Abstract
Objectives
Tiotropium (TIO), Spiriva® Handihaler®, is a well-established bronchodilator, LAMA (long acting muscarinic antagonist), for the treatment of moderate to very severe chronic obstructive pulmonary disease (COPD). Clinical evidence from the SPARK trial suggests that TIO is superior to glycopyrronium (GLY), Seebri® Breezhaler®, in terms of severe exacerbations. This modeling study assessed the cost-effectiveness of TIO versus GLY for Canada (CAN), Spain (ESP), Sweden (SWE), and the UK, making use of this new clinical evidence.
Methods
A Markov cohort model, with moderate to very severe (Global Initiative for Chronic Obstructive Lung Disease II–IV) COPD patients, was populated with efficacy data from the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) and SPARK trials as well as costs, utilities, and epidemiological data relevant for each country. Treatment efficacy was modeled as improvements in lung function, quality-adjusted life years (QALYs), and as a lowering of the risk of exacerbations (rate of exacerbations). Risks of exacerbations differed between cohorts based on data from SPARK. Health and cost outcomes were simulated over an approximate lifetime horizon, starting from the age of 65 years. Robustness of results was validated in deterministic sensitivity analyses.
Results
Over the lifetime horizon, patients treated with TIO accumulated −623 (CAN), 1,066 (ESP), 1,137 (SWE), and −169 (UK), respectively, in incremental costs (€2014). TIO generated better health outcomes compared to GLY in all countries, 0.21 (CAN), 0.25 (ESP), 0.23 (SWE), and 0.23 (UK) in incremental QALYs. The cost per QALY gained was found to be €4,281 and €1,137 for ESP and SWE, respectively, while TIO was found to be cost saving in CAN and the UK. The results were mainly driven by the relative risk of severe exacerbations found in SPARK (GLY/TIO relative risk: 1.43, 95% confidence interval: 1.05–1.97, P=0.025).
Conclusion
The results from this study show that TIO is a cost-effective treatment compared to GLY in moderate to very severe COPD. The cost per QALY is well below the existing implicit and explicit willingness-to-pay thresholds.
Keywords: COPD, exacerbations, tiotropium, glycopyrronium, cost-effectiveness, Markov cohort model
Introduction
Background
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease affectin ~5%–10% of the adult population.1–4 In the European Union, COPD stands for 56% of the direct costs associated with respiratory diseases.5 Respiratory diseases, in turn, represent 6% of the total health care budget.5 This means that the estimated total cost of COPD in the European Union is €38.6 billion.5 In the US, the calculated annual direct cost burden of COPD amounts to $30 billion.6 In addition to the high direct cost burden, indirect costs are large and amount to $20 billion in lost production every year.6 Exacerbations, that is, “acute and sustained worsening of the patient’s respiratory symptoms beyond normal day-to-day variations that result in an increased need for medication […]”, are important drivers of COPD-related costs.7 Exacerbations on the acute and severe end of the spectrum are the most common causes for COPD-related hospitalizations in the US and UK, and hospitalizations, in turn, represent 54% of direct COPD-related costs.8–10 Besides costs, exacerbations increase mortality11,12 and decrease health-related quality of life13,14 in COPD patients. Thus, COPD exacerbations represent a significant economic and clinical burden.
Pharmacologic therapy in moderate to severe COPD includes long-acting bronchodilators, long-acting β2 agonists (LABAs), or LAMAs (long acting muscarinic antagonist). Inhaled corticosteroids (ICSs) and antibiotics can be used alone or in combination with bronchodilators to treat and mitigate the effects of exacerbations. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations, the first choice of therapy for patients with moderate to severe COPD is LAMA or LABA therapy alone.5 In high-risk patients, that is, those at risk of exacerbations, ICS can be added to the LABA monotherapy. Short-acting bronchodilators, either in the form of an anticholinergic or a β2 agonist, are typically administered as needed to relieve symptoms in the short term.
Several studies have shown that regular use of LAMAs, LABAs, and ICSs can lower the incidence and effect of exacerbations.15 In a 4-year, randomized, double-blind trial (Understanding Potential Long-term Impacts on Function with Tiotropium [UPLIFT]), tiotropium (TIO) significantly improved lung function (forced expiratory volume in 1 second [FEV1]) and lowered the risk of exacerbations when added to usual non-LAMA care, compared to usual non-LAMA care alone.16 Evidence from recent studies also suggests that TIO is superior to both once-daily17 and twice-daily18 administered LABAs when it comes to preventing exacerbations. Furthermore, in a recent head-to-head trial (SPARK), patients treated with TIO had lower rates of severe exacerbations, that is, those leading to hospitalization, than patients treated with glycopyrronium (GLY).19
TIO has been shown to be a cost-effective treatment compared to usual care in the management of COPD in a number of countries.20 However, evidence on the cost-effectiveness of TIO versus other LAMAs has been scarce. With the publication of SPARK results in addition to previous trials, it is now possible to compare TIO and GLY head-to-head.19 The primary objective of this study was to analyze the cost-effectiveness of TIO compared to GLY in Canada (CAN), Spain (ESP), Sweden (SWE), and the UK using data from the UPLIFT16 and SPARK19 trials.
UPLIFT and SPARK trials
UPLIFT16 was a randomized, double-blind, placebo-controlled study, investigating the efficacy of TIO when added to non-LAMA usual care (placebo) compared to placebo alone. A total of 5,993 patients (mean age, 65±8 years), with an FEV1 of 70% or less, that is, moderate to very severe COPD (GOLD II–IV), after bronchodilation and a ratio of FEV1 to forced vital capacity of 70% or less, were studied over a 4-year period. Co-primary end points were the rate of decline in mean FEV1 before and after bronchodilation beginning on day 30. Secondary end points were, among other things, rates of exacerbations, measures of forced vital capacity, changes in St George’s Respiratory Questionnaire responses, and mortality. The main findings were that mean absolute improvements in FEV1 in the TIO group were maintained throughout the trial as compared to placebo (P<0.001). In addition, the number of exacerbations per patient-year declined in favor of TIO.16
SPARK19 was a randomized parallel group study investigating the relative efficacy of QVA149 (GLY and indacaterol combination) versus GLY monotherapy and TIO monotherapy. A total of 2,224 patients were enrolled, aged 40 years or more, with severe or very severe COPD (GOLD III–IV) and one or more moderate COPD exacerbations in the last year. Results showed that there was no significant difference between GLY and TIO in preventing mild (GLY/TIO relative risk [RR]: 0.99, P=0.90) and moderate or severe (GLY/TIO RR: 1.03, P=0.68) exacerbations. However, in the case of severe exacerbations (SevEx) alone, TIO was superior to GLY (GLY/TIO RR SevEx: 1.43, P=0.025).
