United States | Three community-based family medicine clinics. | RCT | The programme’s primary purpose was to determine the effect of pharmaceutical care on the prevention, detection and resolution of drug-related problems in high-risk patients in a rural community. | I: 33 C: 36 |
I: 64.4 ± 13.7 C: 66.7 ± 12.3 |
I: 63.6% C: 72.2% |
I: 6.3 ± 2.2 C: 5.7 ± 1.7 |
Percentage of inappropriate prescriptions according to MAI. | Percentage of inappropriate prescriptions according to MAI. | Quality of life (SF-36): no significant difference between groups. Hospitalisations and emergency department (ED) admissions: fewer hospitalisations (2 vs 11, p = 0.003) and ED visits (4 vs 6, p = 0.044) in the intervention group compared to the control group. Compared to the control group, the intervention group was more likely to have controlled blood pressure (p = 0.001), HbA1C (p = 0.001), LDL cholesterol (p = 0.001) and INRs (p = 0.048). Medication compliance: this score improved in the intervention group but not in the control group (p = 0.115). Medication knowledge: this score improved in the intervention group but decreased in the control group (p = 0.000). |
ARR: absolute risk reduction; C: control; I: intervention; INR: international normalised ratio; ITS: interrupted times series; LDL: low-density lipoprotein; MAI: Medication Appropriateness Index; Meds: medications; N/A: not applicable; No.: numbers; RRR: relative risk reductions; Rx: prescription; SD: standard deviation.
Characteristics of included studies
Four of the studies involved a pharmacist carrying out a medication review and providing feedback to patients or their family physician.25–28 One study evaluated the effect of a computerised tool that alerted pharmacists at the point of dispensing when older patients were newly prescribed PIMs.29
Bryant et al. involved pharmacists carrying out clinical medication reviews and providing feedback to the patient’s physicians, while the control group received usual care which was not defined. A total of 269 patients were enrolled in the intervention group. The MAI score improved more in the intervention group than in the control group (mean change in MAI score −2.0 in the intervention group; −0.3 in the control group, p < 0.001). There were more medicines started in the control group than the intervention group (p < 0.0001), while there were more dosage reductions and medicine switches in the intervention group than in the control group (p = 0.037).25
Hanlon et al. evaluated the effect of a pharmacist-led review of patient’s medical charts, followed by a clinical recommendation to the family physician in 105 cases. The researchers also provided compliance strategies to the patients. Patients in the control group had their medications reviewed by a clinical nurse; however, the clinical pharmacist had no interaction with the patients or their clinicians during the study period. The MAI score improved more in the intervention group than in the control group (mean change in MAI score −4.9 in the intervention group; −0.9 in the control group, p < 0.001). There was no significant difference between groups regarding ADEs, p = 0.19, or Veterans Affairs (VA) medicines prescribed, p = 0.83.26
Taylor et al. examined the effect of a pharmacist intervention that provided medication education to patients and therapeutic recommendations to their family physicians following a medication review in 33 older adults. A pharmacist evaluated the pharmacotherapy of each patient in the control group; however, no recommendations were reported to the patient or their physician. The percentage of inappropriate prescriptions decreased in all 10 MAI domains in the intervention group and increased in five domains in the control group. Clinical outcomes such as hypertension, diabetes mellitus, dyslipidaemia, anticoagulation, hospitalisations and emergency department (ED) admissions were reported. Compared to the control group, the intervention group was more likely to have controlled blood pressure (p = 0.001), HbA1c (p = 0.001), low-density lipoprotein (LDL) cholesterol (p = 0.001) and international normalised ratio (INR) (p = 0.048). There were fewer hospitalisations (2 vs 11, p = 0.003) and ED visits (4 vs 6, p = 0.044) in the intervention group compared to the control group.27
Richmond et al. developed pharmaceutical care plans among pharmacists and family physicians. The pharmaceutical care model involved pharmacists carrying out medication reviews and collaborating with physicians, patients and carers to identify issues with compliance and adverse drug reactions. It was hypothesised that the review process would also serve to encourage the prescribing of generic medicines and reduce health costs. Following this, pharmacists conducted monthly medication reviews with feedback to the physicians. The usual care group consisted of patients within each of the five primary care trusts (PCTs) on a waiting list to receive pharmaceutical care. A total of 551 participants completed the study. Results demonstrated that the pharmaceutical care model did not affect the appropriateness of prescribing (mean change in UK-MAI score from baseline to the end of the intervention was −0.26, p > 0.05). Also, the pharmaceutical care model was not associated with any of the reported serious adverse events.28
Finally, Raebel et al. estimated the effect of a computerised tool that alerted pharmacists at the point of dispensing when older patients were newly prescribed PIMs. Pharmacists and physicians collaborated to develop a targeted medication list for the intervention group based on the Beers, Zhan and Kaiser Permanente Care Management Institute lists of medications to be avoided in older people.30–32 The intervention group consisted of 29,840 patients. When a patient randomised to the intervention group was prescribed a new PIM, the pharmacist was notified via a medication alert generated from an electronic database. Pharmacists were required to complete a note on a standard intervention template before printing a label to dispense the prescription. Pharmacists were then instructed to telephone the prescribing physician to suggest alternatives. Patients in the control group received medication prescribing and dispensing according to usual clinical practice. When medications were dispensed, monitoring and patient management proceeded according to the prescriber’s usual procedures. Over the course of the study, 1.8% of intervention group patients versus 2.2% of control group patients had a newly prescribed PIM (p = 0.002). The relative risk reduction (RRR) and absolute risk reduction (ARR) were 16% and 0.3%, respectively. The dispensing rates for amitriptyline (p < 0.001, RRR 37%) and diazepam (p = 0.02, RRR 21%) also differed significantly between groups.29
The appropriateness of sample sizes was addressed by Hanlon et al.26 and Richmond et al.28 Taylor et al.27 and Raebel et al.29 did not carry out a sample size calculation. Finally, Bryant et al.25 calculated the sample size based on the quality-of-life tool, the SF-36; however, it is unclear how many physicians were enrolled in each arm of the study.
