Abstract
The humoral response contributes to the protection against viral pathogens. Although antibodies have the potential to inhibit viral infections via several mechanisms, an ability to neutralize viruses directly may be particularly important. Neutralizing antibody titers are commonly used as predictors of protection from infection, especially in the context of vaccine responses and immunity. Despite the simplicity of the concept, how antibody binding results in virus inactivation is incompletely understood despite decades of research. Flaviviruses have been an attractive system in which to seek a structural and quantitative understanding of how antibody interactions with virions modulate infection because of the contribution of antibodies to both protection and pathogenesis. This review will present a stoichiometric model of antibody-mediated neutralization of flaviviruses and discuss how these concepts can inform the development of vaccines and antibody-based therapeutics.
Introduction
Flaviviruses are positive-stranded RNA viruses that have the potential to cause significant morbidity and mortality in humans. Many viruses of this genus have a global impact on human health including the mosquito-borne dengue virus (DENV), yellow fever virus (YFV), Japanese encephalitis virus (JEV) and West Nile virus (WNV), and the tick-borne encephalitis viruses (TBEV). Flaviviruses are endemic in many regions of the globe. For example, it has been estimated that 390 million human DENV infections occur each year, with 3.6 billion people at risk of infection in more than 100 countries (1). DENV is now the leading arthropod-borne viral disease in the world. Sporadic intense local transmission of flaviviruses also may be a serious threat to public health as illustrated by WNV activity in the United States in over the past decade (2). Flaviviruses cause a variety of disease manifestations including encephalitis and paralysis, massive hepatic injury, and hemorrhagic and plasma leakage syndromes associated with visceral organ injury. At present, there is no specific therapy to treat flavivirus infections; only vaccines have proven effective at blunting the impact of these viruses on public health.
Multiple strategies have been employed for generating effective flavivirus vaccines (reviewed by (3)). The live-attenuated YFV-17D vaccine, created by Max Theiler and colleagues in 1938, was created by extensive passage of the virulent Asibi strain ex vivo (reviewed by (4, 5)). While more than 600 million doses of this highly effective vaccine have been administered, several hundred thousand human infections (and ~30,000 deaths) still occur annually, primarily in Africa and South America (6, 7). Numerous vaccines have been created for JEV (reviewed by (8)). First generation vaccine strategies used inactivated mouse brain preparations of antigen. The live-attenuated SA14-14-2 was developed in China in the late 1980’s and has been deployed extensively in several JEV-endemic countries. The development of second and third generation JEV vaccines remains an active area of study as reflected by the introductions of a formalin-inactivated Vero cell-derived vaccine (IXIARO) (9) and the licensing of a live-attenuated JEV-YF-17D chimeric vaccine (IMOJEV) (10). The clinical impact of tick-borne encephalitis virus (TBEV) has been reduced substantially in regions where the vaccine is used; a formalin-inactivated viral vaccine has been administered widely in Austria and is estimated to be 96–99% effective (11, 12). Combined with the success of veterinary vaccines against WNV (13), these experiences suggest flavivirus-induced disease can be prevented. However, vaccines are not yet available for all flaviviruses that impact human health. Despite numerous promising candidates, no vaccine for WNV is available yet for use in humans (reviewed by (13)). Critically, vaccines are not yet approved to protect against the four groups (serotypes) of dengue viruses circulating among much of the human population. Indeed, the most advanced tetravalent live-attenuated DENV vaccine candidate showed a poor efficacy rate in DENV-naïve individuals in a recently published phase 3 clinical trial (14).
Neutralizing antibodies have been shown to correlate with protection against several flavivirus infections following vaccination (4, 15), and are a critical component of immunity to natural infections (reviewed by (16)). However, the role of antibodies in DENV pathogenesis is more complex. Pioneering studies by Sabin demonstrated that DENV infection could be prevented by vaccination with a single serotype (17). Although short-lived (~6 months) protection was observed against all four DENV serotypes, long-term protection was generated only against the DENV of the same serotype. Because several epidemiological studies identify heterologous DENV infection as a significant risk factor for severe, potentially fatal, clinical manifestations of disease (reviewed in (18)), monovalent vaccines are not tenable and a tetravalent approach is considered necessary. The current generation of live-attenuated, inactivated, and subunit-based DENV vaccine candidates target all four serotypes for antigenic responses (reviewed by (19)).
Although an understanding of the underlying mechanisms by which DENV infection by a given serotype sensitizes an individual to more severe disease after infection with a heterologous DENV serotype is incomplete, several lines of evidence support a pathogenic role of pre-existing or rapidly induced antibodies (reviewed in (20)). Antibody-dependent enhancement (ADE) of infection describes a marked increase in the efficiency of infection of cells expressing Fcγ-receptors in the presence of sub-neutralizing amounts of antibody (21, 22). Cross-reactive mouse, monkey, and human antibodies against the structural proteins have been shown to increase virus burden in mouse and primate models of DENV (23–27). Because a vaccine-induced humoral response has at least the theoretical potential to contribute to disease, understanding how antibodies interact with flavivirus virions is a critical area of study. In this review, we discuss how advances in our quantitative understanding of antibody neutralization of flavivirus infection provide insight into the types of antibodies that protect against or contribute to pathogenesis.
Flavivirus structure
The positive-sense genomic RNA of flaviviruses encodes a single open reading frame that is processed co- and post-translationally by cellular and viral proteases into ten different proteins. Flaviviruses are spherical virions (~50 nm diameter) composed of the structural proteins capsid (C), envelope (E), and pre-membrane (prM), a lipid membrane derived from the endoplasmic reticulum, and a ~11kb genomic RNA (reviewed by (28)). High-resolution atomic structures have been solved of the three structural proteins that comprise the virus particle (reviewed by (29)). The flavivirus E protein is an elongated molecule with three domains composed principally of β–strands that are connected to the viral membrane by a helical stem and two transmembrane domains (Figure 1). The E protein has a central role in virus attachment to cells, entry, and membrane fusion. Domain III (DIII) is an immunoglobulin-like domain hypothesized to interact with cellular attachment factors that enhance the efficiency of virus entry, such as heparin sulfate (30, 31). Domain II (DII) is composed of two elongated fingers that contribute many of the contacts required for the dimerization of E proteins on the surface of mature virus particles (32). Importantly, the distal end of DII contains a hydrophobic fusion loop (DII-FL) that is highly conserved among flaviviruses (33, 34). A central domain I (DI) is connected to both DII and DIII via flexible linkers that facilitates rotation among the three domains required for E protein function. The E protein may be glycosylated at one or two positions (DI or DI and DII, respectively) in some flavivirus strains.