Methods
COPD models
Chronic and slowly progressing diseases such as COPD are well characterized in a simple Markov cohort framework. Indeed, most cost-effectiveness models in the published COPD literature are Markov cohort models.7,21 In most cases, model health states are constructed around the established GOLD classification of severity of airflow limitation.22 This classification is based on four groups: mild (GOLD I FEV1 ≥80%), moderate (GOLD II 50%≤ FEV1 <80%), severe (GOLD III 30%≤ FEV1 <50%), and very severe (GOLD IV FEV1 <30%) COPD, each representing an interval of post-bronchodilator FEV1, a measure of airflow.5 Some models23,24 also incorporate costs and effects of exacerbations, typically modeled as events occurring within the health (GOLD) states. This makes it possible to add baseline costs and outcomes associated with each health state as well as additional event-specific costs and outcomes associated with exacerbations. This study used data obtained from published sources of UPLIFT and SPARK trials. These trials had all the necessary regulatory and ethical approvals in place. As this is a modeling study without any primary data collected, we do not need regulatory/ethical approval.
Model description
The Markov cohort model, shown in Figure 1, was built in TreeAge Pro Suite 2014 and has been described before in an analysis for SWE.25 The model was based on three health states (GOLD II, GOLD III, and GOLD IV), between which the cohort of patients could move with varying probabilities. Patients were modeled from the age of treatment initiation (65 years old) until they deceased or reached the end of the simulation (100 years). Two cohorts were analyzed, one receiving standard treatment with TIO 18 µg (Spiriva® HandiHaler®) and one receiving standard treatment with GLY 44 µg (Seebri® Breezhaler®). Upon entering the model, both cohorts of patients were distributed across the separate states according to the observed distributions found in UPLIFT (GII: 48%, GIII: 44%, GIV: 8%).16 Within each 3-month cycle, the patients could then experience any one of the following three events: 1) no exacerbation, 2) nonsevere exacerbation, or 3) severe exacerbation. Each event occurred with certain state-specific probabilities. Based on trial data from UPLIFT16 and SPARK,19 these probabilities were varied across the treatment alternatives in order to reflect relative efficacy of the treatment alternatives. Each state and event combination had different costs and effects associated with that particular combination. Subsequent to each event, the patients could either die or transition to one of the other two health states for a new 3-month cycle to begin. Within each state, a patient can experience any one of the following events: 1) no exacerbation (No), 2) nonsevere exacerbation (Ex), 3) severe exacerbation (Sev).
Figure 1.
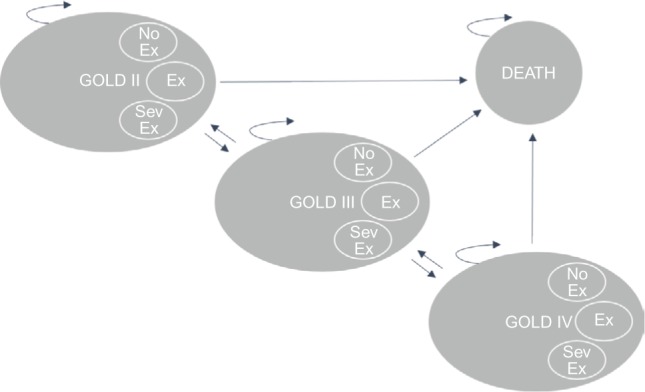
Markov model state transition diagram.
Note: The arrows reflect the fact that a patient can remain in the same state for several cycles.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD II, GOLD classification indicating moderate COPD; COPD, chronic obstructive pulmonary disease; GOLD III, GOLD classification indicating severe COPD; GOLD IV, GOLD classification indicating very severe COPD; No Ex, no exacerbation; Ex, exacerbation; SevEx, severe exacerbation.
Target patient population
The base case model population was assumed to be similar to that of the UPLIFT16 trial in terms of age (65 years), disease state (GOLD II–IV), risks of exacerbations, and the usual (non-LAMA) care received besides the two treatment interventions modeled. In addition, to accurately reflect the real-world characteristics of COPD patients, the model was populated with mortality data representative for each country’s patient population.
Clinical data
Transition probabilities and risks of exacerbations were mainly obtained from Hettle et al,26 who in turn derived estimates from UPLIFT.16 GLY was assumed to be equivalent to TIO in terms of effects on overall lung function (FEV1). The rationale for this assumption is well founded. Recent trials (GLOW1–2)27 and indeed SPARK,19 as well as network meta-analyses,28,29 have shown comparability in terms of the effect on FEV1 between TIO and GLY and other LAMAs. Thus, efficacy data (FEV1) from the TIO arm of the UPLIFT trial was used to model disease progression in both the TIO and the GLY cohorts. However, data on RRs of exacerbations for TIO versus GLY were obtained from the SPARK19 trial. Table 1 provides an overview of the sources used to model treatment efficacy.
Table 1.
Source overview of clinical inputs
| Clinical input | TIO | GLY |
|---|---|---|
| Transition probabilities | ||
| Cycles on treatment (year 0–4) | UPLIFT (TIO arm) | UPLIFT (TIO arm)a |
| Cycles off treatment (≥year 4) | UPLIFT (usual care arm)b | UPLIFT (usual care arm)b |
| Probabilities of exacerbations | ||
| Baseline risks of exacerbations | UPLIFT (TIO arm) | UPLIFT (TIO arm) |
| RRs | (N/A baseline applied) | SPARK RR TIO/GLYc |
Notes:
Several trials have shown comparable efficacy between tiotropium and glycopyrronium in terms of overall lung function (FEV1).