Characteristics of the pharmacist’s interventions
The characteristics of the criteria applied, healthcare professionals involved in each study and details of the pharmacist interventions are summarised in Table 2. The MAI criteria were used in four of the studies.14,25–28 In the Raebel et al.29 study, the Beers criteria and Zhan criteria were used.30–32 According to Richmond et al.28 and Raebel et al.,29 pharmacists did not have access to medical notes. A medication review was conducted in four studies.25–28 In two studies, Hanlon et al.26 and Taylor et al.27 involved pharmacists providing patients with written educational materials as part of the intervention. Pharmacists in four of the studies provided feedback to the physicians orally or in the written format.25–27,29 Two of the studies provided feedback via both methods of communication.26,27 It is not clear from the Richmond et al. 28. study how feedback was communicated to physicians. Finally, one study involved an educational meeting with physicians.28
Table 2.
Characteristics of the pharmacist’s interventions.
| Author | Criteria applied | List of healthcare professionals involved | Number of healthcare professionals involved | Access to lab data | Access to medical notes | Medication review carried out | Patient counselling undertaken | Patients given educational material | Written communication with physicians | Oral communication with physicians | Educational meeting with physicians |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bryant et al. 25 | MAI | Pharmacists, physicians | Two | No | Yes | Yes | Yes | No | Unclear | Yes | No |
| Hanlon et al. 26 | MAI | Pharmacists, physicians and nurses | Three | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Raebel et al. 29 | Beers, Zhan and KPCMI | Pharmacists, physicians | Two | No | No | No | No | No | No | Yes | No |
| Richmond et al. 28 | MAI | Pharmacists, physicians | Two | No | No | Yes | Unclear | No | Unclear | Unclear | Yes |
| Taylor et al. 27 | MAI | Pharmacists, physicians and nurses | Three | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
KPCMI: Kaiser Permanente Care Management Institute, MAI: Medication Appropriateness Index.
Results of the risk of bias assessment
The results of the risk of bias are presented in Tables 3 and 4. Common sources of bias included inadequate sample size, performance bias and spectrum bias. Overall, the authors considered four of the five studies to be at high risk of bias.
Table 3.
Methodological quality of RCT studies included in the review.
| Author | Selection bias |
Performance bias |
Detection bias |
Attrition bias |
Reporting bias |
Other bias |
Risk of bias |
|
|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Overall risk of bias | |
| Bryant et al.25 | High risk | High risk | Unclear | Low risk | High risk | Low risk | Unclear | High |
| Hanlon et al.26 | Low risk | Unclear | High risk | Low risk | Low risk | Low risk | High risk | High |
| Raebel et al.29 | Low risk | Unclear | Low risk | Unclear | Low risk | Low risk | Unclear | Unclear |
| Taylor et al.27 | Unclear | Unclear | Unclear | Unclear | Low risk | Low risk | High risk | High |
RCT: randomised control trial.
Table 4.
Methodological quality of ITS study included in the review.
| Author | Was the intervention independent of other changes? | Was the shape of the intervention effect pre-specified? | Was the intervention unlikely to affect data collection? | Was knowledge of the allocated interventions adequately prevented during the study? | Were incomplete outcome data adequately addressed? | Was the study free from selective outcome reporting? | Was the study free from other risks of bias? | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Richmond et al.28 | Low risk | Low risk | High risk | Low risk | Unclear risk | High risk | High risk | High |
ITS: interrupted time series.
Synthesis method
Due to the heterogeneity of the interventions and outcome measures reported, a narrative synthesis was carried out.
Discussion
Statement of principal findings
This systematic review examined the impact of pharmacist-led interventions on appropriateness of prescribing in older adults in primary care. Interventions across the five studies consisted of structured medication reviews or computer alerts. Three of the five studies reported an improvement in the MAI score in the intervention group compared to the control group.25–27 Raebel et al.29 reported a reduction in newly dispensed PIMs. Richmond et al.28 reported that pharmaceutical care undertaken by community pharmacists did not significantly change the appropriateness of prescribing in older patients. One of the limitations from this study was that the pharmacists were unable to gather detailed clinical records for patients. This may have led to an underestimation of the true MAI score.28 In three studies, pharmacists had access to the patients’ medical notes.25–27 This may have impacted on the nature and scope of the medication review by pharmacists. Another limitation was that the pharmacists reported difficulties in accessing patients and physicians in order to prepare and discuss the pharmaceutical care plan. This was despite the fact that joint collaborative training on pharmaceutical care was provided to pharmacists and physicians.28 The two studies that involved pharmacists providing patients with written education materials may have further improved their understanding of and compliance with medicines.26,27 In the study by Bryant et al.25, the medication review was conducted in the pharmacy or at the patient’s home. Although the MAI score improved more in the intervention group than in the control group, approximately 40% of the pharmacist recommendations were not implemented by the physicians. It is not known whether the non-implementation was due to the physician–pharmacist relationship or whether there were other barriers involved.25 According to Raebel et al.29, the absolute difference in dispensing numbers between intervention and control groups was minimal. This was despite the fact that pharmacists and physicians collaborated to develop a list of medicines for the intervention, specific intervention guidelines and patient counselling scripts. The study highlights the difficulty in modifying prescribing behaviour even though the intervention was fully advocated by the physicians.29 Finally, in the study by Hanlon et al., the target population was elderly male veterans. This may impact on the generalisability of the study findings.26
Clinical significance of MAI change
In the studies carried out by Hanlon et al.26 and Taylor et al.,27 the authors conclude that the clinical significance of the change in MAI remains unclear and highlight this as a potential limitation. Bryant et al.25 suggest that further research be carried out to determine the relationship between the MAI and hospitalisation rates. Finally, in the Richmond et al.28 study, the pharmaceutical care model carried out by pharmacists did not significantly change the appropriateness of prescribing or quality of life in older people.
