Abstract
Lung cancer is the leading cause of cancer mortality around the world. Despite advances in the targeted therapy, patients with lung squamous cell carcinoma(SCC) still benefit few from it, and the search for potential effective therapies is imperative. Here, we demonstrated that deguelin induced significant apoptosis of lung SCC cells in vitro. Importantly, we found deguelin down-regulated the expression of galectin-1, which was involved in a wide range of tumorous physiologic process. Thus, we both over-expressed and down-regulated galectin-1 to perform its role in deguelin-induced apoptosis. We found that increased galectin-1 attenuated apoptosis of SCC cells exposed to deguelin, while galectin-1 knockdown sensitized lung cancer cells to deguelin treatment. Additionally, we observed that down-regulation of galectin-1 resulted in suppression of Ras/Raf/ERK pathway which was involved in deguelin-induced cell apoptosis. We also found that deguelin had a significant anti-tumor ability with decline of galectin-1 in vivo. In conclusion, these findings confirm that deguelin may act as a new chemo-preventive agent through inducing apoptosis of lung SCC cells in a galectin-1 dependent manner.
Keywords: Deguelin, Lung squamous cell carcinoma, Apoptosis, Galectin-1
Introduction
Lung cancer represents one of the most frequently diagnosed cancers worldwide. The data from global cancer statistics indicate that lung cancer is the leading cause of cancer-associated death among males, and has surpassed breast cancer as the leading cause of female cancer mortality in many developed countries1. Approximate 80%-85% of lung cancers are categorized as non-small-cell lung cancer(NSCLC), which contains three main subtypes including adenocarcinoma, SCC and large cell carcinoma2. Although intensive efforts are devoted to improving lung cancer treatment, it still remains a worldwide dilemma for clinical therapy. Targeted therapies such as EGFR-TKIs have been widely applied in patients with adenocarcinoma, however, patients with lung SCC, which account for 30% of NSCLC, benefit few from the treatment. Therefore, potential alternative therapies for lung SCC are encouraging.
Deguelin, a rotenoid extracted from Derris trifoliate Lour. or Mundulea sericea, has been reported to be effective in inhibiting the proliferation, metastasis and invasion of various types of tumors3-6. Previous studies have shown that deguelin can induce the apoptosis of cancer cells by targeting the NF-κB pathway7-9 and PI3K/Akt pathway10, 11. In addition, deguelin has been demonstrated to mediate its chemopreventive effects through targeting cell cycle arrest12, 13 and anti-angiogenesis13, 14. Although the application of deguelin has been explored in several tumors15-17, the effects of deguelin on lung SCC and the cellular mechanisms are still not fully studied.
Recently, Hung-Sheng Shang et al. reported that deguelin could decrease the Ras protein level in human osteosarcoma cells5. H-Ras belongs to the RAS family of GTPases and contributes to numerous cellular functions including control of cell apoptosis and proliferation18. Several reports have shown that H-Ras biological activity requires membrane anchorage which can be strengthened by galectin-119, a β‑galactoside-binding protein, which had been reported to be highly secreted in lots of malignancies and was widely involved in tumorous physiologic processes such as cell proliferation, adhesion, migration and apoptosis 20-24. Increasing clinical and preclinical evidences have confirmed that galectin-1 is correlated with poor prognosis in a variety of tumors25-27, and galectin-1 inhibition may directly lead to anti-proliferation in cancer cells, indicating that galectin-1 may be a promising drug target for cancer treatment28. Although various possible mechanisms related to degelin-induced apoptosis have been proposed, yet the function of galectin-1 in deguelin-treated cancer cells remains to be elucidated.
In the present study, we focused on the potential effects of deguelin on human lung SCC and identified that deguelin can be an apoptosis inducer for lung SCC cells. It is noteworthy that we found deguelin reduced the expression of galectin-1. For further study, we knocked down and overexpressed galectin-1, respectively, and found the expression of galectin-1 was related to apoptotic effect of deguelin by regulating the Ras/Raf/ERK pathway. These results indicated that deguelin could induce apoptosis of lung SCC through regulating expression of galectin-1. Our preliminary studies innovatively explore the role of galectin-1 in deguelin-induced tumor cells apoptosis, and contribute to better understanding with the effect of deguelin on lung SCC cells.
Materials and Methods
Cell Lines and Reagents
Human lung SCC cell SK-MES-1 was purchased from the Committee on Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). NCI-H520 was a kind gift from Dr. Ying (Department of Respiratory Diseases, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China). Both cell lines were maintained in RPMI-1640 (Gibco BRL Co.Ltd., MD, USA), supplemented with 10% fetal bovine serum (FBS, Gibco BRL Co.Ltd., MD, USA), 100U/ml penicillin and 100μg/ml streptomycin (Gibco BRL Co.Ltd., MD, USA) at 37℃ with 5% CO2. Deguelin was obtained from Sigma (St. Louis, MO, USA). Galectin-1 shRNA plasmid, negative control shRNA plasmid, shRNA plasmid transfection reagent and shRNA plasmid transfection medium were purchased from Santa Cruz Biotechnology (CA, USA). Galectin-1 expression plasmid pCMV3-SP-N-His-Gal-1 and empty vector pCMV3-SP-N-His plasmid were from Sino Biological Inc. (Beijing, China). OPTI-MEM was purchased from Gibco BRL Co.Ltd. (MD, USA) and HiPerFect transfection reagent was obtained from Qiagen (Hilden, Germany). Antibodies against rabbit Galectin-1, H-Ras, Raf-1, p-Raf-1 and Raf kinase inhibitor L779450 were purchased from Abcam (Cambridge, MA, USA). GAPDH, Bax, Bcl-2, cleaved caspase-3, cleaved caspase-9, phospho-p44/42 MAPK(T202/Y204), p44/42 MAPK antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell Viability Assay
Cells were seeded in a 96-well plate at a density of 4×103/well. Different concentrations of deguelin were added into each well, and incubated for 12, 24, 48 and 72 hours, respectively. After incubation, WST-8 (Dojindo Laboratories, Tokyo, Japan) was added into each well. The absorbance of solutions was measured spectrophotometrically at 450nm with an automatic microplate analyzer after incubation for an additional two hours.
