Abstract
With the introduction of newborn hearing screening, infants are being diagnosed with hearing loss during the first few months of life. For infants with a sensory/neural hearing loss (SNHL), the audiogram can be estimated objectively using auditory brainstem response (ABR) testing and hearing aids prescribed accordingly. However, for infants with auditory neuropathy spectrum disorder (ANSD) due to the abnormal/absent ABR waveforms, alternative measures of auditory function are needed to assess the need for amplification and evaluate whether aided benefit has been achieved. Cortical auditory evoked potentials (CAEPs) are used to assess aided benefit in infants with hearing loss; however, there is insufficient information regarding the relationship between stimulus audibility and CAEP detection rates. It is also not clear whether CAEP detection rates differ between infants with SNHL and infants with ANSD. This study involved retrospective collection of CAEP, hearing threshold, and hearing aid gain data to investigate the relationship between stimulus audibility and CAEP detection rates. The results demonstrate that increases in stimulus audibility result in an increase in detection rate. For the same range of sensation levels, there was no difference in the detection rates between infants with SNHL and ANSD.
Keywords: Cortical auditory evoked potentials, auditory neuropathy spectrum disorder, infants
Learning Outcomes: As a result of this activity, the participant will be able to (1) describe the relationship between stimulus audibility and the likely presence of a cortical auditory evoked potential in infants with hearing loss, and (2) identify both the benefits and limitations of CAEP testing when used to help manage infants with hearing loss.
Auditory neuropathy spectrum disorder (ANSD) is a hearing loss characterized by elevated or absent auditory brainstem response (ABR) waveforms with evidence of normal cochlear outer hair cell (OHC) function. Surviving OHC function is demonstrated by the presence of otoacoustic emissions (OAEs) and/or the cochlear microphonic.1 2 The prevalence of ANSD is not as low as previously thought with ∼10% of children with congenital hearing loss presenting with the diagnostic features.3 4 5 6
The introduction of newborn hearing screening means that infants with ANSD are being diagnosed within the first few months of life. For infants with a sensory/neural hearing loss (SNHL), the audiogram can be estimated objectively based on wave V thresholds obtained using ABR testing. This information can then be used to determine the appropriate hearing aid fitting for each infant. However, threshold determination using ABR cannot be used for infants with ANSD due to the absence of a clearly defined wave V.
Cortical auditory evoked potentials (CAEPs) are used in clinical practice to assist in the management of infants with hearing loss.7 CAEPs are a series of waves recorded on the scalp that represent the summed neural activity in response to sound at the level of the auditory cortex. In infants, the CAEP is typically dominated by a positive-polarity peak with a latency of around 200 milliseconds and can be recorded from infants within the first few months of life.8 9 10 11
Use of magnetoencephalographic measures generators of P1 in newborns have been localized to auditory centers in the temporal lobe.12 The peak amplitude of P1 of the infant CAEP is relatively large and can be anywhere from 5 to 15 µV depending on the stimulus used,13 compared with ∼0.5 µV for wave V of the ABR.14 Furthermore, the CAEP requires less neural synchrony, with the response extending into hundreds of milliseconds, compared with the ABR, which exhibits small peaks occurring every 1 to 2 milliseconds. This means that small disruptions in neural synchrony can result in abnormalities/absence of the ABR waveforms, but the CAEP waveforms may be preserved. The larger amplitude and lower temporal precision makes CAEPs a particularly relevant measure to consider for the assessment and management of infants with ANSD.
Cone et al investigated the relationship between stimulus sensation level (SL) and the detection of different components of the CAEP in infants with normal hearing using 50-millisecond duration tone burst and speech stimuli presented at a rate of 0.5 per second.13 The N1 component of the CAEP was the most reliably present, with detection rates of 100% at moderate stimulus levels (60 dB sound pressure level [SPL]), and 85% at 30 dB SPL. In comparison, the dominant positive peak (labeled as P2 by Cone et al13) had detection rates of 91 and 77% at the same stimulus levels.