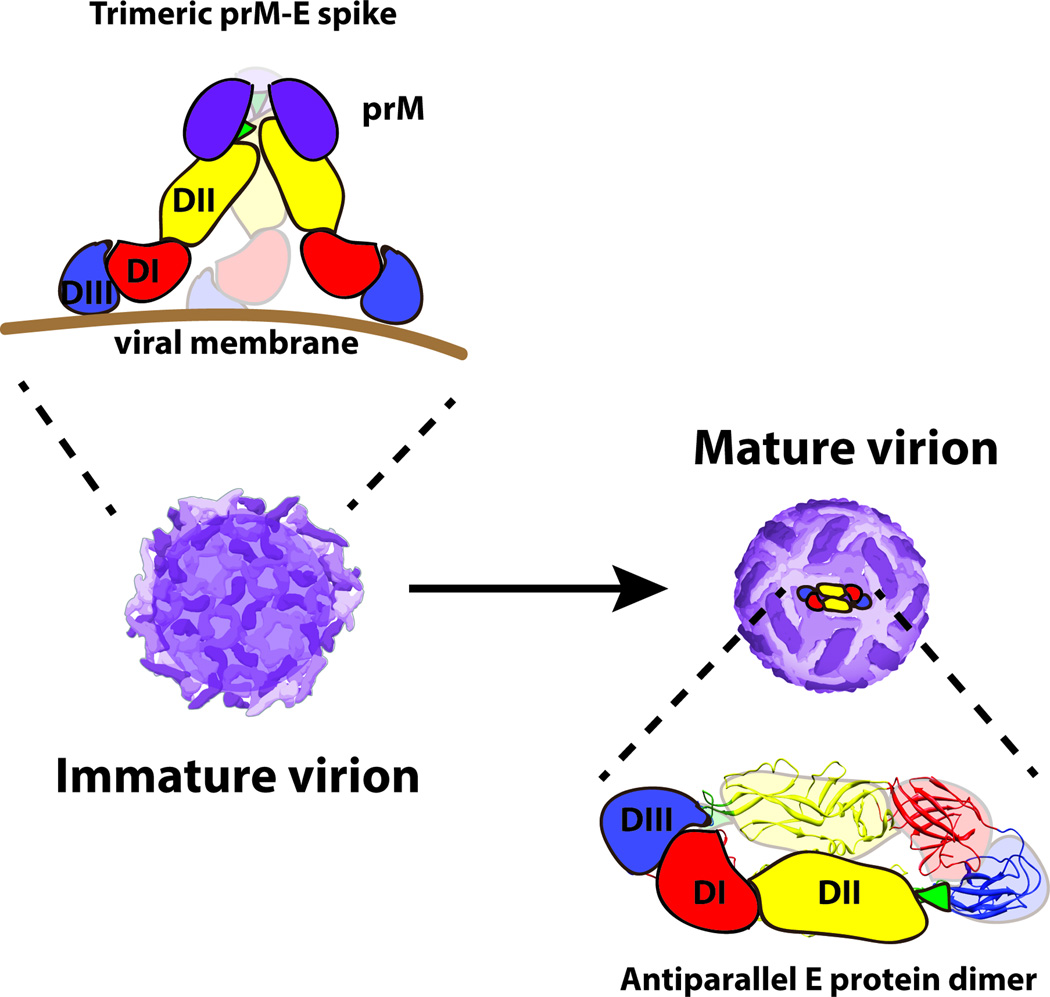
Figure 1. The structure of the flavivirus envelope proteins and their organization on the virus particle.
Flaviviruses are small spherical virions that incorporate a dense array of prM and E proteins that function to promote virus assembly, budding, and entry. The E protein is an elongated three domain molecule that is the principal target of neutralizing antibodies. Domain III (DIII, shown in blue) is thought to interact with receptors on target cells. The conserved 13 amino-acid fusion loop (shown in green) is located at the distal end of domain II (DII, shown in yellow). DIII and DII are connected by the central domain I (DI, shown in red). In this cartoon, the prM protein is depicted as a purple spherical oval. Flaviviruses assemble as immature virus particles on which the E protein exists as sixty trimers of prM-E dimers that project as spikes away from the virion surface. Virus maturation is mediated by cleavage of prM by a cellular furin protease. On mature virions, the E protein exists as anti-parallel homodimers that lie flat against the surface on the virion. The arrangment of E proteins on the mature DENV virion is depicted. Each virus particle is composed of 30 rafts of three antiparallel dimers in a herringbone pattern.
During the virion assembly process, spikes composed of three prM-E heterodimers are incorporated into immature virions in the lumen of the endoplasmic reticulum (Figure 1) (35, 36). Fully immature virus particles are non-infectious (37). prM functions to restrict structural transitions and control the oligomeric state of the E protein and thereby prevent adventitious fusion and inactivation of the virus particle during egress from infected cells (38, 39). Flaviviruses undergo a pH-dependent maturation process during transit through the trans-Golgi network defined by the cleavage of prM by a host furin-like serine protease (40, 41). The product of the maturation process is a mature virus particle that retains only the M peptide; the larger “pr” portion of prM remains with the particle throughout the secretory pathway and then dissociates from the virus particle upon release into the neutral pH of the extracellular space (39, 40).
The structure of mature flavivirus virions have been studied extensively by cryo-electron microscopy (28). Kuhn and Rossmann demonstrated that DENV virions are relatively smooth virus particles, with E protein dimers arranged in an unusual herringbone fashion (42). In this configuration, the 180 E proteins incorporated into the virion exist in three different chemical environments defined by their proximity to the three icosahedral symmetry axes of the virus particle. This dense arrangement of E proteins on the surface of the virion represents a complex surface for antibody recognition. Not all epitopes recognized by neutralizing antibodies are equally accessible on the E proteins in the three different symmetry environments of the mature virion. For example, the WNV DIII-reactive MAb E16 cannot bind its epitope on E proteins at the 5-fold symmetry axis due to steric constraints (43, 44). Thus, only 120 of 180 E proteins on the mature WNV virion can bind Fab fragments of the MAb E16 at saturation. Several potently neutralizing monoclonal antibodies (MAbs) against WNV and DENV recently have been shown to recognize complex epitopes formed from residues on more than one E protein of the mature virus particle (45–48); these antibodies typically do not have the capacity to bind monomeric E proteins. Studies of murine and human MAbs have revealed that prM and all three domains of E can be recognized by antibodies although the frequency with which specific epitopes are targeted by the humoral response varies among species (49–52). Moreover, the functional properties of anti-E antibodies that bind the virus particle vary substantially (for examples, see references (49, 53–55)).
A multiple-hit model for the neutralization of flaviviruses
Interest in the mechanism and stoichiometry of antibody-mediated neutralization dates back almost one hundred years, long before the concept and identity of antibody molecules was understood (reviewed in (56)). Early debate focused on the stoichiometric requirements for neutralization: how many antibodies are required to neutralize virus infectivity? One concept was that viruses could be neutralized following engagement by a single antibody molecule (57, 58). This “one-hit” hypothesis rested principally upon negative data obtained from kinetic neutralization experiments; the absence of a measureable lag phase of virus neutralization following the addition of antibody was interpreted as a requirement for binding by only a single antibody. From this perspective, virions were thought to contain sites of vulnerability that result in non-infectious virions upon binding by antibody. Several limitations of this model have been discussed, including that antibodies bind antigens rapidly relative to the rate at which biological outcomes are measured (59). As an example, high affinity WNV-reactive antibodies bind virus in solution in seconds, whereas the half maximal binding of WNV to cells expressing a highly efficient virus attachment factor is 45 minutes (60, 61)! The alternative “multiple hit” model assumes that virions become decorated with antibody and are neutralized only at a critical occupancy (21, 59). The number of antibodies required to neutralize viruses with different structures has been hypothesized to vary markedly (reviewed by (56, 62)).
‘The early literature describing antibody-mediated neutralization of flaviviruses arose from efforts to distinguish among a growing number of antigenically-related viruses (63, 64). Many early observations supported the concept that the docking of multiple antibody molecules was required for the neutralization of flaviviruses. This evidence was presented in an outstanding review by Della-Porta and Westaway (59). Since that time the large number of studies on flavivirus interaction with antibodies has improved our understanding of the basis of neutralization and refined models of the relationship between antibody occupancy and virus inactivation.
A neutralization resistant population of flaviviruses
One prediction of a multiple-hit model of neutralization is that infectious virions can be decorated by antibody with a stoichiometry insufficient for neutralization even under conditions of antibody excess or saturation. Neutralization resistant viruses may express epitopes in small numbers (small number of viral protein targets/virion) or display them in an inaccessible manner. Several factors may limit epitope accessibility on the virion, such as steric constraints among densely arranged viral proteins (43, 65), proximity to the viral membrane (66), or the presence of carbohydrates that shield antibody-binding determinants (67). Neutralization profiles of some flavivirus-reactive immune sera or MAbs reveal a plateau effect in which a subset of virions remains resistant to neutralization despite saturating antibody concentrations (Figure 2A). In this context, a fraction of virus particles are not neutralized despite the presence of high concentrations of flavivirus-reactive immune sera or MAbs, whereas the remaining fraction of virions is fully neutralized. The existence of a neutralization resistant fraction of virions could be explained by structural heterogeneity that translates into differences in the maximal number of antibodies bound to an individual virion, or the existence of a subset of viruses in the stock encoding mutation(s) at epitopes recognized by neutralizing antibodies.