Cell Apoptosis Assay
With deguelin treatment for 24 hours, cells were harvested and detected for apoptosis by using FITC Annexin V Apoptosis Detection Kit (BD Biosciences, NJ, USA) according to the manufacture's protocols. Briefly, cells were washed twice with cold PBS and resuspended in binding buffer at a concentration of 1×106 cells/ml. Then, cells were stained with 5ul of FITC Annexin V and 5ul of Propidium Iodide followed by incubation for 15 minutes in dark at room temperature. Finally, cells apoptosis were analyzed by BD FACSVerse (BD Biosciences, NJ, USA).
Quantitative real-time PCR
Total cellular RNA was extracted by using RNeasy Mini Kit (Qiagen, Hilden, Germany) in accordance to the manufacturer's protocol and the cellular RNA concentration was measured by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA). The reverse transcription into complementary DNA was performed by using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China), and for quantitative RT-PCR analysis, cDNA amplification was performed by QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany). The comparative Ct method (2-ΔΔCt) was used to analyze data. The specific primers of RT-PCR are shown in Table 1.
Table 1.
Primer sequences used for real-time PCR
| mRNA | Oligonucleotides (5' to 3') |
|---|---|
| Bax-F | 5-CCCGAGAGGTCTTTTTCCGAG-3 |
| Bax-R | 5-CCAGCCCATGATGGTTCTGAT-3 |
| Bcl-2-F | 5-GGTGGGGTCATGTGTGTGG-3 |
| Bcl-2-R | 5-CGGTTCAGGTACTCAGTCATCC-3 |
| Galectin-1-F | 5-TCGCCAGCAACCTGAATCTC-3 |
| Galectin-1-R | 5-GCACGAAGCTCTTAGCGTCA-3 |
| GAPDH-F | 5-GGAGCGAGATCCCTCCAAAAT-3 |
| GAPDH-R | 5-GGCTGTTGTCATACTTCTCATGG-3 |
Immunoblotting
Cells were lysed with lysis buffer, the lysate supernatants were harvested in 1.5ml microcentrifuge tubes and boiled for 10 minutes in loading buffer. Protein concentration was measured by using Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA) according to the manufacture's instructions. Cell lysates were subjected to SDS-PAGE, and then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) for immunoblotting analysis. After blocking with 5% nonfat milk for 1 hour, membranes were incubated with the appropriate primary antibodies overnight at 4℃ followed by incubation with the corresponding secondary antibody for 2 hours at room temperature. Immunoreactive bands were visualized using the enhanced chemiluminescence (ECL) detection system.
Cell Transfection
NCI-H520 and SK-MES-1 cells were seeded in six-well plates before transfection. In order to down-regulate galectin-1 expression, NCI-H520 and SK-MES-1 cells were transfected with galectin-1 shRNA or negative control shRNA using shRNA plasmid transfection reagent according to the procedures provided by Santa Cruz Biotechnology (CA,USA). For galectin-1 overexpression, 0.7μg pCMV3-SP-N-His-Gal-1 plasmid or negative control pCMV3-SP-N-His plasmid and 20μl HiPerFect transfection reagent were diluted separately in 40μl OPTI-MEM at room temperature for 5 minutes. The two mixtures were re-mixed and incubated for 30 minutes. Finally, the complex was added to the wells along with 2ml of medium. After incubation for another 48 hours, western blotting was performed to test the expression level of galectin-1 to confirm transfection efficiency. In order to obtain stable transfected cell lines, the positive transfectants were selected and expanded for further study.
Colony Formation Assay
Cells transfected with shGal-1, negative control shRNA, galectin-1 plasmid vector and empty plasmid vector were counted and seeded in six-well plates at a density of 400 cells/well. After incubation of 24 hours, the cells were treated with 10μM deguelin. Meanwhile, the culture medium was refreshed every three days. After 14 days, the cells were stained with crystal violet, and the number of colonies was counted only if they contained more than 50 cells. The following equation: (number of colonies/ number of seeded cells) ×100% was performed for the rate of colony formation29.
Co-immunoprecipitation Assay
Mock- or deguelin-treated cells were washed twice with cold PBS and lysed with NP-40 lysis buffer (50mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol) with 1mM PMSF for 30 minutes. The lysate was centrifuged at 12000 rpm at 4℃ for 10 minutes and the supernatants were incubated with primary antibodies or non-specific IgG as a negative control at 4℃ overnight, then the Protein A+G Sepharose beads were applied and rotated for 3 hours at 4℃. The immunocomplexes were washed for 5 times with lysis buffer followed by boiling with SDS-PAGE buffer and then analyzed by western blotting.
Animal Experiments
Six-week-old female BALB/c-nude mice obtained from Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) were used for human tumor xenograft models. After one week's acclimatization, they were randomized and allocated into groups of six mice. The control group was injected subcutaneously into the left armpit with 3.5×106 NCI-H520 cells per mouse. Other groups were injected with an equal number of NCI-H520 cells, NCI-H520 cells transfected with galectin-1 plasmid vector or NCI-H520 cells transfected with empty plasmid vector, respectively. When palpable tumors (~200-300mm3) arose, the control group was orally treated with physiological saline, while other groups were treated with deguelin (4 mg/kg) by oral gavage on days 1, 3 and 5 of each week for three weeks. Tumor size was measured by caliper through measurements of the two perpendicular diameters every three days using the formula: Volume = (width2 × length)/2. All procedures were performed according to the Regulations for the Administration of Affairs Concerning Experimental Animals. The experiments were approved by the State Council of the People's Republic of China and the Experimental Animal Ethics Committee of Zhejiang University.
Statistical Analysis
All data are presented as mean ± SD of three independent experiments. SPSS 19.0 (SPSS Inc., Chicago, IL, USA) were used for statistical analysis. Data were analyzed using the Student's t-test, and significance was chosen as P values < 0.05.