As there is little persistence in the effect of LAMAs after stopping treatment, transition probabilities were assumed to return to the placebo arm (usual care) probabilities in the cycle after stopping treatment.
Applied to baseline risks of exacerbations from UPLIFT (TIO arm). Adapted from Wedzicha et al19 and Hettle et al.27
Abbreviations: TIO, Tiotropium; GLY, glycopyrronium; UPLIFT, Understanding Potential Long-term Impacts on Function with Tiotropium; N/A, not applicable; RR, relative risk; FEV1, forced expiratory volume in 1 second.
Hettle et al26 derived sets of transition probabilities for TIO and usual care arms using data from UPLIFT. A separate set of transition probabilities was obtained for the first cycle on treatment in order to capture the initial 30-day effect of TIO on FEV1.26 In the base case analysis, patients were assumed to be on treatment for 4 years from the start of simulation, based on the observed treatment duration in UPLIFT.16 The assumption was made to ensure that results were not driven by unrealistic assumptions about treatment duration (some share of patients will in practice discontinue treatment). As there is little persistence in the effect of LAMAs after stopping treatment, transition probabilities were assumed to return to the placebo arm (usual care) probabilities from UPLIFT in the cycle after stopping treatment. The sets of transition probabilities used in the model are shown in Table 2.
Table 2.
Model transition probabilities by GOLD states
| First cycle | Subsequent cycles | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| To | To | ||||||
|
| |||||||
| GOLD II | GOLD III | GOLD IV | GOLD II | GOLD III | GOLD IV | ||
| TIO/GLY | |||||||
| From | GOLD II | 0.92 | 0.08 | 0.00 | 0.92 | 0.08 | 0.00 |
| GOLD III | 0.17 | 0.80 | 0.03 | 0.08 | 0.88 | 0.04 | |
| GOLD IV | 0.03 | 0.28 | 0.69 | 0.00 | 0.12 | 0.88 | |
| Off treatment | |||||||
| From | GOLD II | 0.86 | 0.13 | 0.01 | 0.91 | 0.09 | 0.00 |
| GOLD III | 0.13 | 0.81 | 0.06 | 0.08 | 0.88 | 0.04 | |
| GOLD IV | 0.02 | 0.22 | 0.76 | 0.00 | 0.13 | 0.87 | |
Notes: For TIO and GLY cohorts, usual care probabilities were assumed to hold when off treatment. Probabilities have been recalculated to reflect 3-month probabilities. Death has been excluded, as this model carries separate mortality rates derived for each country’s population. Based on own calculations using data from Hettle et al27 (derived from UPLIFT16).
Abbreviations: GOLD II, GOLD classification indicating moderate COPD; COPD, chronic obstructive pulmonary disease; GOLD III, GOLD classification indicating severe COPD; GOLD IV, GOLD classification indicating very severe COPD; TIO, Tiotropium; GLY, glycopyrronium; UPLIFT, Understanding Potential Long-term Impacts on Function with Tiotropium.
Rates of exacerbations for usual care and TIO were obtained from Hettle et al26 (derived from UPLIFT) and recalculated to reflect 3-month risks. RRs of exacerbations for TIO versus GLY were obtained from SPARK.19 Usual care probabilities were applied in cycles off treatment.
Table 3 shows the risks of different types of exacerbations by GOLD state for the two treatment alternatives as well as for cycles off treatment (usual care). The efficacy of TIO versus GLY was modeled solely based on the risks of exacerbations observed in SPARK (GLY/TIO RR non-SevEx: 0.98 and GLY/TIO RR SevEx: 1.43).
Table 3.
Model probabilities of exacerbations by GOLD states
| Event/GOLD | Off treatment | Tiotropium | Glycopyrronium | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| II | III | IV | II | III | IV | II | III | IV | |
| No exacerbation | 0.84 | 0.78 | 0.73 | 0.87 | 0.81 | 0.77 | 0.86 | 0.79 | 0.74 |
| Nonsevere exacerbation | 0.14 | 0.17 | 0.18 | 0.11 | 0.14 | 0.15 | 0.11 | 0.14 | 0.14 |
| Severe exacerbation | 0.02 | 0.05 | 0.08 | 0.02 | 0.05 | 0.08 | 0.03 | 0.07 | 0.12 |
Notes: Usual care probabilities from UPLIFT were used in cycles off treatment. Probabilities reflect 3-month risks. Data from Hettle et al27 (derived from UPLIFT), SPARK,19 and own calculations.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; UPLIFT, Understanding Potential Long-term Impacts on Function with Tiotropium.
Epidemiological data
Baseline age-differentiated mortality for the general population in each country was obtained from the national and World Health Organization databases on causes of deaths.30–33 From the databases, it was possible to discern the share of total deaths that were related to COPD and to factor these out of the baseline mortality rates for the general population. The corrected and age-differentiated mortality rates for the general population were then used as baseline rates, on top of which excess mortality related to COPD was added. Point estimates for excess mortality associated with each GOLD state were obtained from Garcia-Aymerich et al11 and extrapolated over age intervals using the change in RR between age groups from Hoogendoorn et al.34 Similarly, age-differentiated excess mortality rates for severe exacerbations were generated using point estimates and changes in RRs between age groups from Hoogendoorn et al.34 Finally, it was assumed that “nonsevere exacerbations” did not additionally impact mortality, that is, the state-specific mortality rates were assumed to hold in cycles where these events occurred.
Health economic data
Drug costs for TIO and GLY were obtained from relevant sources in each country. The drug costs (€2014) per patient per day for each country are displayed in Table 4.
Table 4.