In other studies, an increase in stimulus SL has also been shown to increase the detection rate of CAEPs in infants with normal hearing and SNHL.15 16 For infants with normal hearing, using the 30-millisecond speech stimuli /m/ and /t/ at a presentation rate of about once a second, Carter et al found that the detection rate increased from 27.3 to 54.5 and 77.3% at stimulus SLs of +10, +20, and +30 dB SL, respectively.15 A reanalysis of the Van Dun et al data into nonoverlapping SL ranges demonstrated higher detection rates in infants with SNHL compared with those with normal hearing.16 In these infants, the detection rate using the speech stimuli /m/ (30 milliseconds), /ɡ/ (21 milliseconds), and /t/ (30 milliseconds) increased from 64 to 72 and 77% at stimulus SLs of 1 to 10, 11 to 20, and >20 dB SL, respectively. It is important to note that CAEP responses were absent in over 20% of babies in these two studies, despite using stimulus SLs ≥20 dB SL. This means clinicians need to be cautious in how they interpret the results because an absent CAEP does not necessarily mean the infant is unable to hear the sound.
In infants with ANSD, Gardner-Berry et al reported detection rates of 35.7, 44, and 38.2% at stimulus SLs of 1 to 10, 11 to 20, and >20 dB SL, respectively.17 The detection rate at >20 dB SL was significantly lower than those reported for infants with SNHL by Van Dun et al16; however, there was no significant difference between the groups at lower SLs. CAEP testing was performed on different systems for each of these studies, which may explain some of the differences between results. Nevertheless, it raises the question of whether amplification may result in a deterioration of performance in some infants with ANSD. A greater understanding of the relationship between stimulus audibility and CAEP detection rates is needed to ensure CAEP results are interpreted appropriately when used in the clinical setting.
Rance et al performed CAEP testing in children with hearing loss using a 440-Hz tone burst (200 milliseconds) and the speech stimulus /dæd/ (200 milliseconds) presented at a rate of 0.75 per second at comfortable listening levels.18 For children with SNHL, the CAEP detection rate was 94% for 440 Hz and 83% for /dæd/. A lower detection rate was reported for children with ANSD of 61% for the 440 Hz tone and 61% for /dæd/. This was despite similar levels of stimulus audibility as calculated by the aided articulation index.18
Rance et al also reported a significant relationship between the presence or absence of CAEPs and speech perception scores in children with ANSD but not in children with SNHL.18 The average aided speech perception score for children with ANSD and absent CAEPs using the 440-Hz tone was 6% compared with 60% for those who had present CAEPs, and a similar relationship was seen for the stimulus /dæd/. This relationship is important because one of the unique features of ANSD is that some patients can present with speech discrimination scores that are poorer than expected given the degree of the hearing loss.18 19 20 21 22 A greater understanding about the relationship between CAEP detection and speech perception ability is also needed so clinicians can keep in mind the potential impact of both audibility and sound distortion on the presence or absence of a CAEP.
Given that infants with ANSD are being identified within the first few months of life and that concerns remain about the effectiveness of amplification for some infants in this population, an objective measure of aided benefit is needed to assist with optimal early management. This study aims to investigate the relationship between stimulus audibility and CAEP detection rate for different speech stimuli and to compare the results between infants with ANSD and SNHL. There are several systems available to record CAEP responses. To ensure consistency in recording parameters and analysis of the CAEP responses, data for this study were collected retrospectively from clinics using the HEARLab system (Frye Electronics, Tigard, OR). This system uses preprogrammed speech stimuli and automated statistical analysis of the responses recorded, making it possible to consolidate results across sites.
The primary research question examines whether the proportion of CAEPs detected (CAEP detection rate) increases with increasing stimulus SL for children with hearing loss (SNHL and ANSD). The secondary research question examines whether the relationship between the CAEP detection rate and SL varies according to whether children have SNHL or ANSD. Based on current knowledge, we hypothesize that the CAEP detection rate would increase with increasing stimulus SL for children with hearing loss. Second, we hypothesize that the CAEP detection rate would be lower for infants with ANSD compared with those with SNHL at equivalent SLs.
Methods
Participants
Data are reported for a sample of 46 children (SNHL = 29, ANSD = 17) drawn from clinical measurements conducted between 2008 and 2014 at Australian Hearing pediatric hearing centers across Australia (SNHL = 29, ANSD = 7) or the Children's Hearing Foundation (CHF) in Taiwan (SNHL = 0, ANSD = 10). All infants were diagnosed with a hearing loss at birth. The inclusion criteria were: infants with congenital hearing loss who underwent CAEP evaluations using the HEARLab system under 3 years of age with recordings of short speech sounds /m/, /ɡ/, or /t/ at one or more presentation levels. Table 1 gives the background information of participants.