Results
Deguelin induced the apoptosis of human lung SCC cells
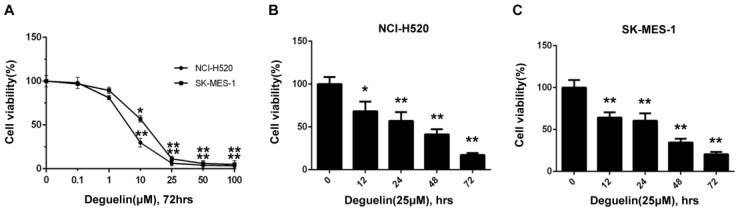
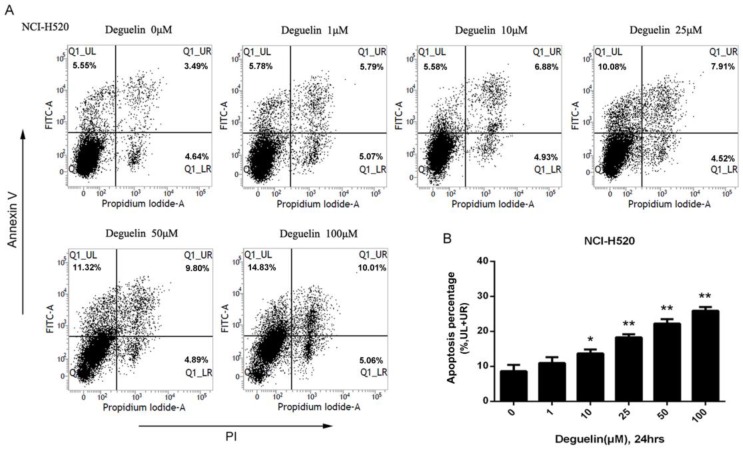
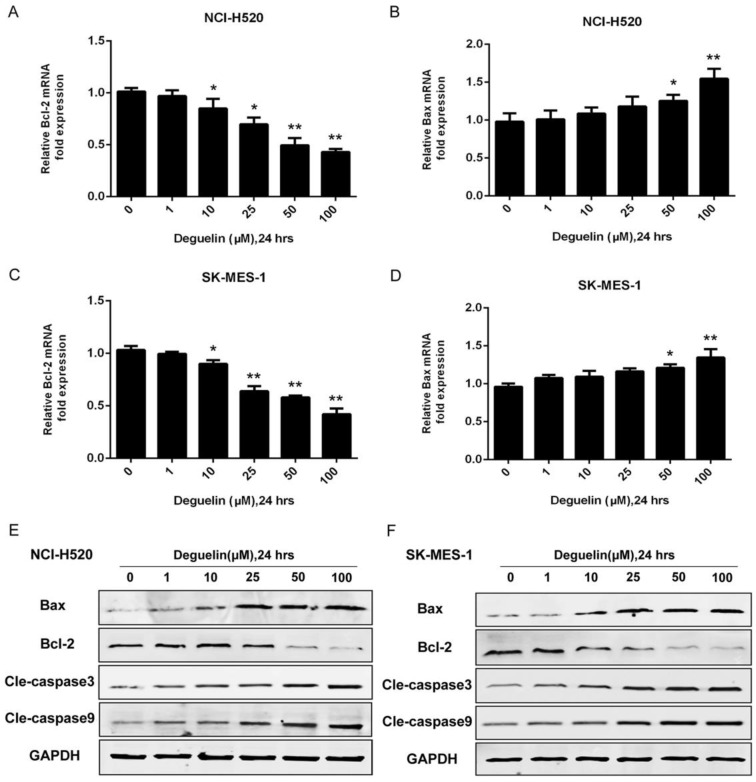
In two subpopulations of human lung SCC cells, NCI-H520 and SK-MES-1, deguelin administration greatly inhibited the cell proliferation in both dose- (Fig. 1A) and time-dependent (Fig. 1B and 1C) manners. Flow cytometry was applied to identify the cellular apoptosis with deguelin exprosure, as shown in Fig. 2, a significant cell apoptosis was observed in NCI-H520 cells, similar result was obtained from SK-MES-1 (data not shown). Next, real-time PCR analysis indicated that deguelin down-regulated the expression of anti-apoptotic factor Bcl-2 (Fig. 3A and 3C), while apoptotic factor Bax was up-regulated (Fig. 3B and 3D) in mRNA level. Furthermore, consistent with the RT-PCR results, similar results were shown by immunoblotting that deguelin exposure increased Bax activity while caused caspase-3 and caspase-9 cleavaged and down-regulated Bcl-2 expression (Fig. 3E and 3F). Thus, these results indicated that deguelin was able to induce the apoptosis of lung SCC cells.
Figure 1.
Deguelin inhibited the lung SCC cells proliferation in both dose-dependent and time-dependent manners. (A) NCI-H520 and SK-MES-1 cells were incubated with 0.1-100μM deguelin for 72 hours. (B) NCI-H520 cells were exposed to deguelin (25μM) for different time points (12, 24, 48 and 72 hours). (C) SK-MES-1 cells were exposed to deguelin (25μM) for different time points (12, 24, 48 and 72 hours). Cell viability was measured using WST-8 assays. Each experiment was performed in triplicate, thrice independently. The data are presented as mean±SD. *P<0.05, **P<0.01 vs Control.
Figure 2.
Deguelin induced the apoptosis of human lung SCC cells. (A) NCI-H520 cells were treated with indicated concentrations of deguelin for 24 hours. Flow cytometry was used for analysis of cell apoptosis. (B) Apoptosis percentages of NCI-H520 cells from three independent experiments are presented as mean±SD. *P<0.05, **P<0.01 vs Control.
Figure 3.
Deguelin exposure down-regulated the expression of anti-apoptotic factors while apoptotic factors were up-regulated. (A, B) NCI-H520 cells were treated with various concentrations of deguelin for 24 hours. Total cellular RNA was extracted and analysed by real-time PCR. (C, D) SK-MES-1 cells were treated with various concentrations of deguelin for 24 hours. Total cellular RNA was extracted and analysed by real-time PCR. The data from three independent experiments are presented as mean±SD. *P<0.05, **P<0.01 vs Control. (E, F) NCI-H520 and SK-MES-1 cells were incubated with different concentrations of deguelin for 24 hours, cell lysates were harvested and the indicated proteins were determined by western blotting.