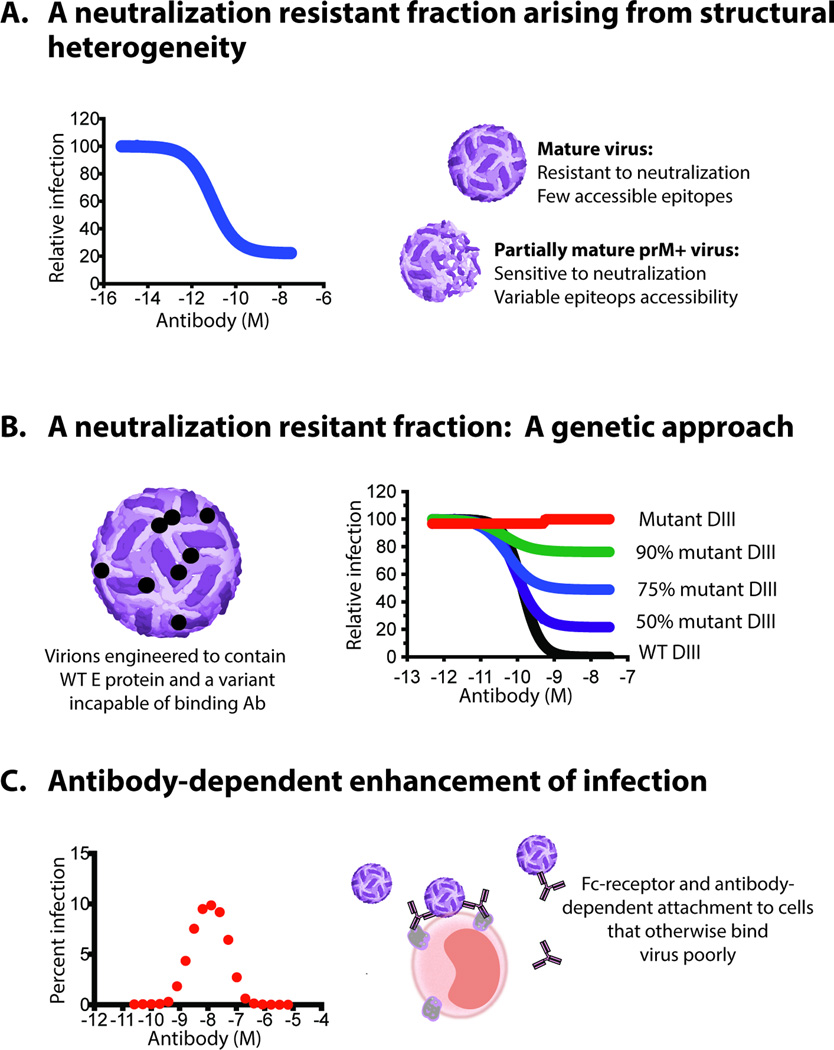
Figure 2. Evidence in support of a multiple-hit requirement for neutralization.
Several types of experimental studies support a multiple-hit model of antibody neutralizatin of flaviviruses. A. In some instances, neutralization assays reveal a plateau effect at high concentrations of antibody. For flaviviruses, the neutralization resistant fraction is a consequence of the significant structural heterogeneity of viruses released from infected cells. Mature virions in the population that do not display enough epitopes for a given antibody with a stoichiometry sufficient for neutralization are resistant to inhibition. Thus, infectious virions can be decorated by antibody with a stoichiometry insufficient for neutralization even under conditions of antibody excess or saturation. B. A fraction of WNV virions resistant to neutralization can be created experimentally by reducing the number of epitopes on the average virion using genetic approaches in which E proteins with mutations (in this instance on DIII) that do not bind a given epitope are increasingly expressed on virions. C. ADE describes a phenomenon in which antibody binds at an occupancy that is insufficient for neutralization yet capable of enhancing the efficiency of virus attachment and entry into cells via interactions with Fcγ-receptors expressed on cells. Because incubation with sub-neutralizing concentrations of antibody can enhance the infectivity via ADE, docking of a single antibody to the virion is insufficient for neutralization.
Insight into the mechanisms responsible for the neutralization of the resistant fraction of flaviviruses was obtained through a series of studies with reporter virus particles (RVPs). RVPs are pseudo-infectious virions produced by genetic complementation of a sub-genomic replicon with the structural proteins of the virus in trans (68, 69). Importantly, the production of RVPs by complementation with cDNA expression constructs eliminates the possibility that neutralization escape mutations are present in virus stocks. A neutralization resistant fraction of WNV and DENV RVPs has been documented in experiments with many E- and prM-reactive MAbs. For example, studies with the WNV E protein-specific MAb E53 indicated that a significant fraction of WNV RVPs produced in HEK-293T cells were resistant to neutralization at concentrations of antibody sufficient to result in antibody occupancy of all accessible epitopes on the virus particle (70). The size of the E53 resistant fraction varied among different RVP preparations (70) and when assayed using different target cells (71). The resistant fraction could be manipulated by changing the completeness of virion maturation, which modulates the number of prM and DII-fusion loop epitopes (among others) accessible for antibody recognition. The appearance of a resistant fraction reflects the subset of virions in the population that do not display epitopes with a stoichiometry sufficient to support neutralization (Figure 2A). In agreement with this interpretation, a resistant fraction of WNV can be created experimentally by reducing the number of epitopes on the average virion using genetic approaches (21) (Figure 2B). Collectively, these findings suggest that neutralization of infectious virions requires engagement of flaviviruses by multiple antibody molecules.
Antibody-dependent enhancement of flavivirus infection
Early studies by Halstead and colleagues demonstrated that under some circumstances flavivirus infection is enhanced by virus-reactive antibodies (72, 73). Initially, DENV immune sera were shown to enhance the infection of primary blood mononuclear cells in a concentration-dependent fashion. ADE was dependent upon the Fc-portion of the antibody molecule and could be blocked by antibodies against Fcγ-receptors (74). The cellular mechanism of ADE relates to an increase in the efficiency of virus attachment and entry into cells via interactions between antibodies bound to virions and Fcγ-receptors expressed on cells (Figure 2C) (75–77); antibody-dependent signaling through Fcγ-receptors also has been proposed to affect the permissiveness of the target cell by modulating antiviral and pro-inflammatory responses through a process termed “intrinsic ADE” (reviewed by (78)). That incubation with non-neutralizing concentrations of antibody may enhance infectivity via antibody-mediated attachment to target cells demonstrates that docking of a single antibody to the virion is not sufficient for neutralization and is in agreement with a “multiple-hit” concept of antibody-mediated neutralization. The stoichiometric requirements for ADE will be discussed in more detail below.
The stoichiometry of neutralization and enhancement of flaviviruses
Although early studies provided compelling evidence in support of a “multiple-hit” model for neutralization of flaviviruses (59), the stoichiometric requirements for neutralization of these viruses remained unknown. Quantitative insights into the requirements for neutralization are of value for defining characteristics of antibodies with significant neutralization potential, as well as those that might readily enhance infection of cells expressing Fcγ-receptors. To estimate the number of antibody molecules required for neutralization of WNV, two complementary experimental approaches were employed using a small panel of E DIII-reactive antibodies (21).
