Abstract
Background
“McConnell’s sign” (McCS), described as hypo- or akinesis of the right ventricular (RV) free wall with preservation of the apex, is associated with acute pulmonary embolism (aPE). However, the sensitivity of McCS for the detection of aPE is limited. We sought to evaluate in patients with McCS, whether echocardiographic parameters of global and regional RV function could differentiate between patients with and without aPE.
Methods
We reviewed echocardiograms of 81 patients with McCS, who underwent CT or V/Q studies for suspected PE, and 40 normal controls (NL). Echocardiograms were analyzed to measure pulmonary artery systolic pressure (PASP), tricuspid regurgitation (TR) by vena contracta width, conventional indices of RV function, and speckle tracking–derived longitudinal free wall strain. ROC analysis was performed to evaluate the diagnostic accuracy of these parameters for diagnosis of aPE.
Results
Fifty-five of eighty-one (68%) had PE (McCS + PE), while 26 of 81 (32%) did not (McCS – PE). Compared to NL, global and segmental RV strain were lower in patients with McCS, contrary to the notion of normal apical function. In McCS + PE, compared to McCS – PE: (1) PASP, fractional area change and TR were significantly lower; (2) strain magnitude was significantly lower globally and in basal and apical segments. Individual parameters had similar diagnostic accuracy by ROC analysis, which further improved by combining parameters. In McCS – PE, 69% of patients had pulmonary hypertension (PH).
Conclusions
McCS and aPE are not synonymous. RV free wall strain may aid in differential diagnosis of patients with McCS evaluated for aPE. Specifically, McCS should prompt an inquiry for evidence of PH, which would indicate that aPE is less likely.
Keywords: pulmonary embolism, McConnell’s sign, right ventricular function, myocardial strain
Venous thromboembolism (VTE) is a common diagnosis encountered in clinical practice. Within VTE, acute pulmonary embolism (aPE) accounts for over a third of cases and has a high rate of both in-hospital and out-of-hospital mortality.1–3 Of those who survive, the morbidity of VTE is high and the cost is significant both for the patients and to the medical system. Despite advancing methods in treatment and diagnosis, the rate of VTE continues to rise in both the USA and Europe.3,4 The signs and symptoms of aPE vary greatly, and the diagnosis is not always clear at the time of presentation. The diagnosis of aPE is typically made using intravenous contrast computed tomography (CT), ventilation–perfusion (V/Q) nuclear scan, or pulmonary angiography.5 However, other diagnostic testing is frequently also performed prior to any confirmatory tests. Specifically, evidence of “right heart strain” on electrocardiogram, echocardiogram, or cardiac biomarkers might suggest a clinically significant aPE.6
“McConnell’s sign” (McCS) is an echocardiographic description of a hypo- or akinetic mid- and basal right ventricular (RV) free wall associated with a seemingly normal or hyperkinetic RV apical wall motion.7 When McCS is present, the diagnosis of aPE is often suspected. However, the specificity and sensitivity of McCS for the diagnosis of aPE are 94% and 77%, respectively.7 Other studies have reported even lower sensitivity,8–10 limiting the diagnostic power of McCs for the detection of aPE.
New echocardiographic technology using deformation imaging, or myocardial strain, allows a detailed, quantitative assessment of myocardial mechanics.11 In the left ventricle, global longitudinal strain has been shown to be better suited than traditional measures, such as ejection fraction, for the detection of subtle myocardial dysfunction,12 and, as a result, a better predictor of outcomes.13 In patients with pulmonary hypertension, right heart free wall strain has been also shown to provide prognostic information.14 Because aPE may cause right heart dysfunction, we hypothesized that right ventricular (RV) strain measurements in patients with McCS could provide additional diagnostic information to that provided by traditional indices in these patients. Specifically, we sought to test this hypothesis by studying echocardiographic parameters of RV function in patients with McCS, including RV global and regional free wall strain, and by testing their ability to differentiate patients with and without aPE.
Methods
Population
We retrospectively studied 161 consecutive patients who had undergone clinically indicated transthoracic echocardiograms (TTE) for suspected aPE and were determined to have a “McConnell’s Sign.” Confirmed diagnosis of PE was made with either a positive CT or a “high probability for PE” on V/Q scans. Eighty studies were excluded due to lack of adequate RV-focused free wall views of quality suitable for strain analysis (N = 73), as well as equivocal diagnosis of PE by CT or V/Q scans (N = 7). After exclusion, we analyzed a total of 81 patients with McCS. In addition, we studied a group of 40 normal controls, selected from patients who had no known heart disease or cardiac abnormalities on echocardiography. The study was approved by the Institutional Review Board.