Deguelin down-regulated the expression of galectin-1 in lung SCC cells
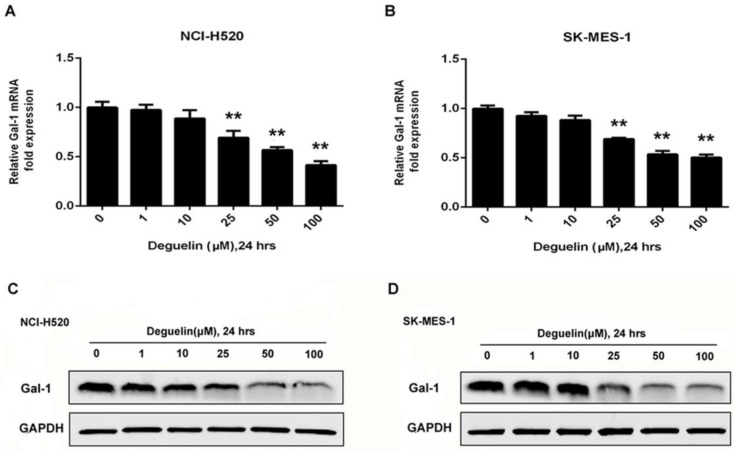
To determine galecitn-1 expression in lung SCC cell lines treated with deguelin, we performed western blotting results as shown in Fig. 4C and 4D that deguelin dose-dependently application could cause galectin-1 degradation. We also analyzed the relative galectin-1 mRNA expression by RT-PCR and the mRNA levels of galectin-1 were prominently down-regulated by deguelin in different doses as shown in Fig. 4A and 4B, which were consist with the immunoblotting results. In summary, these results indicated that deguelin significantly degraded galectin-1 expression at both protein and mRNA levels in lung SCC cells.
Figure 4.
Deguelin down-regulated the expression of galectin-1 in lung SCC cells. (A, B) NCI-H520 and SK-MES-1 cells were treated with various concentrations of deguelin for 24 hours. Total cellular RNA was extracted and analysed using real-time PCR. The data from three independent experiments are presented as mean±SD. **P<0.01 vs Control. (C, D) NCI-H520 and SK-MES-1 cells were incubated with different concentrations of deguelin for 24 hours, cell lysates were harvested and the indicated proteins were determined by western blotting.
Deguelin induced apoptosis of lung SCC cells in a galectin-1 dependent manner
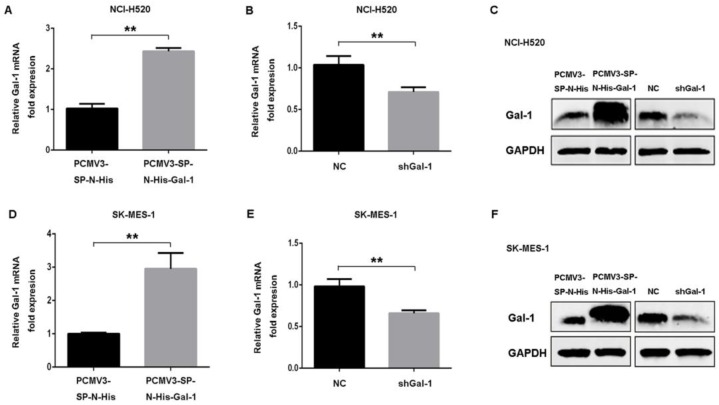
To confirm whether galectin-1 expression level contributed to the apoptotic effect induced by deguelin, we down-regulated galectin-1 expression by transfecting Gal-1 shRNA or negative control shRNA. Meanwhile, NCI-H520 and SK-MES-1 cell lines were also transfected with human galectin-1 plasmid pCMV3-SP-N-His-Gal-1 or empty vector plasmid pCMV3-SP-N-His to increase the expression of galectin-1. Real-time PCR and western blotting were applied to confirm galectin-1 expression at mRNA and protein levels (Fig. 5).
Figure 5.
Construction of stable galectin-1 overexpression and shGal-1-knockdown lung SCC cells. (A) pCMV3-SP-N-His-Gal-1 mediated overexpression and shRNA mediated knockdown of galectin-1 were confirmed by real-time PCR (A, B, D, E) and western blotting (C, F), respectively. The data are representatives of three independent experiments and presented as mean±SD. **P<0.01.
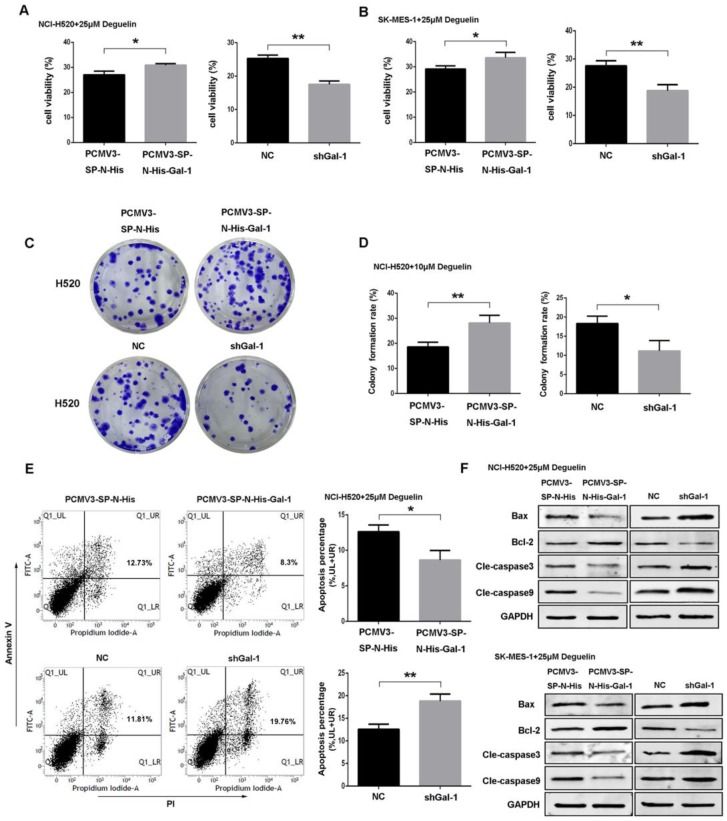
To investigate the role of galectin-1 in deguelin treatment, we performed experiments to determine the proliferation, colony formation and apoptosis of cells exposed to 25μM deguelin. We found that galectin-1 overexpression cells displayed significantly increased proliferation (Fig. 6A and 6B) and colony formation (Fig. 6C and 6D) after deguelin treatment. Meanwhile, galectin-1 knockdown inhibited the cell proliferation and colony forming efficiency compared with the negative control (NC) group. Additionally, flow cytometry analysis showed that shGal-1-knockdown NCI-H520 cells had a higher apoptotic rate with deguelin exposure than NC group while the galectin-1 overexpression had lower apoptotic rate than the equivalent control group (Fig. 6E). The similar results of flow cytometry and colony formation were also observed in SK-MES-1 cell line (data not shown). Next, we performed western blotting to determine the effects of galectin-1 knockdown on apoptosis-related proteins after deguelin treatment. We observed that both in shGal-1-knockdown NCI-H520 and SK-MES-1 cell lines, suppression of galectin-1 was coupled with decreased Bcl-2 and increased Bax, cleaved caspase-3 and cleaved caspase-9 activities. Furthermore, compared with galectin-1 knockdown, we observed that galectin-1 overexpression had contrary results as expected (Fig. 6F), which suggested that deguelin induced lung SCC cells apoptosis via galectin-1 regualtion.