The relationship between antibody occupancy and neutralization
The fraction of epitopes on a virus particle bound by antibody at any given concentration by antibody can be estimated simply if the strength of binding to the virus particle can be measured. The fractional occupancy can be calculated for any antibody concentration using the following equation, provided assays are performed under conditions where free antibody remains in large excess over informative points of the antibody dose-response curve.
Ab bound/Ab boundmax=[Ab]/([Ab]+Kd)
The epitope occupancy requirement for neutralization of WNV was examined by integrating neutralization dose-response profiles of a panel of MAbs with estimates of their functional affinity for virions (21). These results revealed that the fraction of epitopes bound by antibody when WNV was neutralized varied considerably among MAbs that recognized distinct epitopes on the virus particle. Potent neutralization by some MAbs was observed even when a small fraction of epitopes was bound; in these instances, complete neutralization of the population of viruses studied was achieved. Antibodies with these characteristics have a “low” occupancy requirement for neutralization. For example, the WNV-specific MAb E16 completely neutralized WNV infection when less than half of the DIII-lateral ridge (DIII-LR) epitopes were bound (21). Alternatively, neutralization by other antibodies was observed only when a large fraction of accessible epitopes on the virion was engaged. In many instances neutralization was not possible even when all of the accessible epitopes on an individual virions were bound. Antibodies with this type of “high” occupancy requirement may be incapable of neutralizing all the virions added to a neutralization experiment even when added at saturating concentrations. DENV-reactive antibodies with both “low” and “high” occupancy requirements also have characterized in a series of biochemical and genetic studies by Barrett and colleagues (79, 80).
Epitopes recognized by a large number of mouse, monkey, and human anti-flavivirus MAbs have been mapped using biochemical, genetic, and neutralization escape approaches ((49, 50, 81, 82) and references within). Unexpectedly, some of these epitopes are not predicted to be accessible for antibody recognition using static models of the mature virion structure (83–86). Comparison of the occupancy requirements for neutralization by an antibody and predicted accessibility of its corresponding epitope on the mature virus particle revealed an inverse correlation. Antibodies that bind epitopes predicted to be readily accessible on the surface of virions (such as the DIII-LR) neutralize infection by binding a small fraction of the constituent E proteins. In contrast, antibodies with “high” occupancy requirements for neutralization typically bind cryptic epitopes defined by the contribution of amino acids not predicted to be accessible for interaction with the antibody paratope. For many antibodies, the mode of epitope recognition cannot be explained using existing models of virion structure. As detailed below, understanding the structural basis for antibody recognition has expanded our understanding of the ensemble of structures of infectious flaviviruses.
Estimating the stoichiometry of WNV neutralization using mixed virion particles
Cryo-electron microscopic (cryo-EM) reconstruction studies of Fab fragments of WNV E16 docked on WNV revealed that this antibody can bind 120 of 180 E proteins on the virus particle (44), confirming earlier predictions (43). As mentioned above, the DIII-LR epitope recognized by E16 is positioned too close together at the five-fold symmetry axis of the mature virion, so that it cannot be bound due to steric constraints. To estimate the fraction of DIII-LR epitopes bound by E16 when WNV is neutralized, populations of RVPs were produced composed of varying proportions of wild-type (WT) E proteins and a variant encoding a single amino acid substitution (T332K), which prevents antibody binding and epitope recognition (60); indeed, structural studies demonstrated that the T332 is a principal contact residue for E16 recognition (43). Accordingly, antibody dose-response curves from studies with populations of virions composed on both WT and T332K E proteins resulted in the appearance of a fraction of viruses resistant to neutralization (21). The size of the resistant fraction changed as a function of the number of T332K E proteins assembled into the virion (Figure 2B), which were incapable of binding E16 antibody. Inspection of these curves revealed that roughly 50% of virions were sensitive to neutralization when one quarter of the E proteins had an intact DIII-LR epitope, corresponding the a requirement for 30 antibodies for neutralization of WNV (25% of 120 accessible sites). Similar results were obtained with a second DIII-LR antibody, E24 (75).
Is 30 antibodies a reasonable number?
The number of antibodies required to neutralize animal viruses has been studied using multiple approaches (reviewed by (56)). In many instances, the number of antibodies required for neutralization correlates with the size of the virus particle. A requirement for 30 MAbs to neutralize WNV agrees well with the number of antibodies predicted by the antibody “coating theory” of neutralization (62). Unfortunately, a threshold number of 30 antibody molecules by itself does not provide insight into structural or mechanistic requirements for neutralizing virus infectivity, as discussed below.
Experimental and conceptual limitations
In our view, several independent lines of evidence support a multiple-hit requirement for antibody neutralization of flaviviruses. Our estimates of the precise stoichiometry required for neutralization arose from two complementary experimental approaches to study the interaction of antibodies recognizing a single epitope (21). However some caveats and unanswered questions exist:
(i.) Measurement of antibody affinity
Analysis of the occupancy requirements for neutralization requires an accurate estimate of the monovalent antibody affinity for the virion. While highly quantitative measures of antibody binding to soluble recombinant viral envelope proteins have been obtained and been informative in many contexts, these reductionist approaches do not capture the full complexity of the virion structure. With few exceptions, biosensors have not been used to measure the kinetics of antibody binding to flaviviruses due to the challenges of capturing intact virions or the requirement for having precision and costly instruments in a high-level containment facility (87, 88). Instead, a common strategy to study the strength of antibody binding to viral antigens employs an ELISA. Limitations of this approach include the potential to distort the structure of the virion due to the method used to capture virus to plastic, the potential for artifactual bivalent antibody engagement arising from plating density, and quantitative limitations in the assay format. Some of the issues can be mitigated through the use of Fab fragments that are directly labeled (e.g., 125I) to ensure the linearity of the assay.
(ii.) How many accessible epitopes are present on the virion?
Structural studies of the DIII-LR epitope suggest that residues critical for recognition by E16-like antibodies are accessible for binding at 120 locations on the mature virus particle. Sixty DIII-LR epitopes are not bound by Fab fragments of E16 due to steric clashes arising from close proximity of DIII in the five-fold symmetry environment (43, 44). An estimate of the number of DIII-LR antibodies capable of simultaneously binding the virion was a critical piece of information required to estimate the number of number of antibody binding events that define the stoichiometric threshold (21). However, the number of intact antibodies capable of binding the virion may differ from the maximal occupancy of a Fab fragments due to their increased bulk. Indeed, biochemical studies suggest that the number of IgG molecules capable of simultaneously binding virions may be reduced as compared to Fabs (89). A role for the heavy chain of the antibody molecule in defining the stoichiometry of neutralization was hypothesized to contribute to the maturation state-sensitive pattern of neutralization by a DIII-LR antibody (90). To date, the stoichiometry of neutralization by a Fab fragment has not been solved.
(iii.) Beyond the DIII-LR epitope?
Estimates for a stoichiometric threshold of 30 antibodies arise from experiments with antibodies that bind a single epitope (21). To date, experiments with antibodies of differing specificity (e.g., DII-FL MAbs) have been complicated by other factors including whether it is experimentally possible to change the maximal number of epitopes accessible for antibody recognition and retain infectivity.
(iv.) Relating stoichiometry and function
Antibodies have the potential to inhibit virus infection via numerous mechanisms. For flaviviruses, antibodies that block virus attachment have been characterized (43) and may be an important component of the overall neutralizing activity of human sera (91). Antibodies also may inhibit conformational changes in the E protein required for fusion and thus, have the ability to neutralize viruses already attached to cells. Several antibodies, including the WNV-reactive MAb E16, are capable of inhibiting the fusion of antibody-decorated virus particles with synthetic liposomes (88, 92, 93). During infection, virions decorated by E16 are unable to escape from endosomal compartments, consistent with an inability to fuse with membranes (93). Cryo-EM studies of E16 Fab-decorated virions incubated at the acidic conditions that are required to trigger the fusion process suggested that at high concentrations, Fab fragments trap the virus particle at an intermediate step in the fusion process (94). This exciting result highlights the potential for antibodies as tools to understand the mechanisms of a dynamic and quite rapid fusion process.