Study Design
Patients with McCS were divided into two groups, according to the presence or absence of aPE on either CT or V/Q scan. Multiple echocardiographic parameters of RV function, as well as estimated pulmonary artery systolic pressure (PASP) and severity of tricuspid regurgitation (TR), were measured and compared between these two groups. In addition, patients with McCS were compared with the normal controls. Parameters identified as significantly different were subjected to receiver-operating characteristics (ROC) analysis, to identify those with the highest diagnostic accuracy for the detection of aPE, as reflected by the highest area under ROC curve (AUC). Finally, different combinations of parameters were tested to identify the best combination that would improve the echocardiographic diagnostic accuracy of aPE over that of individual parameters.
Echocardiographic Imaging and Interpretation
Transthoracic echocardiograms was performed using the iE33 imaging system with a S-5 transducer (Philips Healthcare, Andover, MA, USA), as either full or limited studies, evaluating RV function. McCS was described based on standard apical and/or subcostal views. Presence of McCS was confirmed by an independent echocardiographer who graded motion in 4 segments of the RV free wall (apex, mid-apical, mid-basal, and basal), using a three-point scale, including: normal (=0), hypokinetic (=1), and akinetic (=2), as in the initial description by McConnell et al.7
Image Analysis
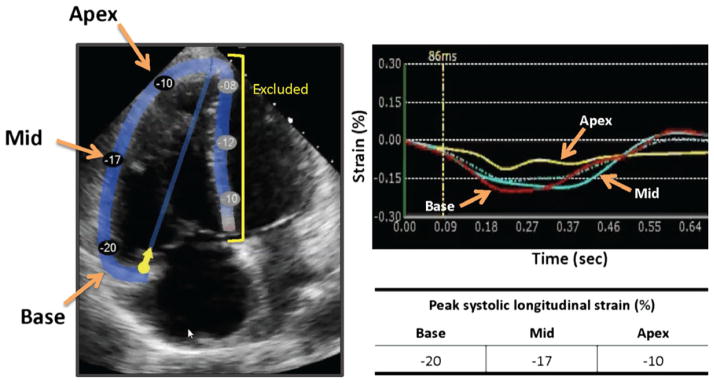
Apical four-chamber RV-focused views were imported into strain analysis software (Echo Insight, Epsilon Imaging). The RV borders were traced at end-diastole by another investigator, blinded to RV free wall motion scores. After adequate tracking was assured throughout the cardiac cycle, RV free wall segmental longitudinal strain was measured using speckle tracking (Fig. 1). In addition, RV fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), early tricuspid annular velocity S′ by tissue Doppler (TDI S′), PASP, and severity of TR were measured, with the latter being quantified by vena contracta (VC) width measured in either the RV inflow or the apical four-chamber view. PASP was estimated using Doppler tracing of tricuspid regurgitation jet with estimated right atrial pressures using inferior vena cava size and collapsibility.15
Figure 1.
Right ventricular free wall segmental longitudinal strain assessment using speckle tracking. After initialization of the RV endocardial boundaries, RV walls are automatically divided into six segments (left): three in the free wall and the other three in the septum, which were excluded in this study. Tracking wall motion throughout the cardiac cycle results in time curves of segmental wall strain over time (right).
Statistical Analysis
Continuous variables were expressed as means and standard deviations. Inter-group comparisons were made using unpaired two-tailed Student’s t-tests. P-values <0.05 were considered significant. ROC curves were generated for parameters that reached statistical significance between the two subgroups of patients with and without aPE by varying the threshold for the detection of aPE over the entire range of each parameter. The resultant curves of sensitivity versus (1-specificity) were used to calculate AUC, a standard measure of diagnostic accuracy independent of specific cutoff values. An optimal cutoff value was then identified for each parameter as the one that maximized the sum of sensitivity and specificity. These sensitivity and specificity and the corresponding overall accuracy values reflected the best-case scenario for each parameter. Finally, we tested combinations of the three parameters that resulted in the highest AUC values by calculating sensitivity, specificity, and accuracy of each combination using the aforementioned optimal cutoff values. These combinations included all possible pairs of parameters (as well as all three together), while requiring for a positive diagnosis that both (or all three) criteria were met, and separately that either one of the two (or three) criteria was met.