Figure 6.
Deguelin induced apoptosis of lung SCC cells in a galectin-1 dependent manner. (A, B) Both galectin-1 overexpression and knockdown cells were incubated with 25μM deguelin for 72 hours and then measured cell viability using WST-8 assays. (C, D) Galectin-1 overexpression or knockdown cells were seeded in six-well plates. After 24 hours of incubation, the cells were treated with 10μM deguelin for 14 days. Then the colony formation efficiencies were summarized. (E) Galectin-1 overexpression or knockdown cells were incubated with 25μM deguelin for 24 hours. Flow cytometry was used for cell apoptosis analysis. (F) Galectin-1 overexpression or knockdown cells were incubated with 25μM deguelin for 24 hours. Cell lysates were harvested and the indicated proteins were determined by western blotting. All data are from three independent experiments and presented as mean±SD. *P<0.05, **P<0.01.
Suppression of galectin-1 resulted in deactivation of Ras/Raf/ERK pathway
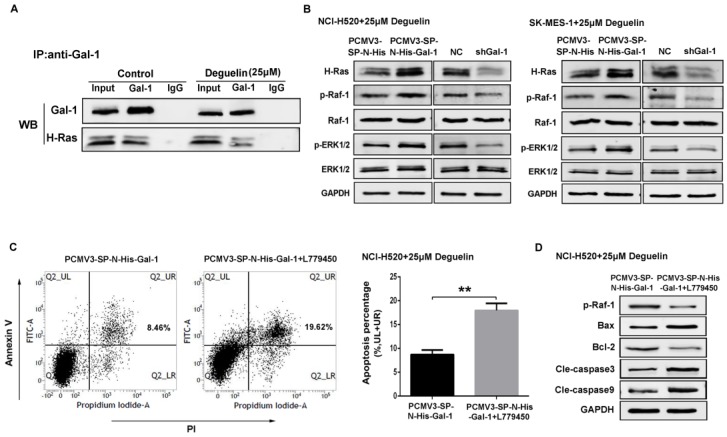
Previously we had demonstrated that galectin-1 was involved in deguelin-induced apoptosis process, and we further explored the galectin-1-related cellular mechanisms. It has been reported that galectin-1 is a selective binding partner of H-Ras19 and mediates a variety of biological functions through direct interaction with H-Ras. To confirm this interaction, co-immunoprecipitation assay was performed and the result indicated that galectin-1 interacted with H-Ras directly in lung SCC cells (Fig. 7A).
Figure 7.
Suppression of galectin-1 resulted in deactivation of the Ras/Raf/ERK pathway with deguelin treatment. (A) Mock- or deguelin-treated cells were lysed for co-immunoprecipitation and then analyzed by western blotting. (B) Galectin-1 overexpression and shGal-1-knockdown cells were incubated with 25μM deguelin for 24 hours, respectively. Cell lysates were harvested and the indicated proteins were determined by western blotting. (C) Galectin-1 overexpression cells with or without L779450 (10μM) were incubated with 25μM deguelin for 24 hours. Flow cytometry was used for cell apoptosis analysis. The data are representatives of three independent experiments and presented as mean±SD. **P<0.01. (D) Galectin-1 overexpression cells with or without L779450 (10μM) were incubated with 25μM deguelin for 24 hours. Then cell lysates were harvested for western blotting.
NCI-H520 and SK-MES-1 cells transfected with Galectin-1 shRNA, negative control shRNA, galectin-1 plasmid vector and empty plasmid vector, respectively, were treated with 25μM deguelin for 24 hours, then cells were harvested and analysed by western blotting. We found that galectin-1 knockdown led to decreased expression of H-Ras, p-Raf-1 and p-ERK1/2, while galectin-1 overexpression resulted in the activation of Ras/Raf/ERK pathway (Fig. 7B).
In order to explore the role of Ras/Raf/ERK pathway in deguelin-induced tumor apoptosis, we then apply a Raf kinase inhibitor L77945030 to block the Ras/Raf/ERK pathway in galectin-1 overexpression cells before deguelin administration. As shown in Fig. 7C, flow cytometry was applied and we observed a significant cell apoptosis in L779450 treatment group. Next, we performed western blotting to detect the expression of apoptosis-related proteins. Bax, cleaved caspase-3 and cleaved caspase-9 expression were up-regulated, while Bcl-2 activity was decreased in deguelin-treated galectin-1 overexpression cells when Ras/Raf/ERK pathway was blocked (Fig. 7D). These results indicated that the Ras/Raf/ERK pathway was involved in the deguelin-induced cells apoptosis.