While considerable insight into the mechanisms of neutralization exists, why ~30 antibodies is required for neutralization is unclear. The surface area contacted directly when 30 antibodies engage the virion is modest (<10%) (21, 43). While Fab fragments have neutralizing activity, the potency of neutralizing antibodies may be influenced significantly by contributions from the heavy chain. Furthermore, antibodies may neutralize via distinct mechanisms when decorated at different occupancies. While E16 has been shown to modestly block attachment at high concentrations (43, 61), at the EC50 inhibition is mediated largely at a post-attachment step (43). Paradoxically, fusion loop antibodies that bind the virion only in the presence of prM efficiently block attachment (43). The relationships between epitope location, mechanism of neutralization, and antibody occupancy require further study.
Factors that modulate the stoichiometry of neutralization
Considering the stoichiometric requirements for neutralization provides a useful platform to explore factors that modulate the potency of neutralizing antibodies. In many instances, epitope mapping studies alone of neutralizing antibodies do not explain how antibodies engage the virus particle. In fact, several well-characterized anti-flavivirus antibodies bind epitopes not predicted to be accessible for recognition using static models of the mature virus particle (42, 85, 86). Epitope accessibility is a critical aspect for defining conditions that allow for occupancy of the virion with a stoichiometry sufficient for neutralization, as detailed above (95). Two properties to the virion have been characterized with the potential to regulate the accessibility of epitopes on the virion.
Virion maturation
Biochemical studies of the composition of flaviviruses commonly reveal the presence of uncleaved prM in virus preparations (reviewed by (96)). The extent of uncleaved prM may vary as a function of the cell type in which virus was propagated and the time of harvest in culture (97)(S. Mukherjee and T. Pierson, unpublished data). The significance of prM in virus stocks cannot be inferred solely from biochemical studies, as they are incapable of distinguishing between two possibilities: uncleaved prM is found solely on non-infectious immature virions or uncleaved prM may be present on virus particles capable of productive entry into cells. Several observations identify the existence of infectious partially mature virions: (i.) prM can promote virus attachment to cells during entry (98), (ii.) changes in the efficiency of prM cleavage modulate the pH at which infectious virus is rendered inactive (99), (iii.) prM-reactive antibodies may enhance infection via ADE (49, 82), and (iv.) the presence of prM alters the sensitivity of virions to neutralization by anti-E protein antibodies (70).
We refer to partially mature viruses as virions on which prM is cleaved incompletely. Despite considerable progress, the structures of this heterogeneous population of virions are not fully understood. Cryo-EM tomographic reconstructions suggest partially mature virions are a mosaic of regions with structural similarity to immature virions (heterotrimeric spikes of prM and E) or mature virus particles (antiparallel E proteins arranged in a herringbone pattern) (100). Immunoprecipitation studies of DENV suggest that most of the E protein in virus-containing supernatants from Vero cells can be bound by prM-reactive antibodies, indicating that under these culture conditions, a majority of secreted virions retain prM (101). The extent of prM cleavage of viruses produced in vivo remains unknown.
The prM content of infectious virions alters the sensitivity of the virus particle to neutralization by many classes of E protein-reactive antibodies (reviewed by (96)). In all instances to date, viruses that retain uncleaved prM are more sensitive to neutralization than fully mature virions; thus, the process of virion maturation also could serve an independent immune evasion function. Because the epitopes recognized by maturation state-dependent antibodies are predicted to be poorly accessible on mature virions, it has been hypothesized that this pattern of maturation state-sensitive neutralization reflects changes in epitope accessibly (70). For example, the antibodies that bind the highly conserved DII-FL only neutralize WNV and DENV particles retaining a significant amount of uncleaved prM. Structural studies confirm that the DII-FL mAb E53 only binds prM+ virions (102). In contrast, the DIII-LR reactive mAb E16 epitope is not sensitive to the maturation state of the virion (70). Of significance, polyclonal mixtures of antibodies also may display maturation state-sensitive patterns of neutralization. Half the recipients of two different candidate WNV vaccines mounted an antibody response less capable of neutralizing mature virions as compared to those that retain uncleaved prM (70). Cross-reactive, but not type specific neutralization DENV antibody responses have been shown to be maturation sensitive (71, 103).
Unexpectedly, a recent study reported that the neutralization potency of a DIII-LR MAb (E33) also is modulated by uncleaved prM on the virion (unlike E16) (90). Like all maturation state-sensitive MAbs characterized to date, E33 neutralizes mature virions at higher concentrations than needed for partially mature virions retaining significant prM. However, Fab and Fab'2 fragments of E33 neutralize wild-type WNV in a maturation state-independent manner. While E16 and E33 bind the virion with similar affinity, extensive epitope mapping experiments suggest they engage the DIII-LR in subtly different manner. These data suggest that the sensitivity of E33 to the prM content of WNV is explained by steric constraints imposed by the Fc domain of the antibody, which in turn governs the availability of an otherwise accessible DIII-LR epitope. The structural basis for this phenomenon requires further study, but highlights how factors beyond epitope accessibility may contribute to maturate state-sensitive patterns of neutralization.
The structural dynamics of virions
Most of the well-characterized epitopes recognized by antibody are not expected to be accessible for binding using current models of the mature flavivirus structure. As detailed above, chances in virus structure associated with virion maturation is one mechanism for modulating epitope accessibility. Changes in epitope accessibility also may arise from changes in virus structure occurring at equilibrium. Proteins are not static structures; their dynamic nature may be a key facet for understanding how proteins (including antibodies) bind their targets (104). Several elegant approaches have been used to demonstrate that picornaviruses sample multiple structures at equilibrium, a process referred to herein as viral “breathing” (105–107). For example, reversible changes in capsid structure have been shown to regulate neutralization by antibodies that target the VP4 protein of poliovirus (108). A role for flavivirus “breathing” initially was suggested by the observation that the complex-specific DENV MAb 1A1D2 binds virions in a temperature-dependent manner and appears to trap E proteins on the virus particle in a unique conformation (65).
A more general role for viral “breathing” in antibody recognition of flaviviruses has been established. Using modified neutralization assay conditions with prolonged incubations in the presence of antibody, the contribution of “breathing” to the overall neutralization sensitivity can be explored (61). Briefly, viruses and antibody are incubated for roughly one hour to allow for steady state binding, at which time the fractional occupancy of epitopes on the virion is defined by antibody concentration and affinity. Further increases in antibody binding will not occur unless the number or presentation of epitopes on the virion changes with time. Experiments with both WNV and DENV demonstrate that further increases in either the time or temperature of incubation results in a marked increase in neutralization potency; the magnitude of this change correlates well with the predicted accessibility of the epitope on the mature virus particle. This pattern of neutralization was not explained by the kinetics of antibody binding, virion aggregation, or the action of complement (61).
To explain these data, it is hypothesized that the accessibility of epitopes changes as viruses sample different ensembles of conformations during viral breathing. Given enough time, even antibodies with limited to almost no neutralizing activity in standard neutralization assays have the potential to dock on the virion with a stoichiometry sufficient for neutralization. In addition to exposing more epitopes, time-dependent changes in the antigenic surface of the virus particle also may allow antibody engagement of the virion with increased avidity via bivalent interactions among E proteins in conformations not present in the average state, as well as cooperative effects during antibody binding (61). A requirement for the presence of antibody during the prolonged incubation suggests that conformational changes that result in increases in epitope exposure on mature virions are reversible (109), as has been reported for picornaviruses (108). Of interest, strain-dependent patterns of neutralization may reflect differences in the structural ensembles sampled by related flaviviruses (53, 84).