A narrative review identified seven studies that evaluated the predictive validity of the MAI in relation to various health outcomes.14 Three studies involved VA outpatients or VA medical centres across the United States.33–35 In these studies, higher MAI scores were significantly associated with unscheduled ambulatory or ED visits and inadequate blood pressure control, ADEs using modified MAI scores and adverse drug reactions by drug–disease interaction criteria.33–35
Results in the context of the current literature
A systematic review by Kaur et al. evaluated various interventions and strategies to reduce inappropriate prescribing in older people in primary and secondary care settings. The review highlighted that pharmacist interventions were successful in reducing inappropriate prescribing. Other interventions that demonstrated positive effects on prescribing included computerised support systems, geriatrician’s services and multidisciplinary team work. However, there were mixed responses for educational interventions aimed at improving inappropriate prescribing due to the variability in assessment methodologies. The effect of regulatory policies as an intervention was also variable.36 A further narrative review appraised prospective and intervention studies that focused on the use of PIMs in community-dwelling older adults. Intervention studies that were included aimed to change the prescribing patterns of physicians. The majority of included studies focused on the prevalence of PIMs. Several others analysed the relationship between PIMs and falls, cognitive function, sleep and quality of life. This narrative review recommends more collaborative multidisciplinary team approaches that include pharmacists to reduce the use of PIMs. It also suggests that mixed-methods research could enhance the quality of interventions to address PIM use.22 A Cochrane Review examined 12 intervention studies including pharmacist-led studies aimed at improving appropriate polypharmacy for older people across healthcare settings.17 In hospital settings, pharmacists provided pharmaceutical care in outpatient clinics and inpatient departments. In primary care, pharmacist interventions included medication reviews with feedback to physicians and medicine education to patients. Finally, in nursing homes, pharmacists worked with other healthcare professionals on case conferences and provided education to staff members. A drug management service was also provided. The post-intervention results demonstrated a mean difference of −3.88 (95% confidence interval (CI) −5.40 to −2.35) in the change in MAI score in favour of the intervention group compared with the control group across the five studies. This updated review published in 2014 was based on a previous Cochrane Review carried out by the same authors. It included two additional studies from the previous review.17
A review by Castelino et al. evaluated 12 interventions involving pharmacists that focused on reducing inappropriate prescribing in older adults across different healthcare settings. The selected studies highlighted pharmacists working independently or as part of multidisciplinary healthcare teams. The services provided by pharmacists commonly involved some form of medication review, highlighting the important role that pharmacists play in optimising medication use for this group of patients.18
The current systematic review has highlighted that pharmacist-led interventions involving access to medical notes and medication reviews conducted in physician practices with feedback to physicians may improve prescribing appropriateness in community-dwelling older adults. The findings are broadly in-keeping with other reviews including a recent systematic review with meta-analysis conducted to determine the effectiveness of pharmacist interventions to reduce PIP in older adults admitted to hospital. The review concluded that pharmacists carrying out medication reviews as part of multidisciplinary patient care teams may improve the quality of prescribing in older hospitalised patients.17,20,22,36
In addition, the findings are consistent with the evidence highlighted that the quality of prescribing for older people in primary care could benefit from pharmacist–physician collaboration.
Clinical implications and areas for further research
This review has implications for clinical practice and future research, in particular, with respect to the emerging role that pharmacists play in moderating medication appropriateness in primary care.
There is a growing need for improved collaboration between physicians and pharmacists in order to optimise prescribing practices in primary care. Collaborative multidisciplinary models can improve the care of older adults with chronic multimorbidities.37 The PINCER trial is an example of such a model. It highlighted the benefit of a pharmacist-led intervention in general practices in the United Kingdom. The practices were cluster randomised to a pharmacist-led information technology intervention group or a control group. This trial demonstrated that the PINCER intervention was effective at reducing medication errors in general practice.38 Schmader et al. demonstrated that compared with usual care, inpatient and outpatient geriatric evaluation and management programmes involving pharmacists reduced suboptimal prescribing in frail elderly patients. The outpatient geriatric teams also reduced serious adverse drug reactions.39
The diverse range of outcome measures used in studies of prescribing appropriateness has made it difficult to make firm conclusions in this field. Ideally, prescribing outcome measures should be linked to important clinical outcomes such as morbidity or mortality. A pan-European study is currently underway and may add clarity to this issue: The OPERAM (Optimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly) study aims to reduce the rates of over- and under-prescribing of medications among multimorbid older European adults. While PIP is one measure among others at measuring optimal prescribing, there is an underreporting of medication errors in the literature. One major component of this large multi-centre randomised clinical trial will be the use of a sophisticated software tool to optimise medication therapy and to determine whether the applied intervention can improve clinical outcomes such as drug-related admissions (DRAs), humanistic outcomes such as quality of life and reduce healthcare costs (https://operam2020.tp21.com).
Educational outreach interventions or ‘academic detailing’ provided by pharmacists (or other clinicians) to physicians in primary care is an area for further research. The term ‘academic detailing’ was coined by Jerry Avorn, MD, over 30 years ago.40 Clinical educators, who are usually pharmacists, nurses or physicians, are trained to provide accurate, balanced, non-commercial and up-to-date synthesis of the best clinical evidence in an engaging format with healthcare physicians.40 Information highlighted to physicians often includes recommendations about alternative treatment regimens or non-pharmacological interventions where appropriate. These recommendations are designed to complement the clinical judgement of a physician and not to replace it.41 This evidence-based strategy has been shown to be an effective means of changing physician behaviour and improving patient care.42 For example, a pharmacist-led intervention comprising academic detailing demonstrated an improvement in statin prescribing in high-risk patients in primary care.43
There is a dearth of research examining the cost-benefit analysis of pharmacist-led interventions to improve prescribing patterns in healthcare. Cowper and colleagues26,44 conducted a cost analysis of a previously reported RCT. The total cost of the clinical pharmacist intervention was US$120 per patient per year. This intervention was a cost-effective means at improving prescribing among elderly outpatients.44 In the PINCER trial, the cost per error avoided was estimated by incremental cost-effectiveness analysis. The pharmacist-led intervention had a 95% probability of being cost-effective at various time points throughout the trial.38 The economic benefits of using validated screening tools in primary care are currently limited and require further research.