Anti-tumor effect of deguelin in NCI-H520 xenograft nude mice model
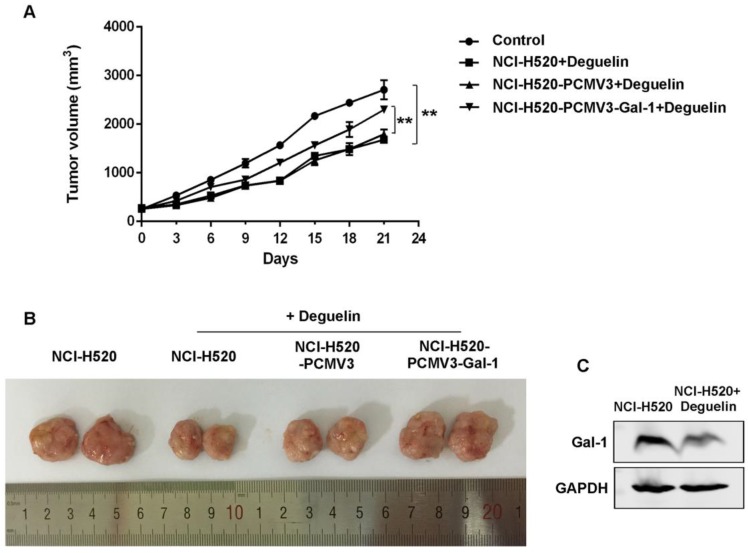
To determine whether the anti-tumor effect of deguelin observed in vitro was consistent with that in vivo, we constructed NCI-H520 xenograft models as mentioned in materials and methods. The NCI-H520 xenograft nude mice group receiving oral deguelin treatment showed a markedly inhibiton in tumor growth, by performance of the reduced tumor size compared with control group receiving physiological saline (Fig. 8A and 8B). Meanwhile, tumors of the group which had been injected with galectin-1 overexpression cells, grew significantly faster than the equivalent group with control plasmid (Fig. 8A and 8B). We then analyzed whether deguelin inhibited the expression of galectin-1 in vivo by using immunoblotting. We found that in the deguelin-treated NCI-H520 xenograft group, galectin-1 was suppressed (Fig. 8C), indicating that deguelin had a significant anti-tumor ability and could reduce the expression of galectin-1 in vivo.
Figure 8.
Deguelin's in vivo anti-tumor effects in NCI-H520 xenograft models. Six-week-old female BALB/c-nude mice were randomized and allocated into groups of six mice followed by injecting subcutaneously with equal numbers of NCI-H520 cells, NCI-H520 cells transfected with pCMV3-SP-N-His empty plasmid or NCI-H520 transfected with pCMV3-SP-N-His-Gal-1 plasmid, respectively. When palpable tumors arose, the control group was orally treated with physiological saline, while other groups were treated with deguelin (4 mg/kg) on days 1, 3 and 5 of each week for three weeks. (A) Tumor volume was determined every three days after the onset of treatment. Data are presented as mean±SD. **P<0.01. (B) On day 21, the tumors were carefully dissected from the mice. (C) Expression of galectin-1 was detected in the harvested tumors by western blotting.
Discussion
Lung cancer, with approximately 24% of cancer-related mortality, remains the single deadliest cancer worldwide. Despite continuous efforts devoted to improving lung cancer treatment, there is no significant improvement in the overall five-year survival rate. Moreover, effective clinical therapies targeted EGFR and EML4-AKL which have been widely applied in adenocarcinoma are rarely associated with patients with lung SCC, another main subtype of NSCLC31. So it is meaningful to find more effective therapeutic agents against lung SCC.
Deguelin is a nature compound of the flavonoid family products extracted from plants including Derris trifoliata Lour. (Leguminosae), Mundulea sericea (Leguminosae) and Tephrosia vogelii Hook.f. (Leguminosae)13. This plant-derived rotenoid has been reported to be an effective cancer chemo-preventive agent suppressing the growth of various types of cancers12, 32-34. Previous studies have shown that the possible mechanisms of anti-tumor effect of deguelin may include DNA damage, reducing DNA repair genes, inhibiting vasculogenic function, and blocking anti-apoptotic pathways13, 14, 35. Although the application of deguelin on tumors has been paid more attention, researches focused on deguelin treatment for lung SCC are still limited.
In the present study, we found that deguelin could induce the apoptosis of lung SCC cells in vitro, which is consistent with the effect of deguelin performed in other cancer cells36, 37. In nude mice xenograft models, we also showed a significant anti-tumor ability of deguelin in vivo. The tumor sizes of mice receiving oral deguelin administration were significantly reduced compared with those receiving physiological saline treatment. These results indicate that deguelin represents as an effective chemopreventive agent against lung SCC. Importantly, our further study shows that deguelin-induced apoptosis is mediated through the regulation of galectin-1 expression.
Galectin-1, a member of the β-galactoside binding protein family, is encoded by the LGALS1 gene located on chromosome 22q1238. It has been reported that the expression of galectin-1 is increased in a wide variety of tumors including breast39, colon40, ovarian41, pancreatic23, prostate42 and lung20, 24 cancers. It is well documented that galectin-1 is involved in multiple processes such as tumor cell proliferation, differentiation, invasiveness and metastasis38, 43, 44. Carlini et al.45 recently reported that NSCLC patients showing high galectin-1 expression were evidenced to have a poorer clinical outcome. Similarly, studies in gastric, colon, breast and epithelial ovarian cancers also showed a positive correlation between galectin-1 expression and poor prognosis25, 39, 40, 46, 47 underscoring the relevance of galectin-1 as a possible biomarker or therapeutic target in cancers.
Here, we explore the effect of deguelin on galectin-1 and put forward that deguelin can reduce the galectin-1 expression level both in vivo and in vitro. These results imply deguelin as a potential inhibitor of galectin-1. To confirm the role of galectin-1 in deguelin-induced apoptosis, we silenced and over-expressed galectin-1 in NCI-H520 and SK-MES-1 cell lines. After exposured to deguelin, we found that galectin-1 knockdown sensitized lung cancer cells to deguelin treatment, while galectin-1 overexpression cells were insensitive to deguelin compared with control cells in vitro. Besides, a similar result was also observed in nude mice xenograft models that the mice group injected with galectin-1 overexpression NCI-H520 cells showed significantly increased tumor volumes compared with mice injected with NCI-H520 control cells. These results suggest that the anti-tumor effect of deguelin on lung SCC cells is performed by down-regulating galectin-1 expression.
It has been reported that galectin-1 can interact with H-Ras and strengthen its memberane anchorage19. In agreement with previous studies, we performed co-immunoprecipitation assay and demonstrated a direct interaction between H-Ras and galectin-1 in NCI-H520 cells. Besides, silencing of galectin-1 resulted in down-regulation of H-Ras, p-Raf-1 and p-ERK1/2 in both deguelin-treated NCI-H520 and SK-MES-1 cells, while galectin-1 overexpression could up-regulate H-Ras, p-Raf-1 and p-ERK1/2, indicating that galectin-1 can regulate the Ras/Raf/ERK pathway through the interaction with H-Ras. For further study, we inhibited the activity of Raf-1 to block the Ras/Raf/ERK pathway. We found the apoptosis ratio of galectin-1 overexpression NCI-H520 cells after deguelin administration was significantly increased when the Ras/Raf/ERK pathway was blocked. Thus, we demonstrate that deguelin can induce the lung SCC cells apoptosis by inhibition the Ras/Raf/ERK pathway through suppressing galectin-1 expression.