Table 1. Participants' Background Information (n = 46).
| Variable | SNHL, n = 29 (61.7%) | ANSD, n = 17 (36.2%) |
|---|---|---|
| Sex | ||
| Male | 13 (44.8%) | 10 (58.8%) |
| Female | 16 (55.2%) | 7 (41.2%) |
| Degree hearing loss | ||
| Mild (20–40 dB) | 1 (3.4%) | 4 (23.5%) |
| Moderate (41–60 dB) | 13 (44.8%) | 6 (35.3%) |
| Severe to profound (>60 dB) | 15 (51.7%) | 7 (41.2%) |
The average age at CAEP testing was 6.6 months (standard deviation [SD] 2.9) for infants with SNHL and 11.2 months (SD 8.5) for infants with ANSD. The average age at which behavioral hearing thresholds were obtained was 11.8 months (SD 3.0) for infants with SNHL group and 16.1 months (SD 7.2) infants with ANSD.
Amplification
Amplification was provided by pediatric audiologists at Australian Hearing or the Children's Hearing Foundation. The SNHL group were fitted using the National Acoustic Laboratories Non-linear 1 (NAL-NL1) prescription algorithm based on estimated hearing thresholds converted from electrophysiologic measurements. The ANSD group was fitted to either the NAL-NL1 (n = 7) or Desired SL (DSL) (n = 10) prescription. The infant fitting protocol for ANSD of Australian Hearing was applied, which included the use of behavioral observation audiometry.23 Behavioral observation audiometry was performed using speech sounds and noise makers of known frequency content and intensity to determine whether infants were responding to sounds presented above age-appropriate levels and to establish whether responses to some frequencies were elevated in relation to others.
Stimuli and Equipment
Stimuli were presented and CAEPs were recorded using the HEARLab system (Frye Electronics, Tigard, Oregon). The test stimuli /m/, /ɡ/, and /t/ were the same as those used by Golding et al, and were chosen as they have spectral emphasis in the low (250 Hz), mid (1,250 Hz), and high frequencies (3,150 Hz), respectively.24 The duration of the stimuli was 30 milliseconds for /m/ and /t/ and 21 milliseconds for /ɡ/. Stimuli were presented with alternating onset polarity and interstimulus interval of 1125 milliseconds, presented via a loudspeaker positioned at 0 degrees azimuth, ∼1.8 m from the infant's head. Stimulus levels were calibrated at 75 dB SPL in the sound field using a built-in calibration method in the presentation system and a calibration microphone at subject position.
Recording electrodes were positioned at Cz referenced to the left mastoid and forehead as ground. EEG was amplified 1210 times and online filtered between 0.3 and 30 Hz. Artifact rejection occurred for each epoch between ± 110 µV.
Procedure
Tympanometry and otoscopy were performed and infants with middle-ear pathology were excluded from the analysis. Hearing aid coupler measurements were performed on the day of CAEP testing using speech-shaped noise at 55, 65, and 75 dB SPL.
CAEP testing took place in a sound booth at Australian Hearing or a quiet office room at CHF with the infant sitting on the caregiver's lap or in a high chair. To maintain the infant in a settled state, a distracter engaged with the baby using quiet toys and/or a children's DVD was played with the sound off. The three stimuli were interspersed during the presentation and initially delivered at 65 dB SPL. The intensity was increased to 75 dB SPL for stimuli where no CAEP response was evident, and it was decreased to 55 dB SPL for stimuli where a CAEP response was present at 65 dB SPL.
CAEP Analysis
The presence of CAEP responses was defined by an automated statistical criterion for generating probability levels (p values) as described by Golding et al and Carter et al, which is incorporated in the HEARLab system.15 24 In the present study, each speech stimulus was presented until the criterion for stopping EEG acquisition was met (p < 0.05). During the acquisition of EEG responses, the residual noise was monitored to assess the quality of the averaged CAEP responses. Recordings with residual noise levels lower than 3.2 µV were considered acceptable by the system.
Behavioral Testing
Behavioral thresholds were obtained using visual reinforcement audiometry to warble tones for at least two of the four stimuli 500, 1,000, 2,000, and 4,000 Hz. For some infants, behavior thresholds were obtained on the same day of CAEP testing, and for others, the thresholds were obtained at a later time when the infant was developmentally ready to perform this test.