Drug costs (€2014) per patient per day for each country
| Therapy/country | CAN | ESP | SWE | UK |
|---|---|---|---|---|
| Spiriva Handihaler (tiotropium 18 µg) | 1.48 | 0.97 | 1.40 | 1.39 |
| Seebri Breezhaler (glycopyrronium 44 µg) | 1.21 | 0.94 | 1.15 | 1.14 |
Notes: Ex-rates 2014: CAN (C$ to €: 0.68), Bank of Canada40; SWE (SEK to €: 0.11), Sveriges Riksbank41; UK (£ to €: 1.24), Bank of England.42 Dosing: one capsule per day. Adapted from Ontario Drug Benefit Formulary35 (CAN), botplus + deductions36,37 (ESP), the Dental and Pharmaceuticals Benefits Agency (TLV)38 (SWE), and MIMS39 (UK).
Abbreviations: CAN, Canada; ESP, Spain, SWE, Sweden; SEK, Swedish krona; MIMS, monthly index of medical specialties; TLV, Tandvårds- och läkemedelsförmånsverket [dental and pharmaceutical benefits agency].
Direct costs, presented in Table 5, were assigned to the different state–event combinations. Each cycle that a patient spent in one of the GOLD states had an associated maintenance cost plus an extra cost for the particular event (no exacerbation, nonsevere exacerbation, or severe exacerbation) occurring during that cycle. The costs of exacerbations were obtained from relevant sources in each country.
Table 5.
Direct costs (€2014) by GOLD state and type of event
| Event/GOLD | CAN43,44 | ESP45,46 | SWE47,48 | UK26 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| II | III | IV | II | III | IV | II | III | IV | II | III | IV | |
| No Ex | 95 | 284 | 416 | 366 | 1,060 | 1,751 | 141 | 333 | 472 | 150 | 321 | 531 |
| Non-SevEx | 391 | 580 | 712 | 669 | 1,363 | 2,054 | 486 | 678 | 817 | 153 | 474 | 684 |
| SevEex | 7,108 | 7,297 | 7,428 | 1,822 | 2,516 | 3,207 | 3,058 | 3,250 | 3,389 | 4,832 | 5,153 | 5,363 |
Notes: Ex-rates 2014: CAN (C$ to €: 0.68)40; SWE (SEK to €: 0.11)41; UK (£ to €: 1.24).42 Where necessary, costs have been inflated to 2014 year prices and recalculated to reflect 3-month cycle.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD II, GOLD classification indicating moderate COPD; COPD, chronic obstructive pulmonary disease; GOLD III, GOLD classification indicating severe COPD; GOLD IV, GOLD classification indicating very severe COPD; CAN, Canada; ESP, Spain, SWE, Sweden; No Ex, no exacerbation; Non-SevEx, nonsevere exacerbation; SevEx, severe exacerbation; SEK, Swedish krona.
The baseline utility weights for each GOLD state were obtained from relevant sources in each country. In the case of CAN, UK weights were used due to the lack of data on Canadian COPD patients. Decrements associated with nonsevere exacerbations (1.66%) and severe exacerbations (4.82%) were obtained from Hoogendorn et al.34 The decrements refer to the annual utility loss of nonsevere and severe exacerbations, respectively. The utility weight associated with each state–event combination was recalculated to reflect the cycle length (3 months), and it was assumed that the decrements lasted for the entire duration of the model cycle. Table 6 shows the annual utility weights associated with each state–event combination.
Table 6.
Utility weights by GOLD state and type of exacerbation
| Event/GOLD | CAN/UK | ESP | SWE | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| II | III | IV | II | III | IV | II | III | IV | |
| No Ex | 0.79 | 0.75 | 0.65 | 0.82 | 0.72 | 0.57 | 0.73 | 0.74 | 0.52 |
| Non-SevEx | 0.77 | 0.74 | 0.64 | 0.81 | 0.71 | 0.56 | 0.72 | 0.73 | 0.51 |
| SevEx | 0.74 | 0.70 | 0.61 | 0.78 | 0.69 | 0.54 | 0.69 | 0.70 | 0.49 |
Notes: Weights for mild and moderate exacerbations were merged (averaged) if necessary to form “nonsevere exacerbation”. Utility weights were derived using the EQ-5D questionnaire. Data from Hettle et al26 (CAN/UK), Miravitlles et al49 (ESP), and Stahl et al50 (SWE).
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD II, GOLD classification indicating moderate COPD; COPD, chronic obstructive pulmonary disease; GOLD III, GOLD classification indicating severe COPD; GOLD IV, GOLD classification indicating very severe COPD; CAN, Canada; ESP, Spain, SWE, Sweden; No Ex, no exacerbation; Non-SevEx, nonsevere exacerbation; SevEx, severe exacerbation; EQ-5D, Euroqol 5 dimension.
Result
Base case
Base case results are presented in Table 7. TIO added 0.21 (CAN), 0.25 (ESP), 0.23 (SWE), and 0.23 (UK) quality-adjusted life years (QALYs) compared to GLY over the lifetime of the average patient. The incremental costs (€2014) compared to GLY were −€623 (CAN), €1,066 (ESP), €264 (SWE), and −€169 (UK). Thus, in the case of UK and CAN, TIO was found to be cost saving, as it was both more effective and less costly than GLY. For ESP and SWE, the cost per QALY (incremental cost-effectiveness ratios [ICERs]) was positive but small, at €4,281 and €1,137, respectively. The estimated ICERs are well below the existing willingness-to-pay thresholds for ESP (€30,000) and SWE (~ €50,000–€100,000).
Table 7.