Results
Of the 81 patients with McCS, 55 (68%) had aPE, while the remaining 26 (32%) did not. Visual wall-motion scores assigned to the different RV free wall segments confirmed the presence of McCS as previously described7: with the scores being lower in the apex (0.17 ± 0.44) than in the other segments (mid-apical: 1.71 ± 0.58; mid-basal: 1.71 ± 0.50; basal: 1.29 ± 0.68), reflecting normal apical wall motion.
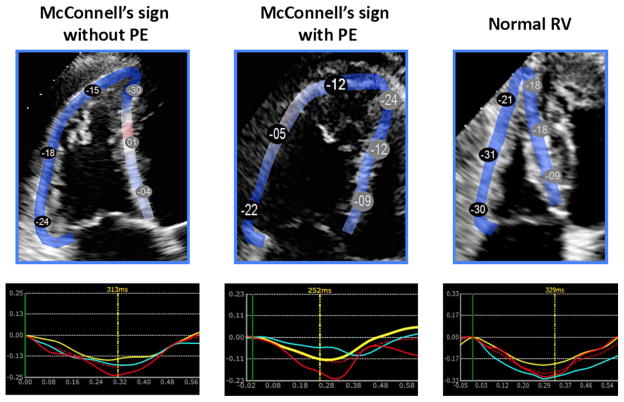
Compared to normal controls, in patients with McCS: (1) conventional parameters of RV function, including FAC, TDI S′, and TAPSE were significantly lower; (2) global RV free wall strain and segmental RV free wall strain were lower in all segments (apex, mid and base) (Table I). Figure 2 shows examples of strain analysis from RV-focused views acquired in three patients: two with McCS, one with and the other without confirmed PE, as well as a patient with normal RV function. In both patients with McCs, peak strain was lower in all three free wall segments, compared to the normal subject.
TABLE I.
Echocardiographic Measurements Obtained in the Patients with McConnell’s Sign (McCS) and Normal Controls (left), as well as in the Subgroups of Patients with McCS Divided by the Presence of Acute Pulmonary Embolism (PE)
| Normal Controls | McCS | McCS + PE | McCS – PE | |
|---|---|---|---|---|
| ES RV area (cm2) | 11 ± 3* | 22 ± 5 | 23 ± 5* | 21 ± 5 |
| ED RV area (cm2) | 18 ± 5* | 27 ± 6 | 28 ± 6 | 26 ± 5 |
| FAC (%) | 36 ± 8* | 18 ± 8 | 17 ± 7* | 22 ± 9 |
| TAPSE (cm) | 2.0 ± 0.6* | 1.1 ± 0.6 | 1.0 ± 0.5 | 1.2 ± 0.7 |
| S′ (cm/sec) | 12 ± 3* | 8 ± 4 | 7 ± 2 | 9 ± 5 |
| Global free wall strain (%) | −21 ± 5* | −10 ± 6 | −8 ± 5* | −13 ± 8 |
| Apical free wall strain (%) | −18 ± 8* | −8 ± 8 | −7 ± 7* | −11 ± 8 |
| Middle free wall strain (%) | −23 ± 8* | −7 ± 9 | −5 ± 7 | −9 ± 12 |
| Basal free wall strain (%) | −24 ± 18* | −15 ± 13 | −12 ± 12* | −20 ± 12 |
| PASP (mmHg) | – | 55 ± 18 | 51 ± 15* | 64 ± 21 |
| TR severity by VC (cm) | – | 0.40 ± 0.30 | 0.32 ± 0.27* | 0.55 ± 0.28 |
| LVEF (%) | 64 ± 4 | 61 ± 12 | 62 ± 10 | 58 ± 16 |
P < 0.05.
Figure 2.
Right ventricular free wall longitudinal strain measurements in two patients with McConnell’s sign: one without pulmonary embolism (left) and the other with pulmonary embolism (middle), and in a normal subject (right), along with the corresponding free wall segmental strain curves (bottom). Note the differences in the magnitude of the segmental peak strain among the three patients (see text for details).
Among the conventional indices of RV function, only FAC was lower in patients with McCS and PE compared to those with McCS without PE (Table II). Also, the magnitude of global strain was significantly lower in patients with aPE, compared to those without (Fig. 2, left and middle). Among the RV free wall segments, the apex and the base were significantly worse (P < 0.01 for both), while the middle RV free wall segment was not different (P = 0.12) in those with PE compared to those without PE (Table I).