The SLs of the CAEP stimuli were estimated as the maximum value (across frequency) of the one-third-octave spectral level of the stimuli minus the behavioral threshold level of the infant at the corresponding frequencies. The SL in the unaided condition was estimated by subtracting the hearing threshold from the stimulus presentation level. The SL of the speech stimulus in the aided condition was derived by adding the hearing aid 2-cc coupler gain and age-appropriate average real ear–to–coupler difference to the unaided SL. Hearing thresholds and stimulus presentation levels were both expressed in units of decibels of SPL in the ear canal in the computations.
Statistical Analyses
The detection rates of CAEP for different SLs were summarized using descriptive statistics. The research questions were investigated using analyses of variance. All statistical analyses were conducted using Statistica v10. In line with standard practice, a type I error rate of α = 0.05 (two-tailed) was adopted.
Results
Data collection was approved in Australia by the Australian Hearing Human Research Ethics Committee, and data collected at the CHF was approved by the Human Research Ethics Committee in Taiwan.
Audiograms
The mean four-frequency average hearing thresholds in the better ear were 67.7 dB HL (SD 22.6) for infants with SNHL, and 57.7 dB hearing level (HL) (SD 22.9) for infants with ANSD. The configuration of the hearing loss was predominantly flat for both groups (see Fig. 1). The breakdown for degree of hearing loss in each group is listed in Table 1.
Figure 1.
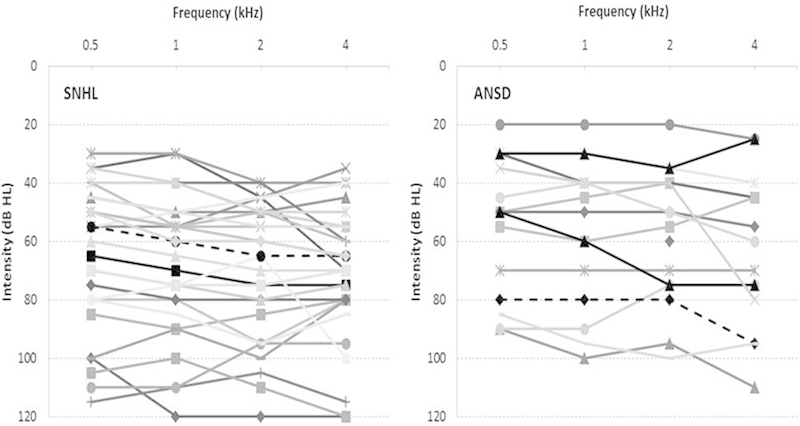
Individual pure tone audiograms for infants with sensory/neural hearing loss (SNHL) and auditory neuropathy spectrum disorder (ANSD).
Relationship between Stimulus Sensation Level and CAEP Detection Rate
Fig. 2 shows the p values for all CAEP recordings as a function of the estimated SL at which the stimuli /m/, /ɡ/, and /t/ were presented. The p values were generated by the automatic detection algorithm in the HEARLab system. Data points below the horizontal line marking p = 0.05 were deemed to have present CAEPs. Regression analysis of the estimated SL of the stimuli with p values (log-transformed) showed a significant relationship (R 2 = 0.17; F[1, 351] = 73.7, p < 0.0001). There was a greater certainty of CAEP detection with increase in SL.
Figure 2.
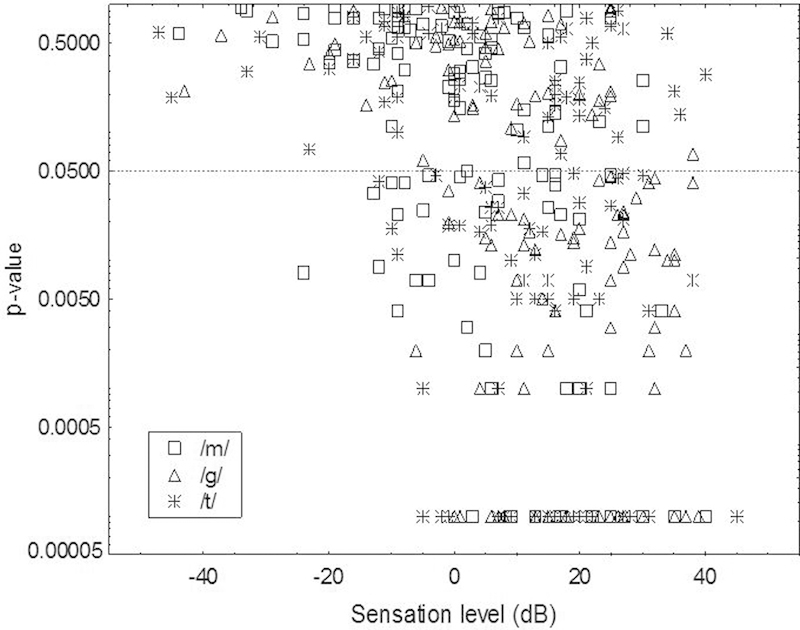
Probability level (p value) as a function of estimated sensation level of the stimuli for 353 cortical auditory evoked potential measures from 46 children. Open circles depict responses elicited using /m/, open triangles depict responses elicited using /t/, and asterisks depict responses elicited using /ɡ/ as stimuli. Note that p values are capped at 0.0001 for display. The dotted line represents the criterion for detection at p = 0.05. Data points below the dotted line were significant at the 5% probability level.