Strengths and weaknesses
This is the first systematic review to focus specifically on the impact of pharmacist-led interventions on prescribing appropriateness in older adults in primary care. An explicit and robust methodology was applied to identify and synthesise the study findings. However, the findings of the review need to be interpreted in the context of the study limitations. First, the methodological quality of the studies was poor overall, limiting the generalisability of the findings. Second, despite applying a comprehensive search strategy, only five studies were eligible for inclusion in the review. Third, the lack of standardised reporting across studies limited the statistical pooling of data. Moreover, three studies reported an improvement in the MAI score for the intervention group compared to the control group; however, the effect sizes are small which highlights the need for further research to assess the impact of pharmacist-led interventions in primary care. Furthermore, the clinical impact of reducing surrogate markers such as the MAI remains unknown and requires further investigation. However, one of the aims of the OPERAM study is to link prescribing outcome measures with clinical outcomes such as hospital admissions. Finally, large cluster-RCTs that are methodologically robust and have a long duration of follow-up are needed to address patient-focused outcomes. In addition, reviews on the appropriateness of prescribing are warranted among other vulnerable populations including paediatric patients, drug users and prisoners.
Conclusion
This review concludes that pharmacist-led interventions involving access to medical notes and medication reviews conducted in physician practices with feedback to physicians may improve prescribing appropriateness in community-dwelling older adults. Interventions where computer alerts that help to inform pharmacists of PIMs may also improve prescribing appropriateness. However, it is unclear if these interventions result in clinically significant improvements in patient outcomes. Furthermore, high-quality research should be conducted to explore the generalisability of these interventions. Finally, the role of a pharmacist working as part of a multidisciplinary primary care team requires further investigation to optimise prescribing in this group of patients.
Appendix
Appendix 1.
Databases and search terms applied.
| Database | Search terms applied |
|---|---|
| CINAHL | AGED OR aged, 65 and over OR middle aged OR elderly AND Primary care OR primary health care OR community AND Pharmacist* AND Prescription OR prescribing |
| ClinicalTrials.gov | (pharmacist or pharmacists) AND (prescribing OR prescription) AND (inappropriate OR strategy OR strategies OR improving OR improve OR optimise OR optimize) AND (primary care OR primary health care OR community OR outpatient care) | Adult, Senior |
| Cochrane Database of Systematic Reviews | ‘aged’ or ‘aged, over 65’ or ‘middle aged’ or ‘elderly’ AND prescribing or prescription or ‘inappropriate prescribing’ AND ‘primary care’ or ‘primary health care’ or ‘outpatient care’ or ‘community’ AND pharmacist* or ‘pharmaceutical care’ or pharmacist* intervention |
| Embase | ‘aged’ OR ‘aged’ OR ‘middle aged’ OR ‘middle aged’ OR ‘aged, over 65’ OR ‘elderly’ OR ‘elderly’ AND ‘primary care’ OR ‘primary health care’ OR ‘outpatient care’ AND prescribing OR prescription* AND pharmacist* OR ‘pharmaceutical care’ |
| MEDLINE (through Ovid) | (prescribing or prescription) AND (aged or middle aged or elderly) AND (primary care or primary health care or elderly care or outpatient care) AND (pharmacist* or pharmaceutical care) |
| metaRegister of Controlled Trials (mRCT) | (pharmacist or pharmacists) AND (prescribing OR prescription) AND (inappropriate OR improving OR improve OR optimise OR optimize) AND (primary care OR primary health care OR outpatient care OR community OR general practice) |
| ProQuest Dissertations & Theses | (aged or elderly OR ‘middle aged’) AND (‘primary care’ or ‘primary health care’ OR community or ‘outpatient care’) AND (prescribing or ‘drug prescribing’ OR prescription* or ‘drug prescription*’) AND (pharmacist* or clinical pharmacist* OR ‘pharmaceutical care’ OR ‘pharmacy intervention’ or ‘clinical intervention’ OR ‘pharmacist intervention’ or ‘clinical pharmacist intervention’) |
| PubMed | (((‘primary care’ or ‘primary health care’ or ‘outpatient care’))) AND ((prescribing or prescription*))) AND ((‘aged’ or ‘middle aged’ or elderly))) AND ((pharmacist* or ‘pharmaceutical care’)) |
| ScienceDirect | aged OR ‘middle aged’ OR ‘elderly’ AND ‘primary care’ OR ‘primary health care’ OR community OR ‘outpatient care’ AND prescribing OR prescription* OR ‘appropriate prescribing’ OR ‘inappropriate prescribing’ OR ‘potentially inappropriate prescribing’ AND ‘clinical pharmacist’ OR pharmacist* OR ‘pharmaceutical care’ OR ‘pharmacy intervention’ or ‘clinical intervention’ OR ‘pharmacist intervention’ or ‘clinical pharmacist intervention’ (All Sources (Medicine and Dentistry, Nursing and Health Professions, Pharmacology, Toxicology and Pharmaceutical Science)). |
| Trip | ‘(title: aged OR middle aged OR elderly AND primary care OR primary health care OR outpatient care) (title: pharmacist* OR pharmaceutical care) (title: usual care OR control) (prescribing OR prescription)’, by quality |
| University of York Centre for Reviews and Dissemination | (aged) OR (‘middle aged’) OR (elderly) AND (prescribing or prescription* or ‘drug prescribing’ or ‘drug prescription*’) AND (‘primary care’) OR (‘primary health care’) OR (‘outpatient care’) AND (pharmacist* or ‘clinical pharmacist*’ or ‘pharmaceutical care’ OR ‘pharmacist* intervention’ OR ‘clinical pharmacist* intervention’) |
| ISI Web of Science | (‘aged’ or ‘middle aged’ or elderly) Timespan=All years, Search language=Auto AND (‘primary care’ or ‘primary health care’ or ‘outpatient care’) Timespan=All years, Search language=Auto AND (prescribing or prescription*) Timespan=All years, Search language=Auto AND (pharmacist* or ‘pharmaceutical care’) Timespan=All years, Search language=Auto |
Appendix 2.
