Abstract
Analysis of cancer family history (CFH) offers a low-cost genetic tool to identify familial cancer predisposition. In middle-income settings, the scarcity of individual records and database-linked records hinders the assessment of self-reported CFH consistency as an indicator of familial cancer predisposition. We used self-reported CFH to identify those families at risk for hereditary cancer syndromes in community-based primary care centers of a low-income Brazilian area. We also evaluated the consistency of the information collected by reassessing CFH five years later. We interviewed 390 families and constructed their pedigrees for genetic cancer risk assessment. We found 125 families affected by cancer, 35.2% with moderate to high risk of familial susceptibility to cancer, a number that represents a relatively high prevalence of potential hereditary cancer syndromes in the overall study sample. Upon reassessment of CFH in 14/20 families that were previously identified as having at least one first-degree and one second-degree relative affected by cancer, and presented moderate to high risk for developing cancer, 90% of initial pedigrees were confirmed. These results demonstrate the reliability of self-reports as a means of early identification of healthy individuals at risk, encouraging the wider use of this method in low- and middle-income primary care settings.
Keywords: Genetic predisposition to disease, Risk factors, Neoplasms, Family, Pedigree
Introduction
Family history is essential for identifying individuals at increased risk for primary and secondary cancers who could benefit from referral to genetics services (Weitzel et al., 2011; Lu et al., 2014). Identification of those individuals is crucial for early diagnosis, family management, and preventive care (Wood et al., 2014), reducing cancer morbi-mortality and health system costs (Rubinstein et al., 2011; Teng; Acheson, 2014).
The analysis of cancer family history (CFH) offers a low-cost, non-invasive genetic tool to track and diagnose familial cancer predisposition (Plat et al., 2009; Valdez et al., 2010; Janssens et al., 2012). Indeed, CFH has been shown to be a strong predictor of disease risk and yet is significantly underused in primary care settings (Doerr and Teng, 2012; Teng and Acheson, 2014).
Reliance on self-reported CFH for use in clinical practice, decision-making regarding surveillance recommendations and the design of preventive interventions depends, however, on knowledge about the reliability and accuracy of this tool. Commonly, medical records, death certificates, and information obtained from cancer registries have been used to determine the accuracy of the self-reported cancer family history (Kelly et al., 2007; Qureshi et al., 2009). However, individual records and database-linked records are rarely available in low- and middle-income settings, making it difficult to establish whether self-reported CFH is a reliable indicator of familial cancer predisposition in these populations (Gomy and Diz, 2013).
In this study we used self-reported family history data to identify those families at risk for hereditary cancer syndromes at five community-based primary care centers in a middle-income area in Brazil. We also sought to evaluate the consistency of the information collected in spontaneous interviews by replicating the data collection five years later. Our results should encourage the wider use of self-reported cancer family history in low- and middle-income primary care settings.
Subjects and Methods
We conducted a longitudinal study divided into two phases. In the first phase (started in 2008), a total of 410 families were selected through simple random sampling among 3,780 families attended at the five community-based primary care centers located in Ribeirão Preto, São Paulo, Brazil. The sample size was calculated considering an expected prevalence of 50% of CFH, and using a 95% confidence interval with α set at 5% considering a likely sampling loss of 15% (Pagano and Gauvreau, 2004).
A research team composed of five research assistants was trained by the first author to visit the families and collect their self-reported family history, with the purpose of selecting families at potential risk for hereditary cancer syndromes. We interviewed one informant per household, who was available at home, volunteered to participate in the study after listening to the research purposes, and then signed the consent form. The informant answered a questionnaire that included variables previously described in the literature (Feerro et al., 2008; Lu et al., 2014), such as personal and/or family history of cancer, degrees of relationship among family members affected by malignancies, gender, age, vital status, age at cancer onset, and type of primary cancer. Data were entered into Progeny pedigree-drawing software (Progeny Software, LLC, Indianapolis, IN, USA), and Statistical Package for the Social Sciences version 17.0 (SPSS Inc., Chicago, Illinois, USA) was used for descriptive statistical analysis.
All pedigrees were analyzed independently by two geneticists, a physician (VEFF), and a nurse (MFS) with expertise in oncogenetics, to perform genetic cancer risk assessment. In case of discordance regarding CFH evaluation, a third geneticist was consulted until consensus was reached, to ascertain data quality. We applied internationally established criteria to classify CFH in sporadic, familial, and hereditary cases, as well as to classify genetic cancer risk as low, moderate, and high (Table 1) (Schneider, 2002; Lindor et al., 2008; Valdez et al., 2010; NCCN, 2014; Vieira et al., 2015).