Base case results
| Tiotropium | Glycopyrronium | Difference | |
|---|---|---|---|
| CAN | |||
| Costs (€) | |||
| Treatment costs | 1,933 | 1,554 | 380 |
| Direct costs | 20,171 | 21,174 | −1,003 |
| Total direct costs | 22,105 | 22,728 | −623 |
| Health outcomes | |||
| QALYs | 6.75 | 6.54 | 0.21 |
| Life years | 9.00 | 8.72 | 0.28 |
| ICER (€) | TIO dom | ||
| ESP | |||
| Costs (€) | |||
| Treatment costs | 1,318 | 1,255 | 63 |
| Direct costs | 39,812 | 38,809 | 1,003 |
| Total direct costs | 41,129 | 40,063 | 1,066 |
| Health outcomes | |||
| QALYs | 7.77 | 7.52 | 0.25 |
| Life years | 10.47 | 10.14 | 0.34 |
| ICER (€) | 4,281 | ||
| SWE | |||
| Costs (€) | |||
| Treatment costs | 1,898 | 1,532 | 366 |
| Direct costs | 18,171 | 18,273 | −102 |
| Total direct costs | 20,069 | 19,805 | 264 |
| Health outcomes | |||
| QALYs | 7.25 | 7.02 | 0.23 |
| Life years | 10.26 | 9.93 | 0.33 |
| ICER (€) | 1,137 | ||
| UK | |||
| Costs (€) | |||
| Treatment costs | 1,865 | 1,503 | 362 |
| Direct costs | 19,285 | 19,816 | −531 |
| Total direct costs | 21,150 | 21,319 | −169 |
| Health outcomes | |||
| QALYs | 7.37 | 7.13 | 0.23 |
| Life years | 9.82 | 9.51 | 0.31 |
| ICER (€) | TIO dom | ||
Notes: Discount rates: all costs and effects are discounted at an annual rate of CAN, 5%; ESP, 3%; SWE, 3%; and UK, 3.5%.
Abbreviations: QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; CAN, Canada; ESP, Spain; SWE, Sweden; TIO dom, tiotropium dominating.
Sensitivity analyses
Deterministic one-way sensitivity analyses were performed on key model parameters, in order to assess their impact on the main results.
Table 8 shows how the ICERs were affected when varying key model parameters one at a time. The scenarios that consistently impacted the ICER the most were a shorter time horizon and a lower RR of severe exacerbations. Shortening the time horizon to 5 years lowered the ICER. Lowering the RR of severe exacerbations to the 95% lower limit found in SPARK (GLY/TIO RR SevEx: 1.05) increased the ICER by >€10,000 in CAN, SWE, and UK.
Table 8.
One-way sensitivity analysis (ICER €2014)
| Variable of interest | Input | CAN | ESP | SWE | UK |
|---|---|---|---|---|---|
| Base case | TIO dom | 4,281 | 1,137 | TIO dom | |
| Discount rates | Costs: 0%, effects: 0% | TIO dom | 4,505 | 1,442 | 81 |
| Costs: 6%, effects: 6% | TIO dom | 4,039 | 814 | TIO dom | |
| Costs: 6%, effects: 0% | TIO dom | 2,328 | 475 | TIO dom | |
| Time horizon | 5 years | TIO dom | 1,174 | TIO | TIO dom |
| 10 years | TIO dom | 3,617 | 227 | TIO dom | |
| 20 years | TIO dom | 4,212 | 1,047 | TIO dom | |
| Treatment duration | 1 year | TIO dom | 4,147 | 1,070 | TIO dom |
| 10 years | TIO dom | 4,458 | 1,316 | TIO dom | |
| Life (35 years) | TIO dom | 4,707 | 1,631 | TIO dom | |
| Sex | Males only | TIO dom | 4,174 | 1,048 | TIO dom |
| Females only | TIO dom | 4,364 | 1,213 | TIO dom | |
| Start age | 40 years | TIO dom | 3,504 | 216 | TIO dom |
| 80 years | 2,293 | 4,384 | 1,201 | TIO dom | |
| GOLD start distribution | All start in GOLD II | TIO dom | 4,136 | 1,402 | TIO dom |
| All start in GOLD III | TIO dom | 4,318 | 997 | TIO dom | |
| All start in GOLD IV | TIO dom | 4,661 | 770 | TIO dom | |
| Subgroup | GOLD II | TIO dom | 1,421 | 1,276 | TIO dom |
| GOLD III | TIO dom | 5,259 | 1,087 | TIO dom | |
| GOLD IV | TIO dom | 11,421 | 2,112 | TIO dom | |
| Mortality | GOLD state mortality = normal mortality (not adjusted for COPD) | TIO dom | 4,384 | 1,255 | TIO dom |
| 20% higher excess mortality (SevEx) | TIO dom | 4,440 | 1,355 | TIO dom | |
| 20% lower excess mortality (SevEx) | TIO dom | 4,088 | 876 | TIO dom | |
| Effect of tiotropium on SevEx | Low 95% CI from SPARKa (RR SevEx: 1.05) | 9,942 | 5,875 | 12,509 | 10,102 |
| High 95% CI from SPARKa (RR SevEx: 1.97) | TIO dom | 4,161 | 308 | TIO dom | |
| RR SevEx: 1.00 for GOLD II patients | TIO dom | 4,579 | 1,691 | TIO dom | |
| Direct costs of SevEx | 20% higher | TIO dom | 3,909 | 611 | TIO dom |
| 20% lower | TIO dom | 4,592 | 1,576 | TIO dom | |
| QoL loss of SevEx exacerbations | 0% loss | TIO dom | 4,309 | 1,145 | TIO dom |
| 20% loss | TIO dom | 4,195 | 1,113 | TIO dom |
Notes:
Base case: RR SevEx GLY versus TIO =1.43.
Abbreviations: ICER, incremental cost-effectiveness ratio; CAN, Canada; ESP, Spain; SWE, Sweden; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GOLD II, GOLD classification indicating moderate COPD; GOLD III, GOLD classification indicating severe COPD; GOLD IV, GOLD classification indicating very severe COPD; COPD, chronic obstructive pulmonary disease; SevEx, severe exacerbation; CI, confidence interval; RR, relative risk; QoL, quality of life; TIO dom, tiotropium dominating; GLY dom, glycopyrronium dominating; TIO, tiotropium; GLY, glycopyrronium.