Previous studies had shown that the activation of Ras could also stimulate the PI3K signaling pathways, which played an important role in Ras-mediated cellular physiological processes like cell survival and proliferation48, 49, and deguelin is well known as a PI3K/AKT inhibitor11, 50. In the present study, we demonstrated that deguelin could down-regulate H-Ras by inhibiting galectin-1 as mentioned previously. Therefore, we infer that deguelin may also regulate the PI3K/AKT pathway through inhibiting galectin-1 expression, which merits further investigation.
Conclusion
In summary, we have shown evidence that deguelin can inhibit the growth of lung SCC by inducing cell apoptosis. Additionally, we found that overexpression of galectin-1 could reduce the apoptotic effects of deguelin through the up-regulation of Ras/Raf/ERK pathway, while suppression of galectin-1 sensitized lung cancer cells to deguelin treatment, suggesting that deguelin induces the apoptosis of lung SCC cells by inhibiting the Ras/Raf/ERK pathway through suppressing galectin-1 expression. This study proposes that deguelin may represent as a novel and effective agent against lung squamous cell carcinoma.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81472171) and Science and Technology Planning Project of Zhejiang Province, China (No. 2012C13022-2).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N, Hatoum H, Dy GK. First line treatment of advanced non-small-cell lung cancer - specific focus on albumin bound paclitaxel. International journal of nanomedicine. 2014;9:209–21. doi: 10.2147/IJN.S41770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta R, Katta H, Alimirah F, Patel R, Murillo G, Peng X. et al. Deguelin Action Involves c-Met and EGFR Signaling Pathways in Triple Negative Breast Cancer Cells. PLoS ONE. 2013;8:e65113. doi: 10.1371/journal.pone.0065113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YP, Lee JJ, Lai TC, Lee CH, Hsiao YW, Chen PS, Suppressive function of low-dose deguelin on the invasion of oral cancer cells by downregulating tumor necrosis factor alpha-induced nuclear factor-kappa B signaling. Head & neck; 2015. [DOI] [PubMed] [Google Scholar]
- 5.Shang HS, Chang JB, Lin JH, Lin JP, Hsu SC, Liu CM. et al. Deguelin inhibits the migration and invasion of U-2 OS human osteosarcoma cells via the inhibition of matrix metalloproteinase-2/-9 in vitro. Molecules. 2014;19:16588–608. doi: 10.3390/molecules191016588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boreddy SR, Srivastava SK. Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model. Oncogene. 2013;32:3980–91. doi: 10.1038/onc.2012.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. Journal of immunology. 2006;177:5612–22. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 8.Geeraerts B, Vanhoecke B, Vanden Berghe W, Philippe J, Offner F, Deforce D. Deguelin inhibits expression of IkappaBalpha protein and induces apoptosis of B-CLL cells in vitro. Leukemia. 2007;21:1610–8. doi: 10.1038/sj.leu.2404788. [DOI] [PubMed] [Google Scholar]
- 9.Kang HW, Kim JM, Cha MY, Jung HC, Song IS, Kim JS. Deguelin, an Akt inhibitor, down-regulates NF-kappaB signaling and induces apoptosis in colon cancer cells and inhibits tumor growth in mice. Digestive diseases and sciences. 2012;57:2873–82. doi: 10.1007/s10620-012-2237-x. [DOI] [PubMed] [Google Scholar]
- 10.Jin Q, Feng L, Behrens C, Bekele BN, Wistuba II, Hong WK. et al. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer research. 2007;67:11630–9. doi: 10.1158/0008-5472.CAN-07-2401. [DOI] [PubMed] [Google Scholar]
- 11.Chun KH, Kosmeder JW 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK. et al. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. Journal of the National Cancer Institute. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 12.Murillo G, Salti GI, Kosmeder JW 2nd, Pezzuto JM, Mehta RG. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. European journal of cancer. 2002;38:2446–54. doi: 10.1016/s0959-8049(02)00192-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Ma W, Zheng W. Deguelin, a novel anti-tumorigenic agent targeting apoptosis, cell cycle arrest and anti-angiogenesis for cancer chemoprevention. Molecular and clinical oncology. 2013;1:215–9. doi: 10.3892/mco.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen MP, Lee D, Lee SH, Lee HE, Lee HY, Lee YM. Deguelin inhibits vasculogenic function of endothelial progenitor cells in tumor progression and metastasis via suppression of focal adhesion. Oncotarget. 2015;6:16588–600. doi: 10.18632/oncotarget.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suh YA, Kim JH, Sung MA, Boo HJ, Yun HJ, Lee SH. et al. A novel antitumor activity of deguelin targeting the insulin-like growth factor (IGF) receptor pathway via up-regulation of IGF-binding protein-3 expression in breast cancer. Cancer letters. 2013;332:102–9. doi: 10.1016/j.canlet.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundela S, Sharma A, Bisen PS. Potential Compounds for Oral Cancer Treatment: Resveratrol, Nimbolide, Lovastatin, Bortezomib, Vorinostat, Berberine, Pterostilbene, Deguelin, Andrographolide, and Colchicine. PLoS One. 2015;10:e0141719. doi: 10.1371/journal.pone.0141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu YC, Chiang JH, Yu CS, Hsia TC, Wu RS, Lien JC, Antitumor effects of deguelin on H460 human lung cancer cells in vitro and in vivo: Roles of apoptotic cell death and H460 tumor xenografts model. Environmental toxicology; 2015. [DOI] [PubMed] [Google Scholar]
- 18.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes & cancer. 2011;2:261–74. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene. 2001;20:7486–93. doi: 10.1038/sj.onc.1204950. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Li D, Wang X, Zhang B, Zhu H, Zhao J. Galectin-1 is overexpressed in CD133+ human lung adenocarcinoma cells and promotes their growth and invasiveness. Oncotarget. 2015;6:3111–22. doi: 10.18632/oncotarget.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh CC, Hsu CH, Shao YY, Ho WC, Tsai MH, Feng WC. et al. Integrated Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) and Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) Quantitative Proteomic Analysis Identifies Galectin-1 as a Potential Biomarker for Predicting Sorafenib Resistance in Liver Cancer. Molecular & cellular proteomics: MCP. 2015;14:1527–45. doi: 10.1074/mcp.M114.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzi M, Bacigalupo ML, Carabias P, Elola MT, Wolfenstein-Todel C, Rabinovich GA, Galectin-1 Controls the Proliferation and Migration of Liver Sinusoidal Endothelial Cells and Their Interaction with Hepatocarcinoma Cells. Journal of cellular physiology; 2015. [DOI] [PubMed] [Google Scholar]