Table 1. Criteria for cancer family history and genetic cancer risk classification.
| Cancer family history | Criteria | Genetic cancer risk |
|---|---|---|
| Hereditary | At least one first and one second-degree relative affected by cancer | High |
| Three or more family members with same or related cancers | ||
| Exhibit classical cancers of hereditary cancer syndromes | ||
| Majority of the cases exhibit an autosomal dominant pattern of inheritance | ||
| Multiple primary cancers in an individual | ||
| Presence of rare cancers | ||
| Excess of bilateral cancers | ||
| Presence of nonmalignant features previously associated with hereditary cancer syndromes | ||
| At least one relative diagnosed at a younger than usual age | ||
| Familial | More cases of cancer within a family than statistically expected | Moderate |
| More distant affected family members | ||
| Does not often exhibit classical features of hereditary cancer syndromes | ||
| Familial cancer clusters without a specific inheritance pattern | ||
| Variable age of onset | ||
| Sporadic | Few or no first- or second-degree relatives affected by cancer | Low |
| Cancer occurs in only one generation | ||
| There is no particular pattern of inheritance | ||
| Later age of cancer onset |
The second phase of the study was conducted five years later. The families that were previously identified as having at least one first-degree and one second-degree relative affected by cancer were visited again, and were re-interviewed by one of the former research assistants (LCLJr.) to confirm the previously reported CFH. The informant interviewed in phase 2 was not necessarily the same person who responded to the questionnaire in phase 1 of the study. All participants were previously contacted by telephone to schedule the interview. At the beginning of the interview, the researcher clarified that the family was contacted again because of its risk to present a familial susceptibility to cancer. Noteworthy, neither the researcher nor the participant had access to the pedigree that was depicted at the first visit. The same questions that were asked in the first interview were asked again. This study was approved by the Institutional Review Board (No.215/CEP/CSE-FMRP-USP).
Results
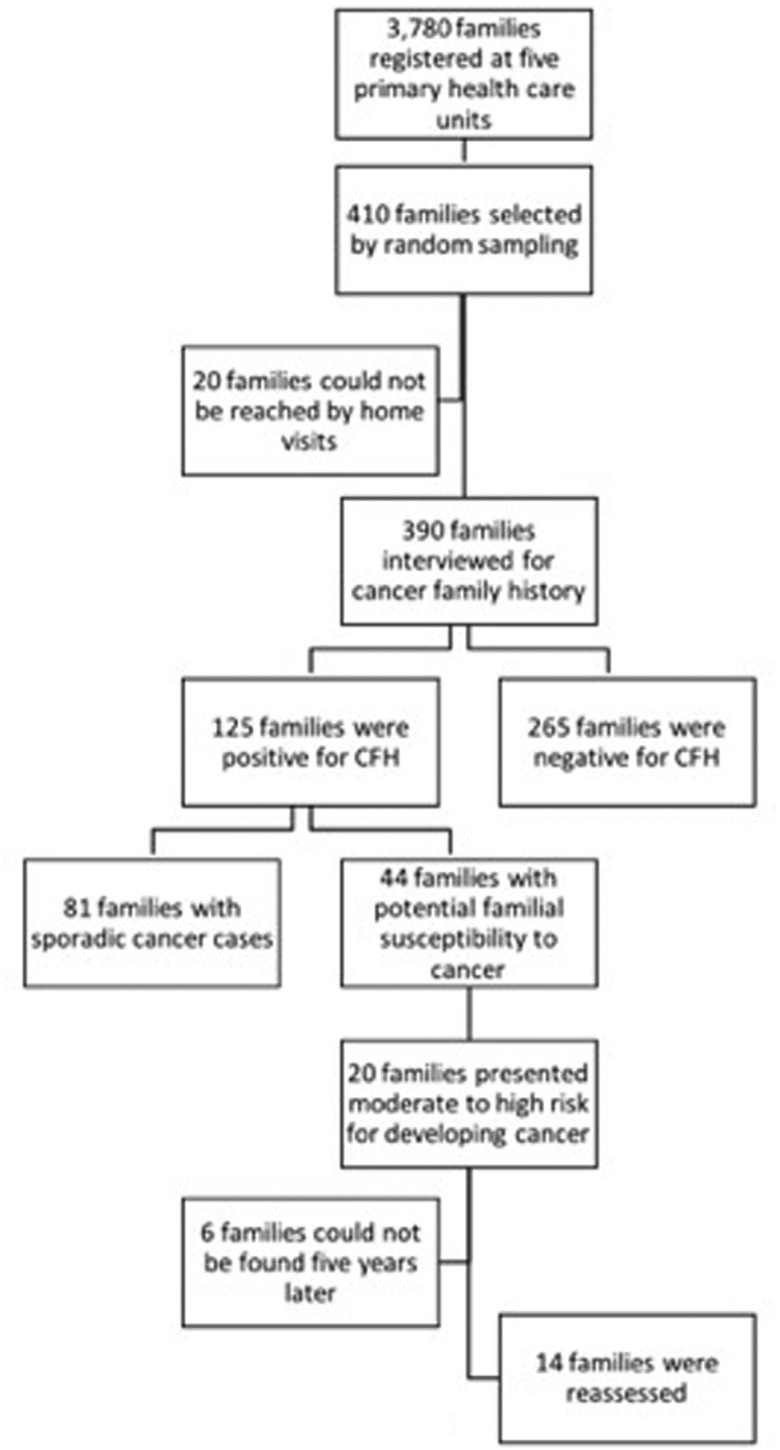
Of the 3,780 families registered in five primary health care units, 410 were randomly chosen for this study. Our final sample was composed of 390 families with which personal contact was possible. The characteristics of their pedigrees are shown in Table 2. Most participants were female (79.5%), and sample mean age was 54.0 ± 16.5 years. Informative familial history - namely, complete information on the occurrence