TABLE II.
Results of Receiver-Operating Characteristics (ROC) Analysis for Echocardiographic Parameters That Were Significantly Different Between Patients with McConnell’s Sign with and without Pulmonary Embolism: Tricuspid Regurgitation (TR) by Vena Contracta (VC) Width, Pulmonary Artery Systolic Pressure (PASP), Right Ventricular Free Wall Global Longitudinal Strain (GLS), and Fractional Area Change (FAC). After Area Under ROC Curve (AUC) and Optimal Cutoff Values Were Obtained, Sensitivity, Specificity, and Overall Accuracy for These Optimal Cutoffs Were Calculated for the Individual Parameters (Upper Portion), as well as for Different Combinations (Lower Portion)
| AUC | Optimal Cutoff | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|
| TR by VC | 0.75 | 0.5 cm | 0.79 | 0.58 | 0.72 |
| PASP | 0.73 | 65 mmHg | 0.84 | 0.50 | 0.75 |
| GLS | 0.71 | −13% | 0.82 | 0.54 | 0.73 |
| FAC | 0.69 | 20% | 0.75 | 0.58 | 0.69 |
|
| |||||
| TR + PASP | 0.71 | 0.75 | 0.72 | ||
| TR or PASP | 0.92 | 0.38 | 0.76 | ||
| TR + GLS | 0.62 | 0.79 | 0.68 | ||
| TR or GLS | 0.98 | 0.33 | 0.76 | ||
| PASP + GLS | 0.63 | 0.88 | 0.69 | ||
| PASP or GLS | 0.98 | 0.25 | 0.78 | ||
| TR + PASP + GLS | 0.53 | 0.88 | 0.63 | ||
| TR or PASP or GLS | 0.97 | 0.19 | 0.74 | ||
With regard to right heart pressures, PASP was significantly higher in patients with McCS and no PE, when compared to those with McCS and PE (Table I). In addition, severity of TR by VC was significantly greater in patients without PE. In the McCS and no PE group, 18 patients (69%) had a diagnosis of primary or secondary pulmonary hypertension (PH). The remaining eight patients (31%) had either acute/chronic pulmonary conditions or valvular heart disease. Left ventricular (LV) ejection fraction was normal in both the non-PE and PE groups (Table I).
Among the parameters that were significantly different between patients with and without PE, ROC curves resulted in highest AUC values for: TR severity, PASP, global longitudinal RV free wall strain (GLS), and RV FAC in descending order (Table II). Optimal cutoff values of these parameters resulted in sensitivities in the range of 0.75 to 0.84, specificity 0.50 to 0.58, and accuracy between 0.69 and 0.75. Combinations of parameters that required either one of the two (or three) resulted in sensitivities >0.90 but low specificities (Table II). In contrast, when two (or three) criteria were required to be met simultaneously, the specificities improved (0.75 to 0.88), while the corresponding sensitivities were reduced (0.53 to 0.71). In both cases, the accuracy was around 0.70, similar to those of the individual parameters. Nevertheless, combinations based on simultaneous criteria had higher specificities than the individual parameters.
Discussion
In cardiovascular disease, VTE is the third most encountered diagnosis in clinical practice. Despite prevention and treatment efforts, the rate of VTE is increasing in the USA and in Europe.3,4 Mortality from aPE ranges from 10% to 17.5%, depending on a variety of factors including age, gender, race, smoking history, or malignancy.1–3 Patients diagnosed and treated for aPE have a high morbidity related to chronic symptoms or to the treatment of the disease, as exemplified by a lower than normal quality of life.16 Of the complications of VTE, right ventricular (RV) dysfunction as a result of PE, is associated with significant morbidity and mortality, yet diagnostic tools for the assessment of RV dysfunction have known limitations.