Fig. 3 shows the CAEP detection rates (percent of stimuli for which a CAEP was detected) for different SL ranges, separately for children with SNHL and those with ANSD in this study. Reanalyzed data from 22 SNHL children reported by Van Dun et al and from 12 children with ANSD reported by Gardner-Berry et al are also shown.16 17
Figure 3.
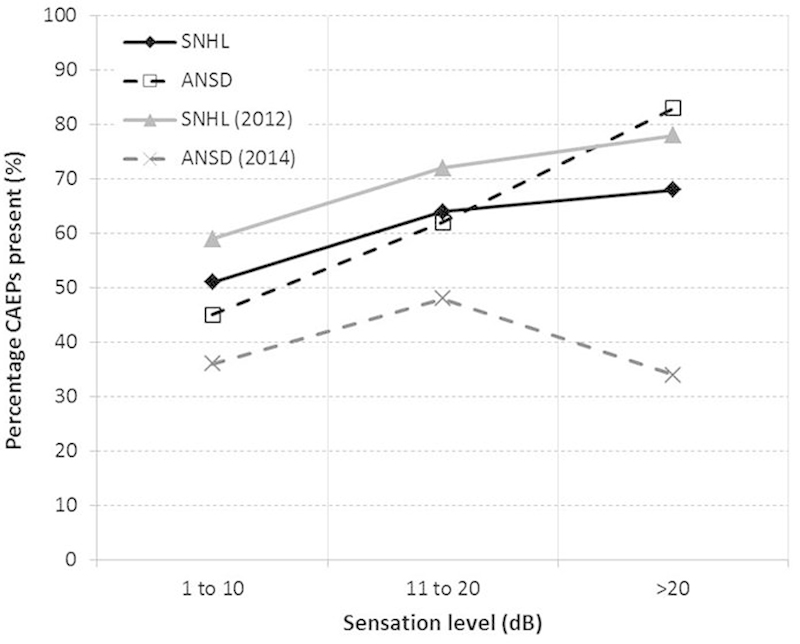
Percentage of cortical auditory evoked potentials (CAEPs) present for three ranges of estimated sensation level. Filled diamonds depict data for 29 infants with sensory/neural hearing loss (SNHL; 243 CAEP measures), and open squares show data for 17 infants with auditory neuropathy spectrum disorder (ANSD; 98 CAEP measures) from the current study. Filled triangles depict data for 22 infants (81 CAEP measures) reported in Van Dun et al.16 Crosses depict data from 12 infants with ANSD (72 CAEP measures) reported in Gardner-Berry et al.17
Regarding the research question of whether the relationship between stimulus SL and CAEP detection was different between children with SNHL and those with ANSD, an analysis of variance was conducted using p values (log-transformed) as the dependent variable, hearing loss type (SNHL versus ANSD), estimated SL (low [1 to 10 dB], mid [11 to 20 dB], high [>20 dB]), and stimuli (/m/ /ɡ/ /t/) as categorical variables. The main effect of SL was significant (F[2, 225] = 3.98, p = 0.02). There were no other significant main effects or interactions.
Discussion
CAEPs are being used in clinical practice to assess aided benefit in infants fitted with hearing aids at a very young age. The aim of this study was to investigate the relationship between estimated SL and CAEP detection rate and to determine whether detection rates differed between infants with SNHL and infants with ANSD.