- 23.Tang D, Gao J, Wang S, Yuan Z, Ye N, Chong Y. et al. Apoptosis and anergy of T cell induced by pancreatic stellate cells-derived galectin-1 in pancreatic cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:5617–26. doi: 10.1007/s13277-015-3233-5. [DOI] [PubMed] [Google Scholar]
- 24.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL. et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:4037–47. doi: 10.1158/1078-0432.CCR-11-3348. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Jeon HK, Cho YJ, Park YA, Choi JJ, Do IG. et al. High galectin-1 expression correlates with poor prognosis and is involved in epithelial ovarian cancer proliferation and invasion. European journal of cancer. 2012;48:1914–21. doi: 10.1016/j.ejca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Szoke T, Kayser K, Baumhakel JD, Trojan I, Furak J, Tiszlavicz L. et al. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology. 2005;69:167–74. doi: 10.1159/000087841. [DOI] [PubMed] [Google Scholar]
- 27.van den Brule FA, Waltregny D, Castronovo V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. The Journal of pathology. 2001;193:80–7. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH730>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S. et al. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer treatment reviews. 2014;40:307–19. doi: 10.1016/j.ctrv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Miao JH, Wang SQ, Zhang MH, Yu FB, Zhang L, Yu ZX. et al. Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncology reports. 2014;32:1497–504. doi: 10.3892/or.2014.3358. [DOI] [PubMed] [Google Scholar]
- 30.Shelton JG, Moye PW, Steelman LS, Blalock WL, Lee JT, Franklin RA. et al. Differential effects of kinase cascade inhibitors on neoplastic and cytokine-mediated cell proliferation. Leukemia. 2003;17:1765–82. doi: 10.1038/sj.leu.2403052. [DOI] [PubMed] [Google Scholar]
- 31.Derman BA, Mileham KF, Bonomi PD, Batus M, Fidler MJ. Treatment of advanced squamous cell carcinoma of the lung: a review. Translational lung cancer research. 2015;4:524–32. doi: 10.3978/j.issn.2218-6751.2015.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HY, Suh YA, Kosmeder JW, Pezzuto JM, Hong WK, Kurie JM. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:1074–9. doi: 10.1158/1078-0432.ccr-0833-3. [DOI] [PubMed] [Google Scholar]
- 33.Udeani GO, Gerhauser C, Thomas CF, Moon RC, Kosmeder JW, Kinghorn AD. et al. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer research. 1997;57:3424–8. [PubMed] [Google Scholar]
- 34.Lee HY, Oh SH, Woo JK, Kim WY, Van Pelt CS, Price RE. et al. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. Journal of the National Cancer Institute. 2005;97:1695–9. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 35.Ji BC, Yu CC, Yang ST, Hsia TC, Yang JS, Lai KC. et al. Induction of DNA damage by deguelin is mediated through reducing DNA repair genes in human non-small cell lung cancer NCI-H460 cells. Oncology reports. 2012;27:959–64. doi: 10.3892/or.2012.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang YL, Ji C, Bi ZG, Lu CC, Wang R, Gu B. et al. Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS One. 2013;8:e54736. doi: 10.1371/journal.pone.0054736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Lee JH, Jung KH, Hong SS. Deguelin promotes apoptosis and inhibits angiogenesis of gastric cancer. Oncology reports. 2010;24:957–63. doi: 10.3892/or.2010.957. [DOI] [PubMed] [Google Scholar]
- 38.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–57R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 39.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP. et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer research. 2013;73:1107–17. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 40.Barrow H, Rhodes JM, Yu LG. The role of galectins in colorectal cancer progression. International journal of cancer Journal international du cancer. 2011;129:1–8. doi: 10.1002/ijc.25945. [DOI] [PubMed] [Google Scholar]
- 41.Chow SN, Chen RJ, Chen CH, Chang TC, Chen LC, Lee WJ. et al. Analysis of protein profiles in human epithelial ovarian cancer tissues by proteomic technology. European journal of gynaecological oncology. 2010;31:55–62. [PubMed] [Google Scholar]
- 42.Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO. et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer research. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 43.He XJ, Tao HQ, Hu ZM, Ma YY, Xu J, Wang HJ. et al. Expression of galectin-1 in carcinoma-associated fibroblasts promotes gastric cancer cell invasion through upregulation of integrin beta1. Cancer science. 2014;105:1402–10. doi: 10.1111/cas.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masamune A, Satoh M, Hirabayashi J, Kasai K, Satoh K, Shimosegawa T. Galectin-1 induces chemokine production and proliferation in pancreatic stellate cells. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G729–36. doi: 10.1152/ajpgi.00511.2005. [DOI] [PubMed] [Google Scholar]
- 45.Carlini MJ, Roitman P, Nunez M, Pallotta MG, Boggio G, Smith D. et al. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung cancer. 2014;84:73–8. doi: 10.1016/j.lungcan.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Tang D, Wang S, Li QG, Zhang JR, Li P. et al. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:2513–9. doi: 10.1007/s13277-013-1332-8. [DOI] [PubMed] [Google Scholar]
- 47.Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ. et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. International journal of cancer Journal international du cancer. 2007;120:2331–8. doi: 10.1002/ijc.22434. [DOI] [PubMed] [Google Scholar]
- 48.Downward J. Targeting RAS signalling pathways in cancer therapy. Nature reviews Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 49.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Current topics in microbiology and immunology. 2010;346:143–69. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Wu Q, Cui GH, Chen YQ, Li R. Deguelin blocks cells survival signal pathways and induces apoptosis of HL-60 cells in vitro. International journal of hematology. 2009;89:618–23. doi: 10.1007/s12185-009-0307-4. [DOI] [PubMed] [Google Scholar]