Ongoing and excluded articles reviewed and reasons for exclusion.
| Study number | Article | Reason for exclusion |
|---|---|---|
| 1. | Developing pharmacist-led research to educate and sensitive community residents to the inappropriate prescription burden in the elderly. Source: ClinicalTrials.gov |
This study is currently ongoing. Study start date: March 2014. Estimated study completion date: September 2016. |
| 2. | Inappropriate prescription in elderly and polypharmacy patients in primary care. PHARM-PC Trial. Source: ClinicalTrials.gov |
This study is not yet open for participant recruitment. Study start date: October 2014. Estimated study completion date: April 2016. |
| 3. | A pilot study to reduce inappropriate anticholinergic prescribing in the elderly. Source: ClinicalTrials.gov |
This study is currently recruiting participants. Study start date: September 2014. Estimated study completion date: December 2015. |
| 4. | Educational intervention to reduce drug-related hospitalizations in elderly primary health care patients. Source: ClinicalTrials.gov |
Emailed author for full paper: 12 December 2014, 10:50 a.m. No reply. Emailed author again on 13 January 2015, 11:35 a.m. No reply. |
| 5. | Minimizing risk and maximizing outcomes in geriatric patients through integrated clinical pharmacy services in an innovative model of community practice. Source: ClinicalTrials.gov |
The recruitment status of this study is unknown because the information has not been verified recently. Observational study. |
| 6. | Study of whether educational visits to primary care professionals improves the quality of care they provide. Source: ClinicalTrials.gov |
Not relevant. The intervention was evaluated using prescribing analysis and cost (PACT) data for antidepressant drugs. |
| 7. | An intervention study to reduce the use and impact of potentially inappropriate medications among older adults. Source: ClinicalTrials.gov |
Emailed author for full paper: 12 December 2014, 11:55 a.m. Author replied on 12 December 2014, 5 p.m. with the following statement: ‘Unfortunately our trial did not involve a pharmacist intervention so would not be relevant for your review’. |
| 8. | Pharmacist-led medicines management outpatient service Source: ClinicalTrials.gov |
Emailed author for full paper: 12 December 2014, 12:05 p.m. Author replied on 5 January 2015 with the following comment: ‘The Medicines Management Outpatient Service research is currently in progress therefore, unfortunately it is not possible to share details at this stage’. |
| 9. | Rationalisation of polypharmacy in the elderly by the RASP instrument Source: ClinicalTrials.gov |
Emailed author for full paper: 12 December 2014, 12:24 p.m. Author replied on 13 January 2015 with following comment: ‘I am afraid that our manuscript concerning the RASP study in hospital setting is still in the makings. We are currently finishing it as we speak. Afterwards we will normally finish a short proof-of-concept study, which was performed in primary care’. |
| 10. | Randomized controlled trial of enhanced pharmacy care in older veteran outpatients Source: ClinicalTrials.gov |
Emailed author for full paper: 12 December 2014, 12:30 p.m. No response. Emailed author again on 13 January 2015 at 11:50 a.m. No response. |
| 11. | Preventing falls through enhanced pharmaceutical care Source: ClinicalTrials.gov |
Full article obtained. The purpose of this study was to assess the effects of a community pharmacy–based falls prevention programme. |
| 12. | Lipton H, Bero L, Bird JA, et al. The impact of clinical pharmacists’ consultations on physicians’ geriatric drug prescribing. A randomized controlled trial. Med Care 1992; 30(7): 646–658. | Full article obtained. Intervention in secondary care. Not a validated tool in 1992. |
| 13. | Elderly people still given inappropriate drugs. Pharmaceut J 2006; 276(7384): 62. | Report from the Pharmaceutical Journal. |
| 14. | Vinks T, Egberts T, De Lange T, et al. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs Aging 2009; 26(2): 123–133. | Full article obtained. No screening tool used. Medication review used. Drug-related problems were identified and validated by reference to national prescribing guidelines such as the practice standards of Dutch general practitioners (GPs) as well as therapeutic handbooks. |
| 15. | Allard J, Hebert R, Rioux M, et al. Efficacy of a clinical medication review on the number of potentially inappropriate prescriptions prescribed for community-dwelling elderly people. CMAJ 2001; 164(9): 1291–1296. | Full article obtained. Potentially inappropriate prescriptions (PIPs) were identified from a list of PIPs developed by the Quebec Committee on Drug Use in the Elderly. Although generated by a panel of experts, this list has never been validated with empirical data. |
| 16. | The Community Pharmacy Medicines Management Project Evaluation Team. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract 2007; 24: 189–200. | Full article obtained. No screening tool used. |
| 17. | Castelino RL, Hilmer SN, Bajorek BV, et al. Drug Burden Index and potentially inappropriate medications in community-dwelling older people: the impact of Home Medicines Review. Drugs Aging 2010; 27(2): 135–148. | From abstract: A retrospective analysis of medication reviews. No control group. Full article not required. |
| 18. | Cowper PA, Weinberger M, Hanlon JT, et al. The cost-effectiveness of a clinical pharmacist intervention among elderly outpatients. Pharmacotherapy 1998; 18(2): 327–332. | Full article obtained. Cost analysis of a previously reported randomised controlled trial. (A randomised controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Hanlon et al. 1996. This paper was included in our final review.) |
| 19. | Davis RG, Hepfinger CA, Sauer KA, et al. Retrospective evaluation of medication appropriateness and clinical pharmacist drug therapy recommendations for home-based primary care veterans. Am J Geriatr Pharmacother 2007; 5(1): 40–47. | Full article obtained: Hard copy only. Retrospective analysis. No control group. |
| 20. | Denneboom W, Dautzenberg MG, Grol R, et al. Treatment reviews of older people on polypharmacy in primary care: cluster controlled trial comparing two approaches. Br J Gen Pract 2007; 57(542): 723–731. | Full article obtained. Treatment review only. No screening tool used. |
| 21. | Faya S. Pharmaceutical care for elderly patients in community pharmacy: analysis and evaluation of community pharmacist interventions in the Randomised Evaluation of Shared Prescribing for Elderly People in the Community over Time (RESPECT) Study. Bradford: University of Bradford, 2009. | Paper produced as part of PhD thesis. Thesis obtained. |
| 22. | Fletcher J, Hogg W, Farrell B, et al. Effect of nurse practitioner and pharmacist counselling on inappropriate medication use in family practice. Can Fam Physician 2012; 58(8): 862–868. | Full article obtained. This study had no control group. |
| 23. | Goodyear-Smith F. Appropriate medications: prescription and use in primary care. J Prim Health Care 2013; 5(3): 178–179. | Full article obtained. Not relevant: Editorial review. |
| 24. | Grymonpre RE, Williamson DA and Montgomery PR. Impact of a pharmaceutical care model for non-institutionalised elderly: results of a randomised, controlled trial. Int J Pharm Pract 2001; 9: 235–241. | Full article obtained. Not related to inappropriate prescribing. |
| 25. | Howard R, Rodgers S, Avery AJ, et al. Description and process evaluation of pharmacists’ interventions in a pharmacist-led information technology-enabled multicentre cluster randomised controlled trial for reducing medication errors in general practice (PINCER trial). Int J Pharm Pract 2014; 22(1): 59–68. | Full article obtained. Pharmacist’s recommendations to manage individual cases of hazardous medicines management. No screening tool used. |
| 26. | Kaufman MB, Brodin KA and Sarafian A. Effect of prescriber education on the use of medications contraindicated in older adults in a managed Medicare population. J Manag Care Pharm 2005; 11(3): 211–219. | Full article obtained. This was a before and after study. No control group. |
| 27. | Krska J, Cromarty JA, Arris F, et al. Pharmacist-led medication review in patients over 65: a randomized, controlled trial in primary care. Age Ageing 2001; 30(3): 205–211. | Full article obtained. The study identified pharmaceutical care issues using medication reviews, however, no screening tool used. |
| 28. | Kwint HF, Faber A and Bouvy ML. The effect of home medication review on the resolution of drug related problems and health-related quality of life. Int J Clin Pharm 2013; 35(5): 896. | Article obtained. Poster presented at 41st European Society of Clinical Pharmacy symposium on clinical pharmacy: Barcelona, Spain. 29–31 October 2012. |
| 29. | Kwint HF, Faber A, Gussekloo J, et al. Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging 2011; 28(4): 305–314. | Full article obtained. Medication review. Implicit and explicit criteria used. Explicit criteria consisted of a list of clinical rules based on Dutch treatment and prescription guidelines. Implicit criteria for identifying Drug-related problems were based on a structural assessment by Cipolle according to a rational order of indication, effectiveness, safety and compliance. |
| 30. | Lipton HL, Bird JA, Bero LA, et al. Assessing the appropriateness of physician prescribing for geriatric outpatients: development and testing of an instrument. J Pharm Technol 1993; 9(3): 107–113. | Full article obtained: Hard copy only. This study was carried out in secondary care. It involved the development and testing of an instrument for drug therapy prescribing problems for geriatric patients. |
| 31. | Lund BC, Carnahan RM, Egge JA, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother 2010; 44(6): 957–963. | Full article obtained. Study utilised data from a previous study. (The Veterans Affairs Enhanced Pharmacy Outpatient Clinic (EPOC) study: a randomised controlled pharmacist–physician intervention trial: Kaboli et al. 2004.) Objective: To determine whether an implicit measure of inappropriate prescribing can predict ADE risk. MAI score not segregated between control and intervention group. |
| 32. | Martin P, Tamblyn R, Ahmed S, et al. An educational intervention to reduce the use of potentially inappropriate medications among older adults (EMPOWER study): protocol for a cluster randomized trial. Trials 2013; 14: 80. | Full article obtained. Educational intervention. Outcome: Cessation of benzodiazepines in the 6 months following receipt of the intervention. No screening tool used. |
| 33. | Milos V, Rekman E, Bondesson A, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs Aging 2013; 30(4): 235–246. | Full article obtained. The majority of the patients in this study were living in nursing homes. The goal of medication reviews has been improved patient safety and quality of medication use, according to the Swedish National Board of Health and Welfare’s indicators for good drug therapy in the elderly. |
| 34. | Mino-Leon D, Reyes-Morales H, Jasso L, et al. Physicians and pharmacists: collaboration to improve the quality of prescriptions in primary care in Mexico. Int J Clin Pharm 2012; 34(3): 475–480. | Full article obtained. Aim: To reduce prescription errors for patients with diabetes and/or hypertension. No screening tool used. |
| 35. | Morley JE. Inappropriate drug prescribing and polypharmacy are major causes of poor outcomes in long-term care. J Am Med Dir Assoc 2014; 15(11): 780–782. | Not relevant. Study carried out in long-term care, for example, nursing homes. |
| 36. | Morrison A and Wertheimer AI. Evaluation of studies investigating the effectiveness of pharmacists’ clinical services (Structured abstract). Am J Health Syst Pharm 58(7): 569–577. | Not relevant: systematic review. |
| 37. | Reboredo-Garcia S, Mateo CG and Casal-Llorente C. Implantation of a program for polymedicated patients within the framework of the Galician Strategy for Integrated Chronic Care. Aten Primaria 2014; 46(Suppl. 3): 33–40. | Full article obtained. Published in Spanish. (A native Spanish speaker was recruited to translate the article into English, Dec 2014.) No control study. |
| 38. | Rossi MI, Young A, Maher R, et al. Polypharmacy and health beliefs in older outpatients. Am J Geriatr Pharmacother 2007; 5(4): 317–323. | Abstract only. This study contained no control group. |
| 39. | Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med 2004; 116(6): 394–401. | Full article obtained. Analysed same patients as inpatient and outpatients. |
| 40. | Sellors J, Kaczorowski J, Sellors C, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients (structured abstract). CMAJ 2003; 169(1): 17–22. | Full article obtained. The intervention focused on drug-related problems. The primary end-point measure was a reduction in the daily units of medication taken, as a surrogate for optimised drug therapy. |
| 41. | Shade MY, Berger AM and Chaperon C. Potentially inappropriate medications in community-dwelling older adults. Res Gerontol Nurs 7(4): 178–192. | Full article obtained. This is a systematic review. |
| 42. | Teichert M, Luijben SN, Wereldsma A, et al. Implementation of medication reviews in community pharmacies and their effect on potentially inappropriate drug use in elderly patients. Int J Clin Pharm 2013; 35(5): 719–726. | Full article obtained. Specifically developed algorithms were used to identify nine potentially inappropriate medicines (PIMs) from the HARM study. |