The results from this study showed that there was a significant increase in CAEP detections with an increase in SL (p < 0.0001). These findings are consistent with previous studies on infants with SNHL,16 25 infants with normal hearing,13 15 and children with SNHL.26 27
Although Cone et al reported increasing detection rates with increasing stimulus intensity, their overall detection rates were considerably higher.13 In the current study, the detection rate was 68.3% for infants with SNHL and 82.6% for infants with ANSD using stimulus SLs >20 dB (21 to 40 dB SL). This compares to over 90% detection in infants with normal hearing using similar SLs (40 dB SPL) reported by Cone et al.13
There are three possible explanations for the differences in detection rate between the current study and that described by Cone et al.13 First, the studies differed in the method of response detection. In the current study, a statistical detection technique was based on a Hotelling's T2 analysis of the amplitude of the response in 50 milliseconds time bins across a window of 450 milliseconds from 101 to 550 milliseconds poststimulus onset. This compares to Cone et al, who used a rule-bound visual detection method that allowed for independent identification of single peaks P1, N1, P2, and N2.13 With the latter method, the detection rate for N1 was the highest at 97% for a 40 dB SPL stimulus, compared with 78% for the positive peak that followed.
The second difference between the studies related to the stimuli used. The current study collapsed the results for three consonants (/m/, /ɡ/, /t/) compared with Cone et al, who collapsed the results for up to five stimuli, with a heavier weighting toward the stimulus /a/.13 Although the results for individual stimuli were not reported, individual examples of waveforms to the stimulus /a/ were 5 µV higher than the example provided for /m/, and N1 was particularly pronounced, which may have contributed to the higher detection rate.
The third difference between the studies relates to the stimulus presentation rate. The current study used an interstimulus interval of 1,125 milliseconds compared with the slower 2,000 milliseconds used by Cone et al.13 Slower stimulus rates have been shown to enhance the amplitude of N1,10 which was one of the dominant features noted by Cone et al, and is therefore likely to have enhanced the detection rate.13
The results from this study showed that there was no significant difference in the CAEP detection rate between infants with SNHL and infants with ANSD. These findings are not consistent with those reported by Gardner-Berry et al, where the detection rate for infants with ANSD was significantly lower at audibility levels of +30 dB SL compared with the group of infants with SNHL.17 The mean age at CAEP testing was less than 12 months for both studies, so maturational changes do not explain the differences between studies.
The mean gestational age of the infants in the study by Gardner-Berry et al was 29 weeks (SD 4), and all the infants had suffered hypoxia.17 The medical history was not available for all infants in the current study, so it is not possible to determine whether the proportion of infants born prematurely or the cause of the ANSD may have differed between studies. Previous studies have shown an increase in amplitude and a decrease in latency of the CAEP over the first 3 months of life, and morphological changes continue to occur throughout childhood.8 A disproportionately higher degree of prematurity could potentially result in a lower CAEP detection rate. Hypoxia can cause selective damage to the inner hair cells, whereas other risk factors associated with ANSD such as severe jaundice can result in damage of neural structures in the auditory pathway.28 Differences in the underlying pathology could potentially result in different behaviors of the auditory system, which may also explain the unusual pattern of results seen in the study by Gardner-Berry et al.17
There are methodological differences between the studies, which may also explain the different results. CAEP recordings for infants with ANSD in the Gardner-Berry et al study were obtained using the Neuroscan (Compumedics, Charlotte, NC) system rather than HEARLab.17 Although the same electrode montage, stimuli, and Hotelling's T2 statistical analysis were used, HEARLab employs a more sophisticated artifact rejection system, which may have improved the signal-to-noise ratio and therefore the detection rate of the CAEP.
In the previous study, a single speech stimulus was presented until the minimum number of acceptable epochs was obtained, after which the next speech stimulus was presented. The HEARLab system changes the stimulus after every 30 epochs, and continues to do so until the minimum number of accepted epochs is obtained for each stimulus. Previous studies have reported a reduction in CAEP amplitude following repeated presentation of the same stimulus and enhancement of the CAEP when the stimulus characteristics are changed.29 The higher detection rate in the current study may therefore be due to the regular changes of stimulus type during the course of the test.