Aside from to the risk for significant morbidity or mortality associated with VTE, the cost burden is similarly high. In the USA, the total cost burden of both PE and DVT is estimated to be between 13.5 and 27 billion dollars annually.17,18 In addition to cost associated with diagnosis and initial hospitalization, the cost of ongoing treatment and complications, including readmissions (5.3% as primary diagnosis or 14.3% secondary), adds significantly to the cost burden.19,20
The gold standard for diagnosis of aPE is pulmonary angiography; however, more recently contrast CT or V/Q scans have become the standard diagnostic tools in clinical practice. RV dysfunction has been established from abnormal ratios of RV size on CT, cardiac biomarkers (including B-natriuretic peptide), “RV strain” on EKG or RV dysfunction on TTE. At times, an abnormal echocardiogram with RV dysfunction, most specifically with the presence of McCS, might suggest PE, or alternatively, a large PE on CT might prompt a TTE evaluation for RV dysfunction as a complication of PE.21
The diagnosis of aPE by TTE advocates a comprehensive approach similar to guidelines for RV assessment independent of etiology. With TTE, several parameters exist to define RV systolic dysfunction, including tissue Doppler methods, FAC, TAPSE, or RV index of myocardial performance; however, each has advantages and limitations. Current guidelines recommend a comprehensive approach for the assessment of RV size and function15 without a specific recommendation of a single parameter for evaluation. In the clinical setting, quantitative parameters are usually combined with the overall qualitative assessment to form an impression on RV function. However, because of the limitations of the quantitative parameters and the challenges of right heart imaging, the assessment is not consistently reproducible.
In the initial description of McCS,7 control patients were compared to those with aPE and a group of patients with “primary PH” now classified as idiopathic/heritable pulmonary arterial hypertension.22 Our study is unique in that: (1) all patients had McCS with or without confirmed aPE, and (2) we compared between these subgroups of patients with McCS as well as between patients with McCS and normal subjects. One important difference between the findings of these two studies is that McConnell et al. reported a very high specificity of McCS for the diagnosis of PE (94%), while in our study group, one-third of the patients with McCS did not have aPE. Although our study design does not allow us to calculate the specificity of McCS, because no patients without it were included, this finding suggests that McCS and aPE are not synonymous. This difference probably stems from the relatively high prevalence of patients with PH in our study. Importantly, it underscores that patients with PH can have McCS without aPE. The clinical implication of this finding is that when McCS is present, it should prompt an inquiry for evidence of PH, once aPE is ruled out.
McConnell et al.7 divided the RV free wall into four segments and used a qualitative wall-motion score as well as a quantitative centerline excursion score. In their analysis, the RV apical segment was “normal” by both qualitative and quantitative analyses, whereas the small group with PH had abnormal wall motion in apical segments. In our visual analysis using similar segmentation and wall-motion scoring system, we found that the RV apical segment did, in fact, appear to be contracting normally. However, using strain measurements, we found the RV apical segment to be lower in patients with McCS, compared to normal controls. This misperception might be explained by an often hyperdynamic left ventricle that shares the ventricular apex with the right ventricle and pulls the hypocontractile RV free wall toward the left in systole in a way that tethers the RV apex such that it appears to be contracting vigorously.
Interestingly, apical segmental strain was more abnormal in patients with aPE than in those with other etiologies of right heart dysfunction, including those with PH. ROC analysis showed that RV free wall strain has diagnostic accuracy for the detection of aPE in the same range as PASP and RV FAC, as reflected by similar AUC values. Importantly, this analysis also allowed us to derive cutoff values for these four parameters for the detection of aPE.
Also, similar to previous studies, our analysis showed a tendency toward lower PASP values in patients with aPE versus those with PH. This presumed mechanism may reflect an insult causing an acute pressure overload, where there is no time for RV remodeling, whereas the chronic pressure/volume overloaded RV has time to remodel, including hypertrophy, and therefore can generate higher pressures. Alternate diagnostic approaches for aPE have been proposed, including the “60/60 sign”10 coined by the authors, in which tricuspid regurgitant gradient ≤60 mmHg and acceleration time ≤60 ms RV are suggestive of aPE. Their prospective analysis showed a low sensitivity and high specificity (25% and 94%, respectively), similar to McCS, and the authors suggested that the combination of McCS and the “60/60 sign” might improve the sensitivity without compromising the high specificity for the diagnosis of aPE.10,23 It is important to remember that echocardiography is overall insensitive to the diagnosis of aPE and thus should not be used as the primary diagnostic modality. However, it may be used to look for RV dysfunction or, in the setting of RV dysfunction, for findings that may point to the underlying cause. It would be expected that any cause of an acute increase in pulmonary vascular resistance could result in McCS, with PE being the most common etiology.