The impact of the dynamic exposure of viral epitopes in vivo remains uncertain. Virtually nothing is known about the relevant concentrations and volumes that govern antibody-virion interactions in tissues and blood where the key events in the pathogenesis of these viruses occur. Because the kinetics of neutralization are increased by an elevated temperature, it is tempting to speculate that certain classes of antibodies, such as those recognizing the DII-FL epitope commonly observed in infected individuals, may function better than anticipated in the context of an acute febrile response.
Complement
The complement system is a family of serum and cell-surface proteins that recognize pathogens and activate inflammatory responses (110). Activation of complement contributes to protection against viral infection through the direct lysis of virions or infected cells, release of inflammatory peptides, opsonization of viruses, and by facilitating antigen presentation and immune cell priming. Opsonization of viruses with classical pathway complement components (C1q, C4b, and C3b) in an antibody-dependent manner can inhibit virus infection by blocking receptor attachment, entry, and fusion or by promoting terminal complement pathway activation and deposition of the C5b-C9 membrane attack components that results in virolysis. Indeed, the absence of classical pathway complement components leads to more severe primary WNV infection (111). Complement has been shown to increase the neutralization potency of antibodies in many systems, including flaviviruses (59, 75, 112–114). Not all IgG subclasses bind C1q avidly and thus complement-augmented neutralization is restricted to certain antibody isotypes (mouse IgG2a and 2b; human IgG1 and 3). Two lines of evidence suggest that C1q has the potential to alter the stoichiometric requirements for neutralization. First, C1q has the potential to markedly reduce the size of (or eliminate altogether) the neutralization resistant fraction of flaviviruses observed in studies of antibodies with a maturation state-dependent phenotype (75). Second, the resistant fraction observed with populations of virions engineered to display varying numbers of intact antibody epitopes also may be reduced (75). Experiments of this type led to the conclusion that the stoichiometric threshold for neutralization of WNV was reduced roughly 50% in the presence of C1q (75). How C1q functions in this regard is not completely understood, by likely involves its ability to cross-link antibodies on the surface of the virion. This mechanism is consistent with the analogous outcome of enhanced neutralization obtained by addition of cross-linking goat anti-mouse IgG secondary antibodies. In addition to its effects on neutralization, complement impacts conditions that support ADE by virtue of its ability to shift the stoichiometric threshold of neutralization below the minimal number of antibodies required to support ADE (discussed below). C1q inhibits ADE by WNV and DENV in vitro on cells that express the Fcγ-receptor CD32 and in vivo.
The stoichiometry of ADE
Insight into the stoichiometric requirements for neutralization provides a biochemical rationale to understand ADE. Analysis of large panels of flavivirus-reactive MAbs suggest that virtually all antibodies that bind the virion and neutralize infection have the potential to promote ADE in vitro, irrespective of their epitope specificity (21, 86). For human cells expressing the Fcγ-receptor CD32, concentrations of antibody that promote maximal enhancement of infection in vitro are similar to those that neutralize half of the virions in parallel studies (21). Thus, neutralization and ADE are phenomena related by the number of antibodies bound to the virion. When half of the virions are engaged by antibody with a stoichiometry sufficient to inactivate virus infection, the other half is not, and remains infectious despite being decorated by antibody. Antibodies that bind the flavivirus virion yet never attach a stoichiometric threshold sufficient for neutralization broadly enhance infection over a wide range of antibody concentrations. Thus, at the upper limit, the number of antibodies that promote ADE is defined by the stoichiometric threshold of neutralization. Because the dose-response curves of virus-antibody binding to cells and ADE are very similar, the minimal number of antibodies required for ADE is likely defined by the requirements for avid attachment to Fcγ-receptor. However, significant gaps in our knowledge exist. Recent studies suggest that neutralization potency is impacted by expression of Fcγ-receptors of target cells, which in turn translates into changes in the antibody concentrations that support ADE (115). Although not yet identified, it remains possible that antibodies exist which bind the surface E or prM protein in a specific geometry (e.g., parallel to the plane of the virus surface) that allows for neutralization but are not accessible to Fcy-receptors and thus cannot support ADE.
Insights into vaccines and therapeutics
During the past decade, structural and quantitative functional studies with MAbs have contributed to a detailed understanding of how antibodies engage virions and block infection of flaviviruses (reviewed in (95)). This information is now being exploited to identify antibodies with the most useful functional properties (24, 116, 117) and to deconstruct the complexity of polyclonal antibody responses to infection and vaccination (49, 54, 103, 118, 119). The limited accessibility of many epitopes recognized by antibodies on flavivirus virions is a critical determinant of the neutralization potency of antibodies. Antibodies that bind accessible epitopes on the virion neutralize infection at relatively low fractional occupancy, and thus low antibody concentrations (21). In contrast, epitopes that are poorly accessible require engagement of a larger fraction of these binding determinants in order to neutralize infection. Finally, not all epitopes support neutralization even at full occupancy (e.g., prM epitopes on a subset of partially mature virions and DII-FL epitopes on mature virions); these classes of antibodies have the potential to support ADE despite high concentrations of antibody (21, 70, 82).
Identifying readily accessible epitopes on the virus particle is an important practical goal as these represent promising targets for antibody-based therapeutics and vaccine responses. For example, the MAb E16 neutralizes infection at a very low fractional occupancy of its accessible DIII-LR epitope (21), is highly protective in passive transfer experiments (60), and has shown promise in animal models as a post-exposure therapeutic agent (117). E16 is capable of binding WNV irrespective of the maturation state of the virus particle (70). Unfortunately, humans do not appear to produce a functionally significant titer of antibodies that recognize this epitope in the context of natural infection (70, 120). Whether the structural and stoichiometric information about flaviviruses can be harnessed using recombinant antigens with novel platforms of epitope display remains to be determined (121, 122). If such programs were successful, safer and more effective vaccines likely could be generated against several species of flaviviruses.