The 17 infants with ANSD in the current study were all tested binaurally, compared with 12 in the study by Gardner-Berry et al, where separate ear data were collected.17 Some of the infants in the previous study demonstrated present CAEPs in one ear but not the other, despite similar levels of audibility. Had binaural testing been performed in the previous study, a slightly higher detection rate may have been calculated based on the response from the “better” ear. However, the lower CAEP detection rate in the previous study was accompanied by lower scores on an assessment of functional auditory behavior as measured using the Parent Evaluation of Aural/Oral Performance in Children diary. This suggests that the discrepancy in detection rates between studies may be due to differences in the infant characteristics between the two groups.
The results from the current study demonstrate a significant relationship between SL and CAEP detection, suggesting that CAEP testing is a valid method of confirming response detection in infants fitted with hearing aids. Stimulus SLs of >10 dB resulted in CAEP detection rates of 66.4% for infants with SNHL and 71.2% for infants with ANSD.
Limitations
It is important to note that for 32% of infants with SNHL and 17% of infants with ANSD, the CAEP was absent despite the calculated audibility being >20 dB SL. Clinicians therefore need to take care not to assume an absent CAEP means the infant is unable to hear the sound. Similarly, the presence of a CAEP alone does not tell the clinician how audible the stimulus is to the infant. In some cases, the stimulus may only just be audible, but it could equally be 40 dB above threshold. More detailed CAEP testing techniques such as amplitude growth functions are required to determine whether there are features of the CAEP response that can provide information about the level of audibility. This information would be of great value to clinicians to assist in the optimal fitting of amplification as early as possible.
The other limitation to this study is that the method used to calculate SL in the current study was not always based on behavioral thresholds obtained at the time of CAEP testing, because not all infants were developmentally ready to perform visual reinforcement orientation audiometry (VROA) until a later time. This assumes that there had been no change in hearing thresholds between the time of CAEP testing and the measurement of behavioral thresholds, which is not necessarily the case. In future studies, it would be beneficial to obtain behavioral thresholds and CAEP recordings at the same time and to test each individual at different SLs to gain a greater understanding of how the auditory system responds with increasing SL in individuals with ANSD.
Conclusion
In conclusion, the present analysis of clinical data indicates a significant relationship between SL and CAEP detection rates, but no significant difference between infants with SNHL and ANSD.
References
- 1.Starr A, Picton T W, Sininger Y, Hood L J, Berlin C I. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- 2.Berlin C I Morlet T Hood L J Auditory neuropathy/dyssynchrony: its diagnosis and management Pediatr Clin North Am 2003502331–340., vii–viii [DOI] [PubMed] [Google Scholar]
- 3.Ching T YC, Day J, Dillon H. et al. Impact of the presence of auditory neuropathy spectrum disorder (ANSD) on outcomes of children at three years of age. Int J Audiol. 2013;52 02:S55–S64. doi: 10.3109/14992027.2013.796532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rance G, Beer D E, Cone-Wesson B. et al. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear. 1999;20(3):238–252. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sininger Y, Oba S. San Diego, CA: Singular; 2001. Patients with auditory neuropathy: who are they and what can they hear? pp. 15–35. [Google Scholar]
- 6.Sanyelbhaa Talaat H, Kabel A H, Samy H, Elbadry M. Prevalence of auditory neuropathy (AN) among infants and young children with severe to profound hearing loss. Int J Pediatr Otorhinolaryngol. 2009;73(7):937–939. doi: 10.1016/j.ijporl.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Punch S, Van Dun B, King A. et al. Clinical experience of using cortical auditory evoked potentials in the treatment of infant hearing loss in Australia. Semin Hear. 2016;37(01):36–52. doi: 10.1055/s-0035-1570331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasman J W, Rotteveel J J, Maassen B, Visco Y M. The maturation of auditory cortical evoked responses between (preterm) birth and 14 years of age. Eur J Paediatr Neurol. 1999;3(2):79–82. doi: 10.1053/ejpn.1999.0186. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzberg D, Hilpert P L, Kreuzer J A, Vaughan H G Jr. Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birthweight infants. Dev Med Child Neurol. 1984;26(4):466–475. doi: 10.1111/j.1469-8749.1984.tb04473.x. [DOI] [PubMed] [Google Scholar]