Strain has been used in left heart disease to follow and diagnose subclinical ventricular dysfunction, such as in patients receiving chemotherapy. Global longitudinal strain can predict mortality, as well or better than ejection fraction or wall-motion score index.13 In the right ventricle, strain imaging has been used to quantify global and regional free wall function in a variety of disease states. In PH, RV free wall strain has been shown to be prognostic of cardiac events and all-cause mortality.14 Specifically for aPE, multiple studies focused on the RV free wall, including several that used speckle-tracking strain analysis, which added a quantitative dimension to the qualitative description. Lopez-Candales et al.24 reported, similar to our findings, abnormal strain patterns in the entire RV free wall in patients with McCS, contrary to the visual perception of normal RV apical wall motion. In a study by Platz et al.,9 when comparing patients with PE and normal subjects, those with McCS had severely abnormal strain patterns, including reduced strain in the RV apex, which correlated with other parameters of RV dysfunction, including higher RV:LV size ratio and lower FAC.
Limitations
One limitation of RV strain analysis is associated with RV imaging challenges, such as suboptimal visualization of its boundaries. Further, RV free wall strain is described in the most recent quantification guidelines as a methodology that is vendor specific and should therefore not be directly compared across different commercial hardware and software.15 The limitations of our study are largely related to its retrospective nature, which limited the number of patients because adequate images of the right ventricle depicting the entire RV free wall throughout the cardiac cycle were not available in all patients. Finally, optimal cutoffs for diagnosis of aPE derived from our cohort were not tested in an independent group of patients, and therefore, the sensitivity, specificity, and accuracy reported here represent the best-case scenario.
Conclusions
McConnell’s sign and aPE are not synonymous. The impression of normal RV apical motion in patients with McCS is likely due to RV tethering by the contracting left ventricular apex that occurs in both acute pulmonary processes such as aPE and more chronic conditions such as PH. However, RV free wall strain measurements in patients with McCS can aid in the differential diagnosis of patients with aPE, because reduced magnitude of apical strain in the absence of evidence of PH may indicate an elevated likelihood of aPE.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3145–3146. doi: 10.1093/eurheartj/ehu393. [DOI] [PubMed] [Google Scholar]
- 6.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 7.McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 8.Casazza F, Bongarzoni A, Capozi A, et al. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005;6:11–14. doi: 10.1016/j.euje.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Platz E, Hassanein AH, Shah A, et al. Regional right ventricular strain pattern in patients with acute pulmonary embolism. Echocardiography. 2012;29:464–470. doi: 10.1111/j.1540-8175.2011.01617.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurzyna M, Torbicki A, Pruszczyk P, et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002;90:507–511. doi: 10.1016/s0002-9149(02)02523-7. [DOI] [PubMed] [Google Scholar]
- 11.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24:277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Jassal DS, Han SY, Hans C, et al. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr. 2009;22:418–424. doi: 10.1016/j.echo.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 14.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Klok FA, van Kralingen KW, van Dijk AP, et al. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138:1432–1440. doi: 10.1378/chest.09-2482. [DOI] [PubMed] [Google Scholar]
- 17.Mahan CE, Holdsworth MT, Welch SM, et al. Deep-vein thrombosis: A United States cost model for a preventable and costly adverse event. Thromb Haemost. 2011;106:405–415. doi: 10.1160/TH11-02-0132. [DOI] [PubMed] [Google Scholar]
- 18.Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: Annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108:291–302. doi: 10.1160/TH12-03-0162. [DOI] [PubMed] [Google Scholar]
- 19.Fanikos J, Rao A, Seger AC, et al. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126:127–132. doi: 10.1016/j.amjmed.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475–486. doi: 10.18553/jmcp.2007.13.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibowitz D. Role of echocardiography in the diagnosis and treatment of acute pulmonary thromboembolism. J Am Soc Echocardiogr. 2001;14:921–926. doi: 10.1067/mje.2001.114390. [DOI] [PubMed] [Google Scholar]
- 22.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Torbicki A, Kurzyna M, Ciurzynski M, et al. Proximal pulmonary emboli modify right ventricular ejection pattern. Eur Respir J. 1999;13:616–621. doi: 10.1183/09031936.99.13361699. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Candales A, Edelman K, Candales MD. Right ventricular apical contractility in acute pulmonary embolism: The McConnell sign revisited. Echocardiography. 2010;27:614–620. doi: 10.1111/j.1540-8175.2009.01103.x. [DOI] [PubMed] [Google Scholar]