Acknowledgments
This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, the Burroughs Wellcome Fund, and NIH grants R01AI073755 and R01A1089591. We apologize to those colleagues whose work we were unable to cite due to the limited scope of this review.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beasley DW, Barrett AD, Tesh RB. Resurgence of West Nile neurologic disease in the United States in 2012: what happened? What needs to be done? Antiviral research. 2013;99:1–5. doi: 10.1016/j.antiviral.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 4.Monath TP, Cetron MS, Teuwen DE. Yellow Fever Vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th. Saunders Elsevier; 2008. [Google Scholar]
- 5.Monath TP. Yellow fever vaccine. Expert review of vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 6.Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. The American journal of tropical medicine and hygiene. 2012;86:359–372. doi: 10.4269/ajtmh.2012.11-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrington CV, Auguste AJ. Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2013;13:198–210. doi: 10.1016/j.meegid.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert review of vaccines. 2011;10:355–364. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 9.Jelinek T. IXIARO updated: overview of clinical trials and developments with the inactivated vaccine against Japanese encephalitis. Expert review of vaccines. 2013;12:859–869. doi: 10.1586/14760584.2013.835638. [DOI] [PubMed] [Google Scholar]
- 10.Appaiahgari MB, Vrati S. IMOJEV((R)): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert review of vaccines. 2010;9:1371–1384. doi: 10.1586/erv.10.139. [DOI] [PubMed] [Google Scholar]
- 11.Heinz FX, Stiasny K, Holzmann H, Grgic-Vitek M, Kriz B, Essl A, Kundi M. Vaccination and tick-borne encephalitis, central Europe. Emerging infectious diseases. 2013;19:69–76. doi: 10.3201/eid1901.120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25:7559–7567. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011;3:269–285. doi: 10.2217/imt.10.93. [DOI] [PubMed] [Google Scholar]
- 14.Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A the CYDSG. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 15.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Suthar MS, Diamond MS, Gale M., Jr West Nile virus infection and immunity. Nature reviews. Microbiology. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- 17.Sabin AB. Research on dengue during World War II. The American journal of tropical medicine and hygiene. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 18.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Archives of virology. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 19.Yauch LE, Shresta S. Dengue virus vaccine development. Advances in virus research. 2014;88:315–372. doi: 10.1016/B978-0-12-800098-4.00007-6. [DOI] [PubMed] [Google Scholar]
- 20.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Advances in virus research. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 21.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell host & microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morens DM, Halstead SB, Marchette NJ. Profiles of antibody-dependent enhancement of dengue virus type 2 infection. Microbial pathogenesis. 1987;3:231–237. doi: 10.1016/0882-4010(87)90056-8. [DOI] [PubMed] [Google Scholar]
- 23.Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. The Journal of infectious diseases. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS pathogens. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell host & microbe. 2010;7:128–139. doi: 10.1016/j.chom.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng JK, Zhang SL, Tan HC, Yan B, Maria Martinez Gomez J, Tan WY, Lam JH, Tan GK, Ooi EE, Alonso S. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS pathogens. 2014;10:e1004031. doi: 10.1371/journal.ppat.1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes and infection / Institut Pasteur. 2011;13:1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perera R, Kuhn RJ. Structural proteomics of dengue virus. Current opinion in microbiology. 2008;11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nature medicine. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 32.Luca VC, AbiMansour J, Nelson CA, Fremont DH. Crystal structure of the Japanese encephalitis virus envelope protein. Journal of virology. 2012;86:2337–2346. doi: 10.1128/JVI.06072-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 34.Allison SL, Schalich J, Stiasny K, Mandl CW, Heinz FX. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. Journal of virology. 2001;75:4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. The EMBO journal. 2003;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. Journal of virology. 2007;81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elshuber S, Allison SL, Heinz FX, Mandl CW. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. The Journal of general virology. 2003;84:183–191. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 38.Moesker B, Rodenhuis-Zybert IA, Meijerhof T, Wilschut J, Smit JM. Characterization of the functional requirements of West Nile virus membrane fusion. The Journal of general virology. 2010;91:389–393. doi: 10.1099/vir.0.015255-0. [DOI] [PubMed] [Google Scholar]
- 39.Yu IM, Holdaway HA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. Journal of virology. 2009;83:12101–12107. doi: 10.1128/JVI.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 41.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. Journal of virology. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufmann B, Nybakken GE, Chipman PR, Zhang W, Diamond MS, Fremont DH, Kuhn RJ, Rossmann MG. West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12400–12404. doi: 10.1073/pnas.0603488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Science translational medicine. 2012;4:139ra183. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 46.Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, Crowe JE, Jr, Lok SM. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO molecular medicine. 2014;6:358–371. doi: 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr, de Silva AM. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18950–18955. doi: 10.1073/pnas.1011036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell host & microbe. 2010;8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE., Jr The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. mBio. 2013;4:e00873–e00813. doi: 10.1128/mBio.00873-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, Wu YC, Lai CY, Lu CH, Huang JH, Chang GJ, Wu HC, Wang WK. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS neglected tropical diseases. 2012;6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crill WD, Hughes HR, Delorey MJ, Chang GJ. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PloS one. 2009;4:e4991. doi: 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukupolvi-Petty S, Brien JD, Austin SK, Shrestha B, Swayne S, Kahle K, Doranz BJ, Johnson S, Pierson TC, Fremont DH, Diamond MS. Functional analysis of antibodies against dengue virus type 4 reveals strain-dependent epitope exposure that impacts neutralization and protection. Journal of virology. 2013;87:8826–8842. doi: 10.1128/JVI.01314-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS neglected tropical diseases. 2011;5:e1188. doi: 10.1371/journal.pntd.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, Diamond MS. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. Journal of virology. 2010;84:10630–10643. doi: 10.1128/JVI.01190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burton DR, Saphire EO, Parren PW. A model for neutralization of viruses based on antibody coating of the virion surface. Current topics in microbiology and immunology. 2001;260:109–143. doi: 10.1007/978-3-662-05783-4_7. [DOI] [PubMed] [Google Scholar]
- 57.Dulbecco R, Vogt M, Strickland AG. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956;2:162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- 58.Taylor HP, Armstrong SJ, Dimmock NJ. Quantitative relationships between an influenza virus and neutralizing antibody. Virology. 1987;159:288–298. doi: 10.1016/0042-6822(87)90466-1. [DOI] [PubMed] [Google Scholar]
- 59.Della-Porta AJ, Westaway EG. A multi-hit model for the neutralization of animal viruses. The Journal of general virology. 1978;38:1–19. doi: 10.1099/0022-1317-38-1-1. [DOI] [PubMed] [Google Scholar]
- 60.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nature medicine. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS pathogens. 2011;7:e1002111. doi: 10.1371/journal.ppat.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klasse PJ, Burton DR. Antibodies to West Nile virus: a double-edged sword. Cell host & microbe. 2007;1:87–89. doi: 10.1016/j.chom.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. The Journal of general virology. 1989;70(Pt 1):37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 64.Calisher CH. Antigenic classification and taxonomy of flaviviruses (family Flaviviridae) emphasizing a universal system for the taxonomy of viruses causing tick-borne encephalitis. Acta virologica. 1988;32:469–478. [PubMed] [Google Scholar]
- 65.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nature structural & molecular biology. 2008;15:312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 66.Gach JS, Leaman DP, Zwick MB. Targeting HIV-1 gp41 in close proximity to the membrane using antibody and other molecules. Current topics in medicinal chemistry. 2011;11:2997–3021. doi: 10.2174/156802611798808505. [DOI] [PubMed] [Google Scholar]
- 67.Pantophlet R. Antibody epitope exposure and neutralization of HIV-1. Current pharmaceutical design. 2010;16:3729–3743. doi: 10.2174/138161210794079182. [DOI] [PubMed] [Google Scholar]