of cancer (and when present, tumor site and age at onset of disease) for at least three generations - was obtained from 219 (56.1%) families. Of the 390 families interviewed, 125 (32.0%) informed to be affected by cancer (Figure 1): in 20 (29.4%) families at least one first- and one second-degree relative of the respondent were affected; in 14 (24.6%) at least one first-degree relative of the respondent was affected; in 48 (70.6%) only second-degree and more distant relatives were affected; and 43 (75.4%) had only more distant family members with cancer. Also, 10 respondents had had cancer themselves.
Table 2. Characterization of family members according to their age, number, and gender of individuals in the pedigrees.
| Variables | Mean ± SD (range) |
|---|---|
| Age of respondents, y | 54.0 (16.5) 18-95 |
| Age of family members affected by cancer, y | 57.5 (13.2) 20-92 |
| Age of youngest family member affected by cancer, y | 50.3 (17.1) 3-92 |
| Number of generations | 3.2 (0.76) 2-6 |
| Number of family members | 16.0 (7.0) 3-40 |
| Number of female family members | 6.9 (3.5) 2-16 |
| Number of male family members | 9.1 (5.0) 2-30 |
| Number of studied families | 390 |
SD, standard deviation; y, years.
Figure 1. Study flowchart including the number of evaluated families which relevant information were obtained in each step of the data collection process.
Based on the analysis of relatedness, age of disease onset, primary tumor sites and clusters, as determined by internationally established criteria for familial cancer susceptibility syndromes, we identified 81 (64.8%) families with sporadic cancer cases, and 44 (35.2% within the families affected by cancer, or 11.3% of the overall study sample) with potential familial susceptibility to cancer. Regarding the hereditary cancer syndromes, we found four families that met the criteria for breast and ovary hereditary cancer, three for Li-Fraumeni syndrome, two families with syndromes that comprised hereditary colorectal cancer, another two families with breast and colon cancer, three families with potential alterations on repair genes, one family probably carrier of hereditary gastric cancer syndrome, and others with familial cancer clusters without a specific inheritance pattern.
Respondents reported cases of breast (n = 29), prostate (n = 29), pelvic (n = 16), colorectal (n = 14), and hematologic (n = 12) cancers, as well as melanoma (n = 6), tumors that are commonly associated with hereditary cancer syndromes (Table 3). Importantly, these results point to the existence of previously unidentified families (11.3% of the overall studied sample healthy population) potentially at risk for familial susceptibility to cancer.