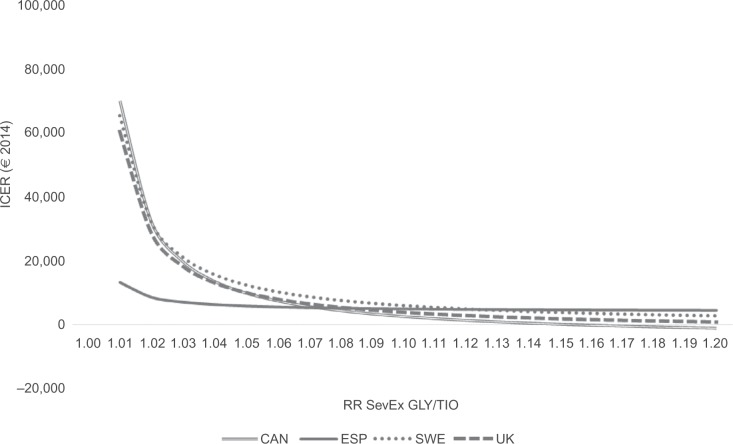
Figure 2 further explores the impact of the most important factor in explaining the results, the RR of severe exacerbations. When it is assumed that TIO and GLY are equally efficacious (RR SevEx: 1.00), TIO is dominated by GLY. This is a trivial result given that the two therapies are equally efficacious in every other regard and that GLY is less expensive. However, for CAN, SWE, and UK, as the RR increases in favor of TIO, the ICER quickly declines. For ESP, the decline is not as large, due to the smaller price difference between TIO and GLY in ESP (3% in ESP vs 22% in the other countries). Nevertheless, already at an RR of 1.02–1.03, TIO can be considered borderline cost-effective by most countries’ willingness-to-pay thresholds. At an RR of 1.43 (found in SPARK19), TIO would certainly be considered a cost-effective alternative to GLY.
Figure 2.
Impact of RR SevEx on ICER.
Abbreviations: RR, relative risk; SevEx, severe exacerbation; ICER, incremental cost-effectiveness ratio; GLY, glycopyrronium; TIO, tiotropium; CAN, Canada; ESP, Spain; SWE, Sweden.
Discussion
The primary objective of this study was to assess the cost-effectiveness of TIO monotherapy compared to GLY monotherapy in moderate to very severe COPD patients in CAN, ESP, SWE, and the UK. TIO was found to be cost-effective compared to GLY in all four countries. The results were to a large extent driven by the lower risk of severe exacerbations observed in the TIO cohort.
The SPARK estimate of relative efficacy of severe exacerbations (GLY/TIO RR SevEx: 1.43, P=0.025) is high compared to existing reviews and network meta-analyses of bronchodilators in COPD.28,51 These studies do not find any large and significant difference between LAMAs (eg, TIO, GLY, and aclidinium) either when it comes to effect on lung function or exacerbations.28,51 However, there are still inherent problems with comparing trials that have differing designs, that allow different co-treatments, and that have varying length of follow-up.52 These problems will be important when comparing exacerbation outcomes indirectly. Comparing SPARK to other relevant trials (eg, GLOW227), SPARK had longer follow-up, was more favorably designed to detect moderate to severe exacerbations, and included more severe patients. This likely affected the ability to detect and record severe events.
Exacerbation prevention is an important goal in the management of COPD and that cost-effectiveness is highly dependent on the ability to prevent exacerbations, particularly severe ones that lead to costly hospitalizations. This study has shown that given the best available head-to-head evidence on exacerbations to date (SPARK), the balance would likely sway in favor of TIO being a cost-effective alternative to GLY. In fact, our findings suggest that a much lower RR of severe exacerbations than the one found in SPARK would suffice to render TIO a cost-effective intervention compared to GLY.
The results presented here compare two dry powder inhalers (Spiriva HandiHaler and Seebri Breezhaler). TIO can also be delivered using the Respimat® inhaler, a soft mist inhaler (inhalation spray). The Tiotropium Safety and Performance in Respimat (TIOSPIR) trial showed that Respimat had a safety profile and exacerbation efficacy similar to that of the HandiHaler device.53 In addition, a head-to-head in vitro dose comparison between Respimat and Breezhaler found that Respimat delivered significantly higher doses of the drug to the lung (Respimat: 60–70 [% not determined]; Breezhaler 50–60 [% not determined]).54 These findings suggest that Respimat can successfully deliver TIO with maintained efficacy and safety and with far less drug deposition in the upper throat. In light of these findings, we expect the cost-effectiveness results to be applicable also to cases where TIO is delivered via the Respimat device.
Extensive sensitivity analyses were conducted to assess the impact of key parameters on the main results. The relative efficacy of the interventions, particularly in preventing severe exacerbations, played an important role in explaining cost-effectiveness. In this regard, it is important to remember the basic design and purpose of the UPLIFT and SPARK trials and how these factors potentially affected the results. The purpose of the UPLIFT trial was to assess the effect of the interventions on overall lung function; exacerbations were only a secondary objective. Additionally, the patients received usual non-LAMA care in both arms. This included LABA and/or corticosteroid therapy in a majority of the patients enrolled. The trial, not being primarily designed to detect exacerbations and the use of alternative inhaled therapy, likely pushed rates of exacerbations downward. This may explain why the study found no significant difference in the rates of severe exacerbations between the two arms.
As opposed to the UPLIFT trial, the SPARK trial was designed specifically to detect moderate and severe exacerbations.16,19 High-risk patients, that is, those having had at least one moderate exacerbation in the past year, were enrolled and randomly assigned to receive either QVA149 (LAMA/LABA combination), TIO, or GLY. Importantly, patients who were receiving LABA therapy discontinued the therapy upon enrolling in the trial, although ICS therapy continued. Indeed, these circumstances suggest that the SPARK trial was more favorably designed to detect and record exacerbations. Having said that, there were only 364 severe exacerbations reported in total across all three arms in SPARK. The low number of observed severe exacerbations naturally introduces some uncertainty in the estimate of the RR between TIO and GLY. It is important to keep in mind that, as opposed to mild and moderate exacerbations, severe exacerbations are infrequent events in COPD. In addition, SPARK did not include a placebo arm, which means that all arms in the trial contained active therapy with the specific aim of lowering rates of exacerbations.19 These circumstances suggest that even if a trial is favorably designed to detect severe exacerbations, it is always going to be difficult to amass a large study population to record many severe exacerbations. Future research should continue to focus efforts on disentangling the relative efficacy of different types of bronchodilators in preventing these rare events, as they are important factors explaining morbidity, mortality, and cost-effectiveness in COPD.