- 68.Pierson TC, Sanchez MD, Puffer BA, Ahmed AA, Geiss BJ, Valentine LE, Altamura LA, Diamond MS, Doms RW. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology. 2006;346:53–65. doi: 10.1016/j.virol.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Khromykh AA, Varnavski AN, Westaway EG. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. Journal of virology. 1998;72:5967–5977. doi: 10.1128/jvi.72.7.5967-5977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, Pierson TC. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS pathogens. 2008;4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukherjee S, Dowd KA, Manhart CJ, Ledgerwood JE, Durbin AP, Whitehead SS, Pierson TC. Mechanism and Significance of Cell Type-Dependent Neutralization of Flaviviruses. Journal of virology. 2014;88:7210–7220. doi: 10.1128/JVI.03690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. The Journal of experimental medicine. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peiris JS, Porterfield JS. Antibody-mediated enhancement of Flavivirus replication in macrophage-like cell lines. Nature. 1979;282:509–511. doi: 10.1038/282509a0. [DOI] [PubMed] [Google Scholar]
- 74.Peiris JS, Gordon S, Unkeless JC, Porterfield JS. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature. 1981;289:189–191. doi: 10.1038/289189a0. [DOI] [PubMed] [Google Scholar]
- 75.Mehlhop E, Nelson S, Jost CA, Gorlatov S, Johnson S, Fremont DH, Diamond MS, Pierson TC. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell host & microbe. 2009;6:381–391. doi: 10.1016/j.chom.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gollins SW, Porterfield JS. Flavivirus infection enhancement in macrophages: radioactive and biological studies on the effect of antibody on viral fate. The Journal of general virology. 1984;65(Pt 8):1261–1272. doi: 10.1099/0022-1317-65-8-1261. [DOI] [PubMed] [Google Scholar]
- 77.Sun P, Bauza K, Pal S, Liang Z, Wu SJ, Beckett C, Burgess T, Porter K. Infection and activation of human peripheral blood monocytes by dengue viruses through the mechanism of antibody-dependent enhancement. Virology. 2011;421:245–252. doi: 10.1016/j.virol.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. The Lancet infectious diseases. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gromowski GD, Barrett ND, Barrett AD. Characterization of dengue virus complex-specific neutralizing epitopes on envelope protein domain III of dengue 2 virus. Journal of virology. 2008;82:8828–8837. doi: 10.1128/JVI.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gromowski GD, Barrett AD. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology. 2007;366:349–360. doi: 10.1016/j.virol.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 81.Diamond MS, Pierson TC, Roehrig JT. Antibody Therapeutics Against Flaviviruses. In: Shi PY, editor. Molecular Virology and Control of Flaviviruses. Caister Academic Press; 2012. [Google Scholar]
- 82.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure. 2012;20:303–314. doi: 10.1016/j.str.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS pathogens. 2012;8:e1002930. doi: 10.1371/journal.ppat.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stiasny K, Kiermayr S, Holzmann H, Heinz FX. Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. Journal of virology. 2006;80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. Journal of virology. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edeling MA, Austin SK, Shrestha B, Dowd KA, Mukherjee S, Nelson CA, Johnson S, Mabila MN, Christian EA, Rucker J, Pierson TC, Diamond MS, Fremont DH. Potent dengue virus neutralization by a therapeutic antibody with low monovalent affinity requires bivalent engagement. PLoS pathogens. 2014;10:e1004072. doi: 10.1371/journal.ppat.1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang ST, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S. Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. Journal of virology. 2013;87:52–66. doi: 10.1128/JVI.02273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiermayr S, Stiasny K, Heinz FX. Impact of quaternary organization on the antigenic structure of the tick-borne encephalitis virus envelope glycoprotein E. Journal of virology. 2009;83:8482–8491. doi: 10.1128/JVI.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee PD, Mukherjee S, Edeling MA, Dowd KA, Austin SK, Manhart CJ, Diamond MS, Fremont DH, Pierson TC. The Fc region of an antibody impacts the neutralization of West Nile viruses in different maturation states. Journal of virology. 2013;87:13729–13740. doi: 10.1128/JVI.02340-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He RT, Innis BL, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. Journal of medical virology. 1995;45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 92.Stiasny K, Brandler S, Kossl C, Heinz FX. Probing the flavivirus membrane fusion mechanism by using monoclonal antibodies. Journal of virology. 2007;81:11526–11531. doi: 10.1128/JVI.01041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes. PLoS pathogens. 2009;5:e1000453. doi: 10.1371/journal.ppat.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaufmann B, Chipman PR, Holdaway HA, Johnson S, Fremont DH, Kuhn RJ, Diamond MS, Rossmann MG. Capturing a flavivirus pre-fusion intermediate. PLoS pathogens. 2009;5:e1000672. doi: 10.1371/journal.ppat.1000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411:306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pierson TC, Diamond MS. Degrees of maturity: the complex structure and biology of flaviviruses. Current opinion in virology. 2012;2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murray JM, Aaskov JG, Wright PJ. Processing of the dengue virus type 2 proteins prM and C-prM. The Journal of general virology. 1993;74(Pt 2):175–182. doi: 10.1099/0022-1317-74-2-175. [DOI] [PubMed] [Google Scholar]
- 98.Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. Journal of virology. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guirakhoo F, Bolin RA, Roehrig JT. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plevka P, Battisti AJ, Junjhon J, Winkler DC, Holdaway HA, Keelapang P, Sittisombut N, Kuhn RJ, Steven AC, Rossmann MG. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO reports. 2011;12:602–606. doi: 10.1038/embor.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, Malasit P, Kuhn RJ, Sittisombut N. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. Journal of virology. 2010;84:8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cherrier MV, Kaufmann B, Nybakken GE, Lok SM, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. The EMBO journal. 2009;28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS pathogens. 2013;9:e1003761. doi: 10.1371/journal.ppat.1003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boehr DD, Wright PE. Biochemistry. How do proteins interact? Science. 2008;320:1429–1430. doi: 10.1126/science.1158818. [DOI] [PubMed] [Google Scholar]
- 105.Phelps DK, Rossky PJ, Post CB. Influence of an antiviral compound on the temperature dependence of viral protein flexibility and packing: a molecular dynamics study. Journal of molecular biology. 1998;276:331–337. doi: 10.1006/jmbi.1997.1542. [DOI] [PubMed] [Google Scholar]
- 106.Lewis JK, Bothner B, Smith TJ, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bothner B, Dong XF, Bibbs L, Johnson JE, Siuzdak G. Evidence of viral capsid dynamics using limited proteolysis and mass spectrometry. The Journal of biological chemistry. 1998;273:673–676. doi: 10.1074/jbc.273.2.673. [DOI] [PubMed] [Google Scholar]
- 108.Li Q, Yafal AG, Lee YM, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. Journal of virology. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. Journal of virology. 2014 doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carroll MC. The complement system in regulation of adaptive immunity. Nature immunology. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 111.Mehlhop E, Fuchs A, Engle M, Diamond MS. Complement modulates pathogenesis and antibody-dependent neutralization of West Nile virus infection through a C5-independent mechanism. Virology. 2009;393:11–15. doi: 10.1016/j.virol.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benhnia MR, McCausland MM, Moyron J, Laudenslager J, Granger S, Rickert S, Koriazova L, Kubo R, Kato S, Crotty S. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. Journal of virology. 2009;83:1201–1215. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–426. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Mayer AE, Johnson JB, Parks GD. The neutralizing capacity of antibodies elicited by parainfluenza virus infection of African Green Monkeys is dependent on complement. Virology. 2014;460–461:23–33. doi: 10.1016/j.virol.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rodrigo WW, Block OK, Lane C, Sukupolvi-Petty S, Goncalvez AP, Johnson S, Diamond MS, Lai CJ, Rose RC, Jin X, Schlesinger JJ. Dengue virus neutralization is modulated by IgG antibody subclass and Fcgamma receptor subtype. Virology. 2009;394:175–182. doi: 10.1016/j.virol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Williams KL, Sukupolvi-Petty S, Beltramello M, Johnson S, Sallusto F, Lanzavecchia A, Diamond MS, Harris E. Therapeutic efficacy of antibodies lacking Fcgamma receptor binding against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies [corrected] PLoS pathogens. 2013;9:e1003157. doi: 10.1371/journal.ppat.1003157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrobial agents and chemotherapy. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Messer WB, de Alwis R, Yount BL, Royal SR, Huynh JP, Smith SA, Crowe JE, Jr, Doranz BJ, Kahle KM, Pfaff JM, White LJ, Sariol CA, de Silva AM, Baric RS. Dengue virus envelope protein domain I/II hinge determines long-lived serotype-specific dengue immunity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1939–1944. doi: 10.1073/pnas.1317350111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Williams KL, Wahala WM, Orozco S, de Silva AM, Harris E. Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo. Virology. 2012;429:12–20. doi: 10.1016/j.virol.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. Induction of epitope-specific neutralizing antibodies against West Nile virus. Journal of virology. 2007;81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou T, Zhu J, Yang Y, Gorman J, Ofek G, Srivatsan S, Druz A, Lees CR, Lu G, Soto C, Stuckey J, Burton DR, Koff WC, Connors M, Kwon PD. Transplanting Supersites of HIV-1 Vulnerability. PloS one. 2014;9:e99881. doi: 10.1371/journal.pone.0099881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, Rupert P, Correnti C, Kalyuzhniy O, Vittal V, Connell MJ, Stevens E, Schroeter A, Chen M, Macpherson S, Serra AM, Adachi Y, Holmes MA, Li Y, Klevit RE, Graham BS, Wyatt RT, Baker D, Strong RK, Crowe JE, Jr, Johnson PR, Schief WR. Proof of principle for epitope-focused vaccine design. Nature. 2014;507:201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]