Table 3. Distribution of malignant neoplasms according to the casuistry of cancer in the affected families.
| Frequency (n) | Total | |||
|---|---|---|---|---|
| Sites of malignant neoplasms | Sporadic | Familial | Potentially hereditary | Neoplasms |
| Head and neck | 31 | 10 | 6 | 47 |
| Lung | 15 | 8 | 7 | 30 |
| Breast | 13 | 9 | 7 | 29 |
| Prostate | 11 | 5 | 13 | 29 |
| Stomach | 5 | 9 | 8 | 22 |
| Pelvic | 2 | 7 | 7 | 16 |
| Colorectal | 4 | 5 | 5 | 14 |
| Womb | 8 | 5 | 1 | 14 |
| Liver | 7 | 2 | 4 | 13 |
| Hematological | 4 | 1 | 7 | 12 |
| Non-melanoma skin | 4 | 6 | 1 | 11 |
| Melanoma | 2 | 2 | 2 | 6 |
| Bone | 5 | 1 | 0 | 6 |
| Pancreas | 3 | 0 | 0 | 3 |
| Esophagus | 1 | 1 | 1 | 3 |
| Ovary | 1 | 0 | 0 | 1 |
| Unknown | 8 | 2 | 10 | 20 |
| Others | 7 | 7 | 14 | 28 |
| Total | 131 | 80 | 93 | 304 |
We next investigated whether self-reported family history is sufficiently consistent for use in genetic risk estimation for inherited cancer. For this, five years later we revaluated 14/20 families that we previously identified as having at least one first- and one second-degree relative affected by cancer and presenting moderate (n = 4) to high (n = 16) risk for developing cancer based on genetic predisposition (Figure 1). Analysis of family history collected in the second interview confirmed 90% of initial pedigrees, regardless of whether the interviewed family member was the same subject (n = 11) from the first interview or not (n = 3). In addition, we observed new cancer red flags in these families, such as benign lesions at early ages and new cancer cases that reinforce the family's high risk for hereditary cancer syndromes (Lindor et al., 2008).
Discussion
Recognizing patterns of familial cancer that indicate increased risk and possible hereditary syndromes can help to identify individuals who may benefit from preventive interventions (Ashton-Prolla, 2013). In this study we used self-reported family history data to examine the risk of hereditary cancer syndromes among middle- and low-income families registered at primary care centers in Brazil. We also revaluated the cancer family history of families at risk five years later to determine the consistency of the information collected through the use of spontaneous self-reports.
Our results showed that among those families affected by cancer, 35.2% had moderate to high risk of familial susceptibility to cancer. This represents a relatively high prevalence of potential hereditary cancer syndromes in the study sample. Likewise, a significant prevalence (6.2%) of hereditary breast cancer syndromes have been reported in a previous study in which CFH was assessed in women from primary health care units of an underserved region in southern Brazil (Palmero et al., 2009). These results point to the likely - yet often neglected - benefits of screening policies aimed at identifying individuals at risk in these settings (Doerr and Teng, 2012; Teng and Acheson, 2014). A pioneering initiative to identify individuals with an increased risk for hereditary breast cancer syndromes was conducted through the successfully development and validation of a simple questionnaire, which is sensitive and specific in primary care setting, as a screening tool to refer at-risk individuals for genetic counseling (Ashton-Prolla et al., 2009).
Given that cancer family history is a dynamic measure that changes significantly over time, its periodic reassessment has been recommended (Lu et al., 2014; Wood et al., 2014). Upon revaluation, we found that cancer family history was confirmed, and expanded, for 90% of the families interviewed. In some cases, we collected data regarding benign lesions that are often found on the clinical spectrum of the hereditary cancer syndromes (Lindor et al., 2008; Lu et al., 2014; NCCN, 2014). These data point to the reliability of self-reports as a means of early identification of healthy individuals at risk of cancer. Indeed, literature data have shown that self-reports of personal cancer history are generally reliable, especially for breast, prostate, and colon cancer (Roth et al., 2009; Scheuner et al., 2010). Most sensitivity values for self-reports of a positive family history of cancer in a first-degree relative range from 70 to 90% (Ziogas and Anton-Culver, 2003; Murff et al., 2007). Our study demonstrated that, even after a long period of time, self-reported CFH is an effective mean to detect not only breast, prostate, and colon cancer, but other important tumors that are considered as part of the spectrum of the hereditary cancer syndromes, such as Li-Fraumeni syndrome, which has shown considerable prevalence in the Brazilian population (Achatz et al., 2007; Giacomazzi et al., 2013).