A potential drawback with this study, like any other utilizing trial data, is that the patient population might be somewhat different from the actual population of COPD patients (GOLD II–IV). Measures were taken to limit this potential bias, eg, by applying country-specific mortality rates. Furthermore, one should interpret differences between countries with caution, as there are substantial differences in the organization of health care and its funding. Differences in the way unit costs are estimated may also play an important role. Finally, it is important to keep in mind that the model presented here evaluates an exploratory scenario where the two cohorts are equal in every other regard except in terms of the risk of experiencing exacerbation.
Conclusion
Clinical data from UPLIFT and SPARK studies were incorporated in a Markov cohort model, which explicitly modeled the effects of exacerbations. Cost-effectiveness of TIO was assessed relative to GLY. TIO was found to be cost-effective relative to GLY given the current state of clinical evidence. The findings were to a large extent driven by TIO being relatively efficacious in preventing severe exacerbations, based on the findings in the SPARK trial.
Acknowledgments
The abstract of this paper was presented at the ISPOR 20th Annual International Meeting, Philadelphia, PA, USA, on May 16–20, 2015, as a poster presentation with interim findings (excluding Spain). The poster’s abstract was published in “Poster Abstracts” in Value in Health May 2015, Vol 18, Issue 3, Pages A173–A174. In addition, an analysis using the same model has been published during 2015.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The study was sponsored by Boehringer Ingelheim (a manufacturer of Spiriva [tiotropium]). OE and FB independently designed and executed the study. FA, JF, AT, MEO, CC, and MB are employees of Boehringer Ingelheim. The authors report no other conflicts of interest in this work.
References
- 1.Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2011;7:457–494. doi: 10.2147/COPD.S32330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 3.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 4.Halbert R, Natoli J, Gano A, Badamgarav E, Buist A, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. GOLD; 2016. pp. 1–111. [Google Scholar]
- 6.Guarascio AJ, Ray SM, Finch CK, Self TH. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mapel DW, Roberts MH. New clinical insights into chronic obstructive pulmonary disease and their implications for pharmacoeconomic analyses. Pharmacoeconomics. 2012;30(10):869–885. doi: 10.2165/11633330-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mapel DW, Robinson SB, Dastani HB, Shah H, Phillips AL, Lydick E. The direct medical costs of undiagnosed chronic obstructive pulmonary disease. Value Health. 2008;11(4):628–636. doi: 10.1111/j.1524-4733.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 9.Mapel DW, Hurley JS, Frost FJ, Petersen HV, Picchi MA, Coultas DB. Health care utilization in chronic obstructive pulmonary disease: a case-control study in a health maintenance organization. Arch Intern Med. 2000;160(17):2653–2658. doi: 10.1001/archinte.160.17.2653. [DOI] [PubMed] [Google Scholar]
- 10.Halpin DM. Health economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(3):227–233. doi: 10.1513/pats.200507-072SF. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Aymerich J, Pons IS, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax. 2011;66(7):585–590. doi: 10.1136/thx.2010.152876. [DOI] [PubMed] [Google Scholar]
- 12.Hoogendoorn M, Hoogenveen RT, Rutten-van Mölken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710. [DOI] [PubMed] [Google Scholar]
- 13.Solem CT, Sun SX, Sudharshan L, Macahilig C, Katyal M, Gao X. Exacerbation-related impairment of quality of life and work productivity in severe and very severe chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:641–652. doi: 10.2147/COPD.S51245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 15.Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012;40(6):1545–1554. doi: 10.1183/09031936.00048912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 17.Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533. doi: 10.1016/S2213-2600(13)70158-9. [DOI] [PubMed] [Google Scholar]
- 18.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 19.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 20.Keating GM. Tiotropium bromide inhalation powder. Drugs. 2012;72(2):273–300. doi: 10.2165/11208620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.van Mölken MR, Goossens L. Cost effectiveness of pharmacological maintenance treatment for chronic obstructive pulmonary disease. Pharmacoeconomics. 2012;30(4):271–302. doi: 10.2165/11589270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Pocket Guide to COPD Diagnosis, Management, and Prevention. GOLD; 2013. p. 30. [Google Scholar]
- 23.Price D, Asukai Y, Ananthapavan J, Malcolm B, Radwan A, Keyzor I. A UK-based cost-utility analysis of indacaterol, a once-daily maintenance bronchodilator for patients with COPD, using real world evidence on resource use. Appl Health Econ Health Policy. 2013;11(3):259–274. doi: 10.1007/s40258-013-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoogendoorn M, Kappelhoff BS, Overbeek JA, Wouters EF, Rutten-van Molken MP. Which long-acting bronchodilator is most cost-effective for the treatment of COPD? Neth J Med. 2012;70(8):357–364. [PubMed] [Google Scholar]
- 25.Eklund O, Afzal F, Borgström F. Cost-effectiveness of tiotropium versus usual care and glycopyrronium in the treatment of chronic obstructive pulmonary disease in Sweden. Cost Eff Resour Alloc. 2015;13(1):1. doi: 10.1186/s12962-015-0040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettle R, Wouters H, Ayres J, et al. Cost-utility analysis of tiotropium versus usual care in patients with COPD in the UK and Belgium. Respir Med. 2012;106(12):1722–1733. doi: 10.1016/j.rmed.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 27.D’Urzo A, Kerwin E, Overend T, D’Andrea P, Chen H, Goyal P. Once daily glycopyrronium for the treatment of COPD: pooled analysis of the GLOW1 and GLOW2 studies. Curr Med Res Opin. 2013;30(3):493–508. doi: 10.1185/03007995.2013.858618. [DOI] [PubMed] [Google Scholar]
- 28.Cope S, Donohue JF, Jansen JP, et al. Comparative efficacy of long-acting bronchodilators for COPD-a network meta-analysis. Respir Res. 2013;14(1):100. doi: 10.1186/1465-9921-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karabis A, Lindner L, Mocarski M, Huisman E, Greening A. Comparative efficacy of aclidinium versus glycopyrronium and tiotropium, as maintenance treatment of moderate to severe COPD patients: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:405–423. doi: 10.2147/COPD.S48967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ons.gov.uk [webpage on the Internet] Deaths Registered in England and Wales 2012. 2012. [Accessed November 3, 2014]. Available from: http://www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--deaths-registered-in-england-and-wales--series-dr-/2012/dr-tables-2012.xls.