- 10.Wunderlich J L, Cone-Wesson B K, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006;212(1–2):185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Kushnerenko E, Ceponiene R, Balan P, Fellman V, Huotilaine M, Näätäne R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13(1):47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- 12.Huotilainen M, Kujala A, Hotakainen M. et al. Auditory magnetic responses of healthy newborns. Neuroreport. 2003;14(14):1871–1875. doi: 10.1097/00001756-200310060-00023. [DOI] [PubMed] [Google Scholar]
- 13.Cone B, Whitaker R. Dynamics of infant cortical auditory evoked potentials (CAEPs) for tone and speech tokens. Int J Pediatr Otorhinolaryngol. 2013;77(7):1162–1173. doi: 10.1016/j.ijporl.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards C G, Durieux-Smith A, Picton T W. Neonatal auditory brain stem responses from ipsilateral and contralateral recording montages. Ear Hear. 1985;6(4):175–178. doi: 10.1097/00003446-198507000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Carter L, Golding M, Dillon H, Seymour J. The detection of infant cortical auditory evoked potentials (CAEPs) using statistical and visual detection techniques. J Am Acad Audiol. 2010;21(5):347–356. doi: 10.3766/jaaa.21.5.6. [DOI] [PubMed] [Google Scholar]
- 16.Van B Dun, Carter L, Dillon H. Sensitivity of cortical auditory evoked potential detection for hearing-impaired infants in response to short speech sounds. Audiol Res. 2012;2(1):e13. doi: 10.4081/audiores.2012.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner-Berry K, Purdy S C, Ching T Y, Dillon H. The audiological journey and early outcomes of twelve infants with auditory neuropathy spectrum disorder from birth to two years of age. Int J Audiol. 2015;54(8):524–535. doi: 10.3109/14992027.2015.1007214. [DOI] [PubMed] [Google Scholar]
- 18.Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear Hear. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Kraus N, Bradlow A R, Cheatham M A. et al. Consequences of neural asynchrony: a case of auditory neuropathy. J Assoc Res Otolaryngol. 2000;1(1):33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng F G, Kong Y Y, Michalewski H J, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- 21.Berlin C I, Morlet T, Hood L J. Aurora, CO.: Bill Daniels Center for Children's Hearing; 2008. Management of individuals with auditory neuropathy spectrum disorder; pp. 35–40. [Google Scholar]
- 22.Berlin C I, Hood L J, Morlet T. et al. Multi-site diagnosis and management of 260 patients with auditory neuropathy/dys-synchrony (auditory neuropathy spectrum disorder) Int J Audiol. 2010;49(1):30–43. doi: 10.3109/14992020903160892. [DOI] [PubMed] [Google Scholar]
- 23.King A M. The national protocol for paediatric amplification in Australia. Int J Audiol. 2010;49 01:S64–S69. doi: 10.3109/14992020903329422. [DOI] [PubMed] [Google Scholar]
- 24.Golding M, Dillon H, Seymour J, Carter L. The detection of adult cortical auditory evoked potentials (CAEPs) using an automated statistic and visual detection. Int J Audiol. 2009;48(12):833–842. doi: 10.3109/14992020903140928. [DOI] [PubMed] [Google Scholar]
- 25.Chang H W, Dillon H, Carter L, van Dun B, Young S T. The relationship between cortical auditory evoked potential (CAEP) detection and estimated audibility in infants with sensorineural hearing loss. Int J Audiol. 2012;51(9):663–670. doi: 10.3109/14992027.2012.690076. [DOI] [PubMed] [Google Scholar]
- 26.Amatuzzi M, Liberman C M, Northrop C. Selective inner hair cell loss in prematurity: a temporal bone study of infants from a neonatal intensive care unit. J Assoc Res Otolaryngol. 2011;12(5):595–604. doi: 10.1007/s10162-011-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glista D Easwar V Purcell D et al. A pilot study on cortical auditory evoked potentials in children: aided CAEPs reflect improved high-frequency audibility with frequency compression hearing aid technology International Journal of Otolaryngology 2012.dx.doi.org/10.1155/2012/982894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinpelu O V, Waissbluth S, Daniel S J. Auditory risk of hyperbilirubinemia in term newborns: a systematic review. Int J Pediatr Otorhinolaryngol. 2013;77(6):898–905. doi: 10.1016/j.ijporl.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Woods D L, Elmasian R. The habituation of event-related potentials to speech sounds and tones. Electroencephalogr Clin Neurophysiol. 1986;65(6):447–459. doi: 10.1016/0168-5597(86)90024-9. [DOI] [PubMed] [Google Scholar]