The inclusion of new health technologies, including genetic risk assessment and genetic testing, remains a challenge for middle-income countries like Brazil and other Latin American countries (Palmero et al., 2009). Therefore, the possibility to use self-reports to unveil familial susceptibility to cancer, as revealed here, may be particularly beneficial in those settings characterized by a lack of electronic health records or individual health history. Notably, no population-based notification system exists in the Primary Health Care Brazilian database (Vieira et al., 2015). It should be emphasized that none of the 20 families that fulfilled the established criteria for familial cancer susceptibility syndromes, and that were identified as having moderate to high risk to hereditary cancer syndromes (Lindor et al., 2008; Valdez et al., 2010; NCCN, 2014; Vieira et al., 2015), had their CFH registered in their medical records at the community-based primary care centers where they were followed.
Although cancer management has been predominantly focused on the individuals affected by disease, not on their families, our results indicate that better management may be achieved by including family screening practices in primary care policies. Therefore, to enhance family practices and adherence to health policies, primary care work force should receive training and ongoing education, which should include essential competencies to collect family history, such as, basic genetics and genomics knowledge, communication skills, ability to establish empathetic interpersonal relationships, and capacity to deal with relevant ethical issues (Flória-Santos et al., 2013).
Even though barriers to incorporating family history taking and hereditary risk assessment in middle-income primary care settings do exist, the wider use of self-reported cancer family history can be a useful tool to achieve this goal.
Acknowledgments
This work was supported by a grant from FAPESP (07/08421-4). The authors would like to thank the staff of the community-based primary care centers for help with participant recruitment, as well as the participants who generously gave their time to take part in this study.
Footnotes
Associate Editor: Patricia Ashton-Prolla
References
- Achatz MI, Olivier M, Le Calvez F, Martel-Planche G, Lopes A, Rossi BM, Ashton-Prolla P, Giugliani R, Palmero EI, Vargas FR, et al. The TP53 mutation, R337H, is associated with Li-Fraumeni and Li-Fraumeni-like syndromes in Brazilian families. Cancer Lett. 2007;245:96–102. doi: 10.1016/j.canlet.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Ashton-Prolla P. Hereditary cancer syndromes: Opportunities and challenges. BMC Proc. 2013;7:K14–K14. doi: 10.1186/1753-6561-7-S2-K14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton-Prolla P, Giacomazzi J, Schmidt AV, Roth FL, Palmero EI, Kalakun L, Aguiar ES, Moreira SM, Batassini E, Belo-Reyes V, et al. Development and validation of a simple questionnaire for the identification of hereditary breast cancer in primary care. BMC Cancer. 2009;9:e283–e283. doi: 10.1186/1471-2407-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr M, Teng K. Family history: Still relevant in the genomics era. Cleve Clin J Med. 2012;79:331–336. doi: 10.3949/ccjm.79a.11065. [DOI] [PubMed] [Google Scholar]
- Feerro WG, Bigley MB, Brinner KM, The Family, Health History Multi-Stakeholder Workgroup of the American Health Information Community New standards and enhanced utility for family health history information in the electronic health record: An update from the American Health Information Community's Family Health History Multi-Stakeholder Workgroup. J Am Med Inform Assoc. 2008;15:723–728. doi: 10.1197/jamia.M2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floria-Santos M, Santos EMM, Nascimento LC, Pereira-da-Silva G, Ferreira BR, Miranda DO, Lopes LC, Junior, Pinto PS. Oncology nursing practice from the perspective of genetics and genomics. Text Context Nursing. 2013;22:526–533. [Google Scholar]
- Giacomazzi J, Selistre SG, Rossi C, Alemar B, Santos-Silva P, Pereira FS, Netto CB, Cossio SL, Roth DE, Brunetto AL, et al. Li-Fraumeni and Li-Fraumeni-like syndrome among children diagnosed with pediatric cancer in Southern Brazil. Cancer. 2013;119:4341–4349. doi: 10.1002/cncr.28346. [DOI] [PubMed] [Google Scholar]
- Gomy I, Diz MDPE. Hereditary cancer risk assessment: Essential tools for a better approach. Hered Cancer Clin Pract. 2013;11:16–16. doi: 10.1186/1897-4287-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens AC, Henneman L, Detmar SB, Khoury MJ, Steyerberg EW, Eijkemans MJ, Mushkudiani N, Oostra BA, van Duijn CM, Mackenbach JP. Accuracy of self-reported family history is strongly influenced by the accuracy of self-reported personal health status of relatives. J Clin Epidemiol. 2012;65:82–89. doi: 10.1016/j.jclinepi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Shedlosky-Shoemaker R, Porter K, Remy A, DeSimone P, Andrykowski MA. Cancer family history reporting: Impact of method and psychosocial factors. J Genet Couns. 2007;16:373–382. doi: 10.1007/s10897-006-9076-x. [DOI] [PubMed] [Google Scholar]