- 31. statcan.gc.ca [webpage on the Internet] [Accessed November 2, 2014];Deaths and Mortality Rate, by Selected Grouped Causes, Age Group and Sex in Canada. 2011 Available from: http://www5.statcan.gc.ca/cansim/a26?id=1020551&retrLang=eng&lang=eng. [Google Scholar]
- 32. socialstyrelsen.se [webpage on the Internet] Database: Causes of Death in Sweden. 2012. [Accessed March 5, 2014]. Available from: http://www.socialstyrelsen.se/statistik/statistikefteramne/dodsorsaker.
- 33. who.int who.int who.int who.int [webpage on the Internet] Causes of Death Query Online Database. 2015. [Accessed March 23, 2015]. Available from: http://apps.who.int/healthinfo/statistics/mortality/causeofdeath_query/definitions/definitions.html.
- 34.Hoogendoorn M, Rutten-van Mölken M, Hoogenveen R, Al M, Feenstra T. Comparing the Cost-Effectiveness of a Wide Range of COPD Interventions Using a Stochastic, Dynamic, Population Model for COPD. Institute of Health Policy & Management, Erasmus University Rotterdam; 2010. [Google Scholar]
- 35. healthinfo.moh.gov.on.ca [webpage on the Internet] [Accessed October 30, 2014];Comparative Drug Index for Ontario Canada. 2014 Available from: https://www.healthinfo.moh.gov.on.ca/formulary/ [Google Scholar]
- 36. botplusweb.portalfarma.com [webpage on the Internet] Online Database of Drug Prices in Spain 2015. 2015. [Accessed March 1, 2015]. Available from: https://botplusweb.portalfarma.com/botplus.aspx.
- 37. msssi.gob.es [webpage on the Internet] Deductions for Medications in Spain. 2014. [Accessed March 1, 2015]. Available from: https://www.msssi.gob.es/profesionales/farmacia.
- 38.tlv.se [webpage on the Internet] Online Database of Reimbursed Drugs in Sweden. 2014. [Accessed November 3, 2014]. Available from: http://www.tlv.se/beslut/sok/lakemedel/
- 39. mims.co.uk [webpage on the Internet] Online Database of Drug Prices in the UK. 2014. [Accessed November 2, 2014]. Available from: http://www.mims.co.uk/
- 40. bankofcanada.ca [webpage on the Internet] [Accessed June 22, 2015];Year Average of Exchange Rates 2014. 2014 Available from: http://www.bankofcanada.ca/stats/assets/pdf/nraa-2014-en.pdf. [Google Scholar]
- 41. riksbank.se [webpage on the Internet] Annual Average Exchange Rates in 2014 in Sweden. 2015. [Accessed June 22, 2015]. Available from: http://www.riksbank.se/sv/Rantor-och-valutakurser/Arsgenomsnitt-valutakurser/?y=2014&m=12&s=Comma#search.
- 42. bankofengland.co.uk [webpage on the Internet] Annual Average Exchange Rates. 2015. [Accessed June 22, 2015]. Available from: http://www.bankofengland.co.uk/statistics/Pages/default.aspx.
- 43.Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–637. doi: 10.2165/00019053-200523060-00008. [DOI] [PubMed] [Google Scholar]
- 44.Mittmann N, Kuramoto L, Seung S, Haddon J, Bradley-Kennedy C, Fitzgerald J. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102(3):413–421. doi: 10.1016/j.rmed.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Miravitlles M, Sicras A, Crespo C, et al. Costs of chronic obstructive pulmonary disease in relation to compliance with guidelines: a study in the primary care setting. Ther Adv Respir Dis. 2013;7(3):139–150. doi: 10.1177/1753465813484080. [DOI] [PubMed] [Google Scholar]
- 46.Sicras A, Huerta A, Navarro R, Ibañez J. Uso de recursos y costes asociados a las exacerbaciones de enfermedad pulmonar obstructiva crónica: estudio retrospectivo de base poblacional [Use of resources and associated costs of chronic obstructive pulmonary disease exacerbations: a population based retrospective study] Semergen/Sociedad Espanola de Medicina Rural y Generalista. 2013;40(4):189–197. doi: 10.1016/j.semerg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respir Med. 2002;96(9):700–708. doi: 10.1053/rmed.2002.1334. [DOI] [PubMed] [Google Scholar]
- 48.Jansson SA, Backman H, Stenling A, Lindberg A, Ronmark E, Lundback B. Health economic costs of COPD in Sweden by disease severity – has it changed during a ten years period? Respir Med. 2013;107(12):1931–1938. doi: 10.1016/j.rmed.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Miravitlles M, Huerta A, Fernández-Villar JA, et al. Generic utilities in chronic obstructive pulmonary disease patients stratified according to different staging systems. Health Qual Life Outcomes. 2014;12(1):120. doi: 10.1186/s12955-014-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stahl E, Lindberg A, Jansson S-A, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3(1):56. doi: 10.1186/1477-7525-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones PW. Long-acting muscarinic antagonists for the prevention of exacerbations of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2015;9(3):84–96. doi: 10.1177/1753465815576471. [DOI] [PubMed] [Google Scholar]
- 52.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–428. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Wise RA, Anzueto A, Cotton D, et al. Tiotropium respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 54.Ciciliani A, Langguth P, Bickmann D, Wachtel H, Voshaar T. vitro dose comparison of Respimat® Soft Mist™ inhaler with dry powder inhalers for COPD maintenance therapy; ISAM 2013 Conference; April 6–10, 2013; Chapel Hill, NC. [Google Scholar]