| 43. | Wong I, Campion P, Coulton S, et al. Pharmaceutical care for elderly patients shared between community pharmacists and general practitioners: a randomised evaluation. RESPECT (Randomised Evaluation of Shared Prescribing for Elderly people in the Community over Time. BMC Health Serv Res 2004; 4(1): 11. | Full article obtained. This paper describes a proposed randomised multiple interrupted time series trial design. |
| 44. | Zermansky AG, Petty DR, Raynor DK, et al. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technol Assess 2002; 6(20): 1–86. | Full article obtained. Primary outcome: the number of repeat medication changes per patient over a 12-month period. Secondary outcome was the effect on the medication costs. |
| 45. | Basger BJ, Moles RJ and Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. Int J Clin Pharm 2015; 37: 1194–1205. | Full article obtained. This study was performed in a small private hospital. (Not a primary care study.) |
| 46. | Rose O, Waltering I, John C, et al. The WestGem study; medication management in the elderly. Int J Clin Pharm 2015; 37(2): 405–406. | Abstract only obtained. The WestGem-Study is still going on. Results will be published by the end of 2015. |
| 47. | Verdoorn S, Kwint HF, Faber A, et al. Majority of drug-related problems identified during medication review are not associated with STOPP/START criteria. Eur J Clin Pharmacol 2015; 71(10): 1255–1262. | Full article obtained. This study has no control group. |
| 48. | Bregnhøj L, Thirstrup S, Kristensen MB, et al. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol 2009; 65: 199–207. | Full article obtained. This was not a pharmacist-led study. The pharmacist analysed the patients’ prescription and medical history and proposed changes in their medication. The pharmacist and clinical pharmacologist discussed these recommendations; however, it was the responsibility of the clinical pharmacologist what was finally recommended. The pharmacist forwarded the feedback to the physicians. The clinical pharmacologists contacted the physicians by telephone to discuss any uncertainties concerning the recommendations given. The clinical pharmacologists delivered the interactive educational interventions. |
| 49. | Monane M, Matthias D, Nagle B, et al. Improving prescribing patterns for the elderly through an online drug utilization review intervention: a system linking the physician, pharmacist, and computer. JAMA 1998; 280(14): 1249–1252. *Handsearched |
Full article obtained. This study has no control group. |
| 50. | Fick DM, Maclean JR, Rodriguez NA, et al. A randomized study to decrease the use of potentially inappropriate medicines among community dwelling older adults in a south-eastern managed care organisation. Am J Manag Care 2004; 10(11 Pt 1): 761–768. *Handsearched |
Full article obtained. Pharmacists suggested a list of potentially inappropriate medicine alternative medicines and performed a peer review of the drugs to be included in the intervention and their corresponding alternative medications. Not a pharmacist-led intervention. |
| 51. | Bucci C, Jackevicius C, McFarlane K, et al. Pharmacist’s contribution in a heart function clinic: patient perception and medication appropriateness. Can J Cardiol 2003; 19(4): 391–396. *Handsearched |
Full article obtained. This pharmacist intervention was carried out at the heart function clinic at Toronto General Hospital. Interventions carried out in secondary care were not included in the review. |
| 52. | Meredith S, Feldman P, Frey D, et al. Improving medication use in newly admitted home healthcare patients: a randomized controlled trial. J Am Geriatr Soc 2002; 50: 1484–1491. *Handsearched |
Full article obtained. There was no validated screening tool used in this study and potentially inappropriate prescribing was not measured as an outcome. |
| 53. | Avorn J and Soumerai SB. Improving drug-therapy decisions through educational outreach. A randomized controlled trial of academically based ‘detailing’. N Engl J Med 1983; 308(24): 1457–1463. *Handsearched |
Full article obtained. The three target drugs were selected on the basis of an analysis of national prescribing practices, for example, Medicaid prescribing records and evidence from published controlled clinical trials. There was no screening tool used in the intervention, and the target population was not specifically aimed at those aged over 65 years. |
Appendix 3.
PRISMA checklist.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including as applicable: background, objectives, data sources, study eligibility criteria, participants and interventions, study appraisal and synthesis methods, results, limitations, conclusions and implications of key findings, systematic review registration number. | 2–3 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 4–6 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS). | 6 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language and publication status) used as criteria for eligibility, giving rationale. | 7 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 6 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 6 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 7 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently and in duplicate) and any processes for obtaining and confirming data from investigators. | 7–8 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made. | 6–8 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 8 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means). | N/A |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis. | N/A |
| Page 1 of 2: risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies). | N/A |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | N/A |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 9, 26 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations. | 9–12, 27–38 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study, and, if available, any outcome level assessment (see Item 12). | 12, 39–40 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot. | 12, 39–40 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | N/A |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | N/A |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)). | N/A |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome, consider their relevance to key groups (e.g. healthcare providers, users and policy makers). | 13–14 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias) and at review-level (e.g. incomplete retrieval of identified research, reporting bias). | 19 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research. | 15–20 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data), role of funders for the systematic review. | N/A |
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; N/A: not applicable.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Health Research Board SPHeRE/2013/1.
References
- 1. Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 2011; 171: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 2. Jano E, Aparasu RR. Healthcare outcomes associated with Beers’ criteria: a systematic review. Ann Pharmacother 2007; 41: 438–447. [DOI] [PubMed] [Google Scholar]
- 3. Spinewine A, Schmader KE, Barber N, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 2007; 370: 173–184. [DOI] [PubMed] [Google Scholar]
- 4. Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151: 1825–1832. [PubMed] [Google Scholar]
- 5. O’Sullivan D, Byrne S, O’Mahony D. An evaluation of the inappropriate prescribing in older residents in long term care facilities in the greater Cork and Northern Ireland regions using the STOPP and Beers’ criteria. Centre for Ageing Research and Development in Ireland (CARDI), 2011. [Google Scholar]
- 6. Cahir C, Fahey T, Teeling M, et al. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol 2010; 69: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradley MC, Fahey T, Cahir C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. Eur J Clin Pharmacol 2012; 68: 1425–1433. [DOI] [PubMed] [Google Scholar]
- 8. Bradley MC, Motterlini N, Padmanabhan S, et al. Potentially inappropriate prescribing among older people in the United Kingdom. BMC Geriatr 2014; 14: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curtis LH, Ostbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164: 1621–1625. [DOI] [PubMed] [Google Scholar]
- 10. Marriott J, Stehlik P. A critical analysis of the methods used to develop explicit clinical criteria for use in older people. Age Ageing 2012; 41: 441–450. [DOI] [PubMed] [Google Scholar]
- 11. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 12. Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009; 68: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galvin R, Moriarty F, Cousins G, et al. Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA). Eur J Clin Pharmacol 2014; 70: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanlon JT, Schmader KE. The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging 2013; 30: 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanlon JT, Artz MB, Pieper CF, et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother 2004; 38: 9–14. [DOI] [PubMed] [Google Scholar]
- 16. Muijrers PE, Knottnerus JA, Sijbrandij J, et al. Changing relationships: attitudes and opinions of general practitioners and pharmacists regarding the role of the community pharmacist. Pharm World Sci 2003; 25: 235–241. [DOI] [PubMed] [Google Scholar]
- 17. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014;10: 1–119. [DOI] [PubMed] [Google Scholar]
- 18. Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother 2009; 43: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 19. Alldred DP, Kennedy MC, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev 2016; 2: CD009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walsh KA, O’Riordan D, Kearney PM, et al. Improving the appropriateness of prescribing in older patients: a systematic review and meta-analysis of pharmacists’ interventions in secondary care. Age Ageing 2016; 45: 201–209. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 22. Shade MY, Berger AM, Chaperon C. Potentially inappropriate medications in community-dwelling older adults. Res Gerontol Nurs 2014; 7: 178–192. [DOI] [PubMed] [Google Scholar]
- 23. Cochrane Collaboration. Suggested risk of bias criteria for EPOC reviews.1–4. [Google Scholar]
- 24. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bryant LJM, Coster G, Gamble GD, et al. The General Practitioner-Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. Int J Pharm Pract 2011; 19: 94–105. [DOI] [PubMed] [Google Scholar]
- 26. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med 1996; 100: 428–437. [DOI] [PubMed] [Google Scholar]
- 27. Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health Syst Pharm 2003; 60: 1123–1129. [DOI] [PubMed] [Google Scholar]
- 28. Richmond S, Morton V, Cross B, et al. Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. Br J Gen Pract 2010; 60: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raebel MA, Charles J, Dugan J, et al. Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc 2007; 55: 977–985. [DOI] [PubMed] [Google Scholar]
- 30. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163: 2716–2724. [DOI] [PubMed] [Google Scholar]
- 31. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Arch Intern Med 1997; 157: 1531–1536. [PubMed] [Google Scholar]
- 32. Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA 2001; 286: 2823–2829. [DOI] [PubMed] [Google Scholar]
- 33. Schmader KE, Hanlon JT, Landsman PB, et al. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother 1997; 31: 529–533. [DOI] [PubMed] [Google Scholar]
- 34. Lund BC, Carnahan RM, Egge JA, et al. Inappropriate prescribing predicts adverse drug events in older adults. Ann Pharmacother 2010; 44: 957–963. [DOI] [PubMed] [Google Scholar]
- 35. Hanlon JT, Sloane RJ, Pieper CF, et al. Association of adverse drug reactions with drug-drug and drug-disease interactions in frail older outpatients. Age Ageing 2011; 40: 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaur S, Mitchell G, Vitetta L, et al. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging 2009; 26: 1013–1028. [DOI] [PubMed] [Google Scholar]
- 37. Topinkova E, Baeyens JP, Michel JP, et al. Evidence-based strategies for the optimization of pharmacotherapy in older people. Drugs Aging 2012; 29: 477–494. [DOI] [PubMed] [Google Scholar]
- 38. Avery AJ, Rodgers S, Cantrill JA, et al. A pharmacist-led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet 2012; 379: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmader KE, Hanlon JT, Pieper CF, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med 2004; 116: 394–401. [DOI] [PubMed] [Google Scholar]
- 40. Hartung DM, Hamer A, Middleton L, et al. A pilot study evaluating alternative approaches of academic detailing in rural family practice clinics. BMC Fam Pract 2012; 13: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maltais R, Schmidt T, Chhina H, et al. Academic detailing to optimize care for RA by family physicians – results of a satisfaction survey. J Rheumatol 2014; 41: 1494–1495. [Google Scholar]
- 42. Van Hoof TJHL, Miller NE, Pappas MS, et al. Characteristics of academic detailing: results from a systematic review. Am Health Drug Benefits 2015; 8: 414–442. [PMC free article] [PubMed] [Google Scholar]
- 43. Lowrie R, Lloyd SM, McConnachie A, et al. A cluster randomised controlled trial of a pharmacist-led collaborative intervention to improve statin prescribing and attainment of cholesterol targets in primary care. PLoS ONE 2014; 9: e113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cowper PA, Weinberger M, Hanlon JT, et al. The cost-effectiveness of a clinical pharmacist intervention among elderly outpatients. Pharmacotherapy 1998; 18: 327–332. [PubMed] [Google Scholar]