- Lindor NM, McMaster ML, Lindor CJ, Greene MH, National Cancer Institute Division of Cancer Prevention and Community Oncology and Prevention Trials Research Group Concise handbook of familial cancer susceptibility syndromes. Second edition. J Natl Cancer Inst Monogr. 2008;38:1–93. doi: 10.1093/jncimonographs/lgn001. [DOI] [PubMed] [Google Scholar]
- Lu KH, Wood ME, Daniels M, Burke C, Ford J, Kauff ND, Kohlmann W, Lindor NM, Mulvey TM, Robinson L, et al. American society of clinical oncology expert statement: Collection and use of a cancer family history for oncology providers. J Clin Oncol. 2014;32:833–840. doi: 10.1200/JCO.2013.50.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murff HJ, Greevy R, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10:174–180. doi: 10.1159/000101759. [DOI] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Principles of Biostatistics. 2nd edition. Thomson Pioneira; São Paulo: 2004. 522 p [Google Scholar]
- Palmero EI, Caleffi M, Schüler-Faccini L, Roth FL, Kalakun L, Netto CBO, Skonieski G, Giacomazzi J, Weber B, Giugliani R, et al. Population prevalence of hereditary breast cancer phenotypes and implementation of a genetic cancer risk assessment program in southern Brazil. Genet Mol Biol. 2009;32:447–455. doi: 10.1590/S1415-47572009005000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plat AW, Kroon AA, Van Schayck CP, De Leeuw PW, Stoffers HE. Obtaining the family history for common, multifactorial diseases by family physicians. A descriptive systematic review. Eur J Gen Pract. 2009;15:231–242. doi: 10.3109/13814780903447572. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Carroll JC, Wilson B, Santaguida P, Allanson J, Brouwers M, Raina P. The current state of cancer family history collection tools in primary care: A systematic review. Genet Med. 2009;11:495–506. doi: 10.1097/GIM.0b013e3181a7e8e0. [DOI] [PubMed] [Google Scholar]
- Roth FL, Camey SA, Caleffi M, Schuler-Faccini L, Palmero EI, Bochi C, Moreira SM, Kalakun L, Giugliani R, Ashton-Prolla P. Consistency of self-reported first-degree family history of cancer in a population-based study. Fam Cancer. 2009;8:195–202. doi: 10.1007/s10689-008-9228-2. [DOI] [PubMed] [Google Scholar]
- Rubinstein WS, Acheson LS, O’Neill SM, Ruffin MT, 4th, Wang C, Beaumont JL, Rothrock N, Family Healthware Impact Trial Group Clinical utility of family history for cancer screening and referral in primary care: A report from the Family Healthware impact trial. Genet Med. 2011;13:956–965. doi: 10.1097/GIM.0b013e3182241d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California health interview survey. Genet Med. 2010;12:726–735. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- Schneider K. Counseling About Cancer: Strategies for Genetic Counselors. 2nd edition. Wiley-Liss; New York: 2002. 333 p [Google Scholar]
- Teng K, Acheson LS. Genomics in primary care practice. Prim Care. 2014;41:421–435. doi: 10.1016/j.pop.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: A genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- Vieira DK, Attianezi M, Esposito AC, Barth A, Sequeira C, Krause N, Oliveira V, Lucidi A, Serao C, Llerena JC., Jr Identification of familial clustering for cancer through the family health strategy program in the municipality of Angra dos Reis, Rio de Janeiro, Brazil. J Community Genet. 2015;6:9–16. doi: 10.1007/s12687-014-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JN, Blazer KR, Mac Donald DJ, Culver OJ, Offit K. Genetics, genomics and risk assessment: State of the art and future directions in the era of personalized medicine. CA Cancer J Clin. 2011;61:327–359. doi: 10.3322/caac.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, Neuss MN, Hughes KS. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measures as part of the American Society of Clinical Oncology Quality Oncology Practice Initiative. J Clin Oncol. 2014;32:824–829. doi: 10.1200/JCO.2013.51.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziogas A, Anton-Culver H. Validation of family history data in cancer family registries. Am J Prev Med. 2003;24:190–198. doi: 10.1016/s0749-3797(02)00593-7. [DOI] [PubMed] [Google Scholar]
Internet Resources
- NCCN National Comprehensive Cancer Network. 2014. [accessed November 17, 2014]. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection.