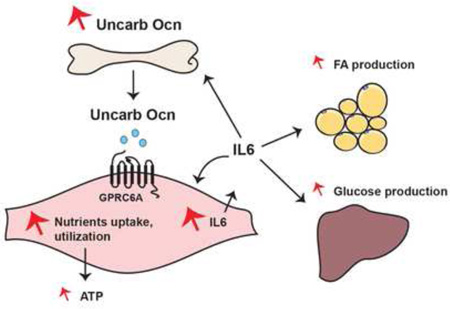
Summary
Circulating levels of undercarboxylated and bioactive osteocalcin double during aerobic exercise at the time those of insulin decrease. In contrast, circulating levels of osteocalcin plummet early during adulthood in mice, monkeys and humans of both genders. Exploring these observations revealed that osteocalcin signaling in myofibers is necessary for adaptation to exercise by favoring uptake and catabolism of glucose and fatty acids, the main nutrients of myofibers. Osteocalcin signaling in myofibers also accounts for most of the exercise-induced release of interleukin-6, a myokine that promotes adaptation to exercise in part by driving the generation of bioactive osteocalcin. We further show that exogenous osteocalcin is sufficient to enhance the exercise capacity of young mice and to restore to 15 month-old mice the exercise capacity of 3 month-old mice. This study uncovers a bone to muscle feed-forward endocrine axis that favors adaptation to exercise and can reverse the age-induced decline in exercise capacity.
Graphical Abstract
Introduction
The ability to perform exercise is an evolutionary conserved function that has been essential for the survival of most vertebrates and has nowadays, significant health benefits (Neufer et al., 2015; Zierath and Wallberg-Henriksson, 2015). During exercise muscle function needs to significantly increase; this requires that the uptake and catabolism of the two main nutrients of myofibers glucose and fatty acids (FAs), markedly rise (Hawley et al., 2014). As an anabolic hormone insulin promotes glucose uptake in myofibers and stores it in the form of glycogen post-prandially (Saltiel and Pessin, 2002). However, insulin does not promote glucose catabolism and its circulating levels decline during exercise (Coderre et al., 1995; Lund et al., 1995). This suggests that the rise in nutrients uptake and in their catabolism in muscle that occurs during exercise may be favored by other secreted molecules, myokines (Catoire and Kersten, 2015) or hormones, the circulating levels of which would increase during exercise. Conceivably, myokines and hormones may cooperate to favor adaptation to exercise.
The ability of bone to sense mechanical forces, the physical proximity of the two tissues and the fact that exercise capacity and bone mass decline at the same time have long suggested that a crosstalk between bone and muscle may exist (Novotny et al., 2015). The recent identification of bone-derived hormones and of their receptors allows to test if bone regulates muscle function at rest or during exercise and to elucidate the molecular bases of this function. Another reason to ask this question arises from a feature of the bone-derived hormone osteocalcin: This hormone favors physiological functions that, like memory and male fertility, greatly decline with age (Oury et al., 2013; Oury et al., 2011). This observation raises the possibility that osteocalcin may regulate other physiological processes severely affected by aging, such as muscle function during exercise (Partridge and Gems, 2002).
In testing this hypothesis we observed that the circulating levels of osteocalcin double during endurance exercise (exercise) in young adult wild-type (WT) mice, decrease sharply before or around mid-life in all species tested and do not increase during exercise in older mice to the same extent than in young mice. Exploring these observations revealed that osteocalcin signaling in myofibers favors adaptation to exercise in part because it promotes the uptake and catabolism of glucose and FAs. These observations explain why exogenous osteocalcin restores the exercise capacity of 15 month-old mice to that of 3 month-old mice. In addition to its regulation of nutrients uptake and catabolism, osteocalcin signaling in myofibers is also responsible for most of the exercise-induced increase in the circulating levels of interleukin-6 (IL-6), a myokine that promotes adaptation to exercise in part by increasing the production of bioactive osteocalcin. Hence, a feed forward regulation linking together the endocrine functions of bone and muscle appears to be necessary and sufficient to favor adaptation to exercise.
Results
Regulation of osteocalcin by exercise and age
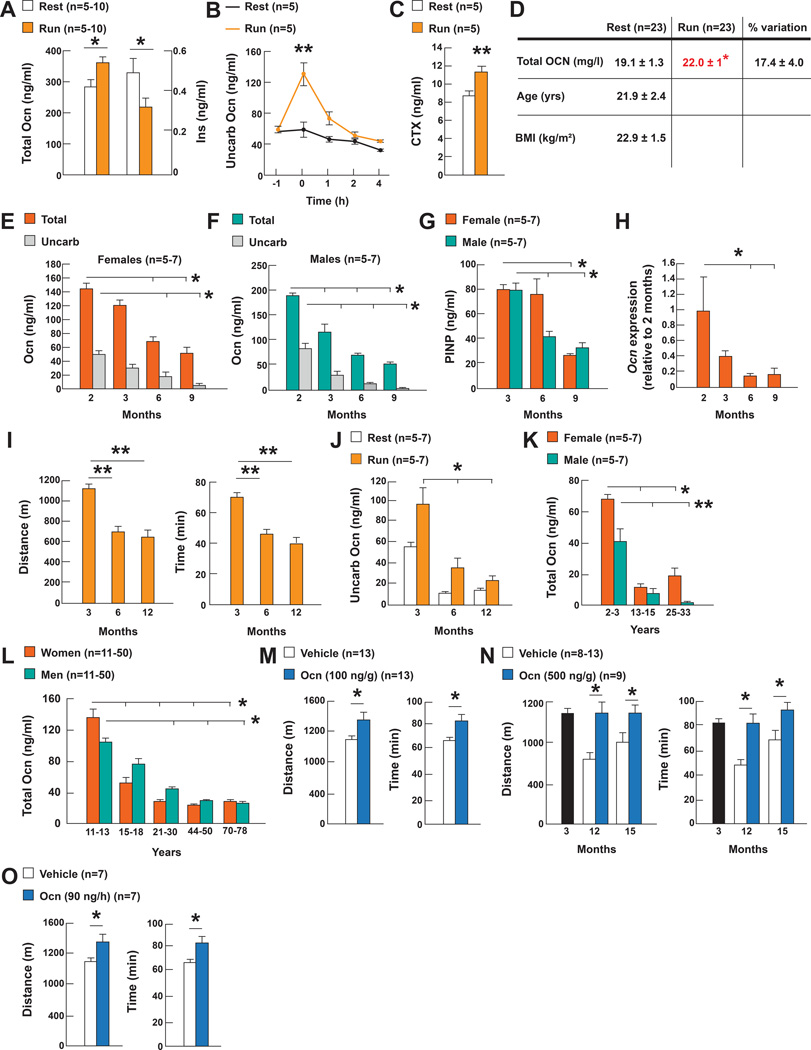
The growing number of functions ascribed to osteocalcin (Karsenty and Olson, 2016) raises the question of whether the circulating levels of this hormone change in various physiological situations. This analysis revealed that a single bout of endurance aerobic-based exercise (40 minutes run on a treadmill at 30cm/s, 80% VO2 max, thereafter referred to as exercise) increased circulating osteocalcin levels of total and undercarboxylated and bioactive two-fold in 3 month-old wild-type (WT) mice while at same time the circulating levels of insulin decreased. This increase in the circulating levels of bioactive osteocalcin was due in part to an increase in bone resorption, the arm of bone remodeling that is responsible of osteocalcin decarboxylation and activation (Ferron et al., 2010) (Figure 1A–C). Circulating osteocalcin levels also significantly increased in young women after a 45 minute-long exercise (Figure 1D).
Figure 1. Regulation of circulating osteocalcin levels by exercise and age.
A. Serum total osteocalcin (Ocn) and insulin (Ins) levels in 3 month-old mice at rest or after exercise.
B. Serum undercarboxylated Ocn (uncarb Ocn) levels in 3 month-old mice at rest (−1), 0, 1, 2 and 4 hours after exercise.
C. Serum CTX levels in 3 month-old mice at rest and after exercise.
D. Serum OCN levels in women at rest and after exercise.
E–F. Serum total and uncarb Ocn levels in female and male mice of various ages.
G. Serum PINP in mice of various ages.
H. Osteocalcin (Ocn) expression in femur in mice of various ages.
I. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 3, 6 and 12 month-old female WT mice.
J. Serum uncarb Ocn levels in 3, 6 and 12 month-old female mice at rest and after exercise.
K. Serum total Ocn levels in 2 to 33 year-old female and male rhesus macaque monkeys.
L. Serum total OCN levels in women and men 11 to 78 year-old.
M. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 3 month-old and N. 12 and 15 month-old WT mice treated with Ocn and O. 10 month-old WT mice receiving Ocn for 28 days.
(Exercise refers to 40 min running at 30cm/s on a treadmill).
In contrast, circulating osteocalcin levels greatly decreased in all species tested, in another physiological situation, aging. Indeed, whether we measure total or undercarboxylated circulating osteocalcin, these levels decreased by 70% in male and female mice between 2 and 9 months of age. This was due to a decline in Osteocalcin expression and in bone formation as measured by serum PINP levels (Figure 1E–H). This decrease in circulating osteocalcin levels occurs at the time the ability of mice to perform exercise declines, and circulating osteocalcin levels do not increase to the same extent in 6 or 12 month-old mice than in 3 month-old mice during exercise (Figure 1I–J). The same decrease in circulating osteocalcin levels was seen in male and female rhesus monkeys between young (2–3 years) and middle age (13–15 years) (Figure 1K). In humans circulating osteocalcin levels reach their lowest point before 30 year-old in women and 50 year-old in men (Figure 1L).
The increase of circulating osteocalcin levels during exercise and their decrease with age when exercise capacity declines, led us to test whether exogenous osteocalcin could increase the exercise capacity of older WT mice. In a proof of principle experiment we first asked whether one intraperitoneal injection of uncarboxylated mouse osteocalcin (osteocalcin) (100ng/g of body weight) immediately before exercise would improve the exercise capacity of 3 month-old WT mice. This injection raised circulating osteocalcin levels without affecting those of insulin (Figure S1A–B), and increased the time and distance these mice run on the treadmill at a constant speed (30cm/s) before exhaustion by over 20% (Figure 1M). In view of these results, 12 and 15 month-old WT mice that have low circulating osteocalcin levels were injected with osteocalcin (500ng/g of body weight) prior to exercise. This injection increased circulating osteocalcin levels more than 4-fold and conferred to these older mice the ability to run the same time and distance than 3 month-old untreated WT mice (Figure S1C and 1N). Chronic delivery of osteocalcin through mini-pumps (90ng/hour) for 28 days also increased circulating osteocalcin levels without affecting those of insulin (Figure S1D–E), as well as the time and distance 10 month-old WT mice run on a treadmill before exhaustion (Figure 1O). Thus exogenous osteocalcin is sufficient to reverse the age-induced decrease in exercise capacity in mice.
Osteocalcin signaling in myofibers is necessary for adaptation to exercise
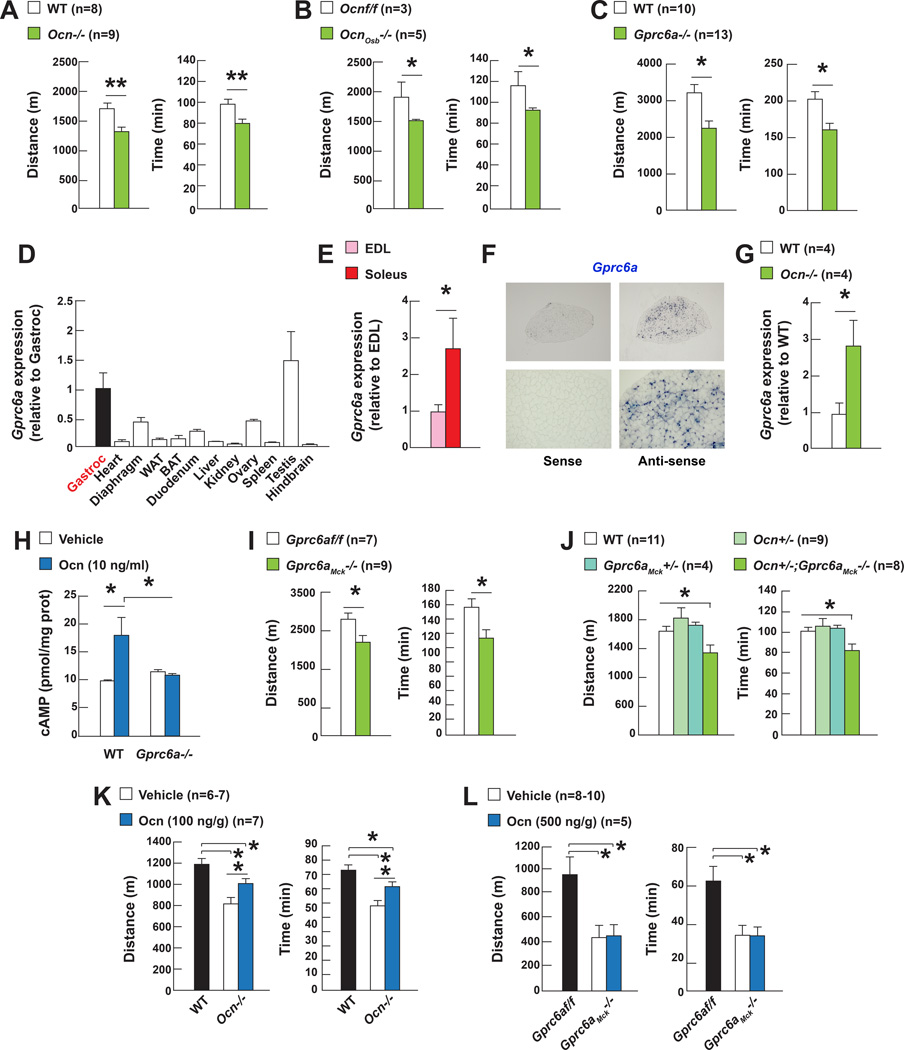
The observations presented above raised the question of whether osteocalcin is necessary for adaptation to exercise. Given the influence of testosterone on physical activity and the low circulating testosterone levels in male Osteocalcin (Ocn)−/− mice, this question was addressed in female mice.
When forced to run on a treadmill at a constant speed until exhaustion, 3, 6 and 9 month-old Ocn−/− mice run 20 to 30% less time and distance than WT littermates (Figure 2A and S2A). Since exercise capacity may vary even in genetically identical animals, experiments testing exercise capacity were conducted in several cohorts of control and mutant mice (n=8–13 per group). This reflected the absence of a signaling event from bone to muscle since the same decline in exercise capacity was observed in mice lacking Osteocalcin only in osteoblasts and post-natally (Figure 2B). Accordingly, this decline in exercise capacity was not observed in 2 month-old Ocn−/− mice indicating that the osteocalcin regulation of exercise capacity is not of developmental origin (Figure S2B). The same decrease in exercise capacity was observed in several cohorts of 3 month-old mice lacking Gprc6a, the osteocalcin receptor (Oury et al., 2011) (Figure 2C).
Figure 2. Osteocalcin signaling in myofibers is necessary for adaptation to exercise.
A. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 3 month-old Ocn−/− and WT mice, B. Ocnf/f and OcnOsb−/− mice and C. Gprc6a−/− and WT mice.
D. Gprc6a expression in various tissues and E. in EDL and soleus muscles.
F. In situ hybridization analysis of Gprc6a expression in soleus muscle.
G. Gprc6a expression in WT and Ocn−/− gastrocnemius muscles.
H. cAMP accumulation in WT and Gprc6a−/− myotubes treated with vehicle or osteocalcin (Ocn).
I. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 3 month-old Gprc6af/f and Gprc6aMck−/− mice and J. Ocn+/−; Gprc6aMck+/− and control mice.
K. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 6 month-old Ocn−/− or L. 12 month-old Gprc6aMck−/− mice treated with Ocn.
Ocn−/− and Gprc6a−/− mice display metabolic and/or behavioral abnormalities that make the interpretation of any phenotype linked to exercise difficult. To exclude these confounding factors and determine if osteocalcin promotes adaptation to exercise by signaling in skeletal muscle, we studied Gprc6a expression in this tissue. A qPCR survey showed that Gprc6a was more highly expressed in skeletal muscles than in most tissues and more so in oxidative muscles (soleus) that are needed for a prolonged effort, than in glycolytic muscles (EDL) and an in situ hybridization analysis demonstrated that Gprc6a is expressed in myofibers (Figure 2D–F). Moreover, Gprc6a expression was three-fold higher in Ocn−/− than in WT muscle and osteocalcin did not increase cAMP production in Gprc6a−/− myotubes as it did in WT ones (Figure 2G–H). In view of these data suggesting that Gprc6a mediates osteocalcin signal in myofibers we deleted Gprc6a from myofibers by crossing mice harboring a floxed allele of Grpc6a with Mck-Cre deleter mice (Bruning et al., 1998). Gprc6a expression was decreased over 50% in skeletal muscles in Gprc6aMck−/− mice (Figure S2C). Body composition, glucose tolerance and insulin sensitivity were similar in Gprc6aMck−/− and control mice (Figure S2D–F).
Starting at 3 months of age, all cohorts of Gprc6aMck−/− mice experienced a decrease in exercise capacity that was of equal severity as the one noted in Ocn−/− mice (Figure 2I). This could not be explained by a disruption of the intrinsic properties of muscle since excitation-contraction coupling and resistance to fatigue were not lower in Gprc6aMck−/− than in control muscles. Fiber type composition and size were also the same in muscles of Gprc6aMck−/− and control mice (Figure S2G–I). That a similar decrease in exercise capacity was seen in compound mutant mice lacking one allele of Osteocalcin and one allele of Gprc6a in myofibers (Ocn+/−;Gprc6aMck+/−) provides a genetic support to the notion that osteocalcin is the ligand of Gprc6a in myofibers that is responsible of Gprc6a regulation of adaptation to exercise (Figure 2J). Moreover, a single injection of osteocalcin increased the exercise capacity of Ocn−/− mice but did not do so in Gprc6aMck−/− mice (Figure 2K–L). At the same time multiple evidences suggest that the ability of osteocalcin to favor adaptation to exercise is not secondary to its signaling in the heart: Gprc6a expression is 20-fold lower in the heart than in skeletal muscle (Figure 2D); heart function is normal in Ocn−/− and Gprc6aMck−/− mice; and deleting Gprc6a only in cardiomyocytes does not affect exercise capacity in mice (Figure S2J–L).
Osteocalcin signaling in myofibers promotes uptake and catabolism of glucose during exercise
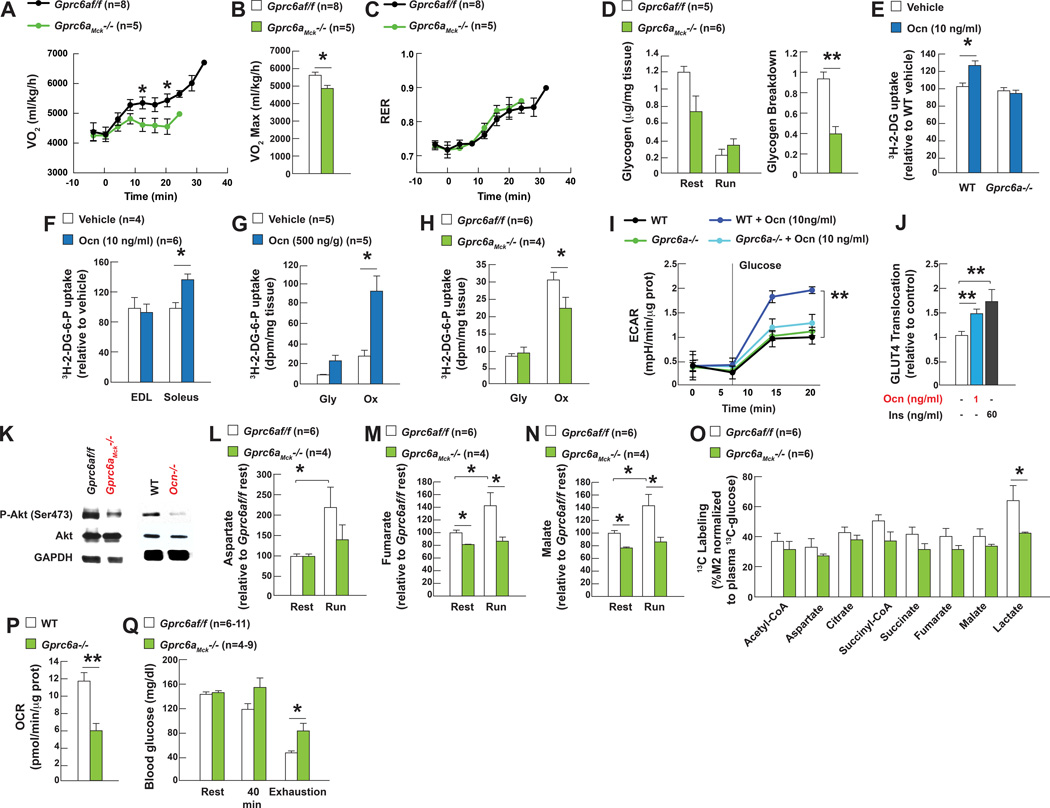
To determine how osteocalcin signaling in myofibers favors adaptation to exercise we used indirect calorimetry to measure nutrient utilization and aerobic capacity Gprc6aMck−/− and control mice running on a treadmill at an increasing speed (initial speed 5cm/s increasing by 3cm/s every minute until exhaustion). In the conditions of this assay the maximal oxygen consumption was significantly decreased in Gprc6aMck−/− mice whereas their respiratory exchange ratio (RER) was not affected (Figure 3A–C). These results suggesting that osteocalcin signaling in myofibers promotes aerobic capacity during exercise prompted us to test if osteocalcin signaling in myofibers affects mitochondrial number/respiration and/or uptake and utilization of nutrients.
Figure 3. Osteocalcin signaling in myofibers promotes uptake and utilization of glucose during exercise.
A. VO2, B. VO2 max and C. RER in 3 month-old Gprc6af/f and Gprc6aMck−/− mice running on a treadmill at increasing speed until exhausted.
D. Glycogen content and breakdown in 3 month-old Gprc6af/f and Gprc6aMck−/− tibialis muscles at rest and after exercise.
E. Uptake of 3H-2-deoxyglucose (3H-2-DG) in WT and Gprc6a−/− myotubes and F. WT EDL and soleus muscles treated with vehicle or osteocalcin (Ocn).
G. Uptake of 3H-2-DG in glycolytic (Gly, white quadriceps) and oxidative (Ox, red quadriceps) muscles after exercise in 3 month-old Gprc6af/f and Gprc6aMck−/− mice and H. 15 month-old WT mice treated with Ocn.
I. Glycolysis, determined by the extracellular acidification of the media (ECAR), in WT and Gprc6a−/− myofibers treated with vehicle or Ocn.
J. GLUT4 translocation in C2C12 myoblasts treated with Ocn determined by optic microscopy.
K. Western blot analyses of Akt phosphorylation (Ser473) in tibialis muscles of 3 month-old Gprc6af/f, Gprc6aMck−/−, WT and Ocn−/− mice after exercise.
L. Aspartate and M–N. TCA cycle metabolites accumulation in quadriceps of 3 month-old Gprc6af/f and Gprc6aMck−/− mice at rest and after exercise.
O. 13C-labeled TCA metabolites and lactate in quadriceps muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− mice receiving a bolus of 13C-glucose prior to exercise.
P. Oxygen consumption rate (OCR) in myofibers cultured in Krebs-Ringer HEPES buffer with 25mM glucose.
Q. Blood glucose levels in 3 month-old Gprc6af/f and Gprc6aMck−/− mice at rest and after running on a treadmill for 40 min or until exhaustion.
(Exercise refers to 40 min running at 30cm/s on a treadmill).
The number of mitochondria in muscles was the same in 3 month-old Ocn−/− mice that already have a poor exercise capacity, and WT littermates. Expression of a transcriptional determinant of mitochondrial biogenesis and muscle adaptation to exercise Pgc1α (Da Cruz et al., 2012; Handschin and Spiegelman, 2008), and of its target genes was similar in muscles of Ocn−/−, Gprc6aMck−/− and control mice after exercise. The activities of the mitochondrial proteins COX and SDH were the same in Gprc6aMck−/− and control muscles after exercise and there was no detectable difference in mitochondrial respiration between WT and Gprc6a−/− myofibers that were cultured in the presence of glucose, pyruvate and amino acids (Figure S3A–G). Given their negative nature these results need to be interpreted cautiously, yet they indicate that the ability of osteocalcin signaling in myofibers to favor adaptation to exercise is not secondary to a measurable effect on mitochondrial number or function. Hence, we turned our attention to nutrients uptake and catabolism during exercise.
The main nutrient used by muscle to generate energy at the onset of exercise is glucose that is stored in myofibers in the form of glycogen (Lehninger et al., 2000). Glycogen breakdown measured by the difference between glycogen content at rest and after exercise was lower in Gprc6aMck−/− and Ocn−/− muscle than in control ones (Figure 3D and S3H). In contrast, liver glycogen breakdown was the same in Gprc6aMck−/− and control mice (Figure S3I). Furthermore, osteocalcin enhanced glucose uptake as determined by the uptake of 3H-2-deoxyglucose (3H-2DG), in WT but not in Gprc6a−/− myotubes. The same was true in WT isolated soleus treated with osteocalcin (Figure 3E–F and S3J). In vivo, the uptake of 3H-2DG was significantly increased in oxidative muscles in WT mice receiving osteocalcin prior to exercise but was decreased in those of Gprc6aMck−/− mice after exercise (Figure 3G–H). Lastly, osteocalcin favored glycolysis, defined by the extracellular acidification of the media (ECAR), in WT but not in Gprc6a−/− myofibers (Figure 3I). Importantly, none of the other proposed ligands of Gprc6a favored glucose uptake or glycolysis in WT myotubes and myofibers (Figure S3K–L).
To promote glucose uptake in muscle osteocalcin does not affect the expression of Glut1 and Glut4 (Figure S3M) but rather favors the translocation of GLUT4 to the plasma membrane of C2C12 myoblasts that express Gprc6a-myc and HA-Glut4-Gfp (Figure 3J). Signaling through GPCRs can promote Akt phosphorylation, an event that is observed in muscles after exercise and that allows the translocation of GLUT4 to the plasma membrane (Deshmukh et al., 2006; Lopez-Ilasaca et al., 1997). We found that Akt phosphorylation was decreased in muscles of Gprc6Mck−/− and Ocn−/− compared to those of control mice after exercise, conversely acute or chronic administration of osteocalcin induced Akt phosphorylation in WT myotubes and muscle (Figure 3K and S3N–P).
To assess the influence osteocalcin signaling in myofibers on activity of the TCA cycle we performed a metabolomics study. This analysis showed that the accumulation of aspartate, a reliable indicator of the cellular levels of the TCA cycle intermediate oxaloacetate, and of fumarate and malate the two TCA cycle intermediates that increase the most during exercise (Gibala et al., 1998; Sahlin et al., 1990), did not rise to the same extent in muscles of Gprc6aMck−/− than in those of control mice after exercise (Figure 3L–N). Moreover, the contribution of 13C-glucose to the labeling of each TCA cycle intermediate measured and of lactate was decreased in muscle of Gprc6Mck−/− mice injected with 13C-glucose prior to exercise (Figure 3O). This latter result suggests that the lower content of TCA cycle intermediates in Gprc6aMck−/− muscles after exercise reflects in part a decrease in the entry of carbon originating from glucose into the TCA cycle. This decreased uptake and catabolism of glucose in muscle of Gprc6Mck−/− mice explains why the oxygen consumption rate (OCR) was two-fold lower in Gprc6a−/− than in WT myofibers when the only nutrient of these myofibers was glucose (Figure 3P). The ability of osteocalcin signaling in myofibers to favor glucose uptake also provides an explanation for the increase in blood glucose levels observed in Gprc6aMck−/− mice running on until exhaustion (Figure 3Q).
Osteocalcin signaling in myofibers favors FAs utilization during exercise
Since FAs uptake and oxidation in muscle progressively increase during exercise (Hawley et al., 2014; Koves et al., 2005), we tested if osteocalcin signaling in myofibers also affects these processes during exercise.
We first measured muscle and plasma levels of acylcarnitines, a reliable indicator of FAs utilization in cells (Overmyer et al., 2015). We found that the accumulation of long and medium-chain acylcarnitines seen in muscles of control mice after exercise did not occur nearly to the same extent in muscles of Gprc6aMck−/− mice (Figure 4A). There was instead a significant rise in acylcarnitines accumulation in the plasma of Gprc6aMck−/− mice (Figure 4B). Moreover, the levels of free L-carnitine that significantly declined in muscles of control mice after exercise did not do so to the same extent in those of Gprc6aMck−/− mice (Figure S4A). These results suggesting that osteocalcin signaling in myofibers is needed for efficient FAs catabolism during exercise explain in part why osteocalcin did not increase 14C-oleate oxidation in Gprc6a−/− myotubes as it did in WT ones, and for why the OCR of Gprc6a−/− myofibers was significantly lower than the one of WT ones when oleate was the only substrate of these myofibers (Figure 4C–D and S4B). Plasma non-esterified fatty acids (NEFA) levels were significantly higher in Gprc6aMck−/− than in control mice after exercise, while glycerol levels were unchanged. These results are consistent with the notion that FAs uptake and catabolism in muscle are decreased in mice lacking osteocalcin signaling in myofibers (Figure 4E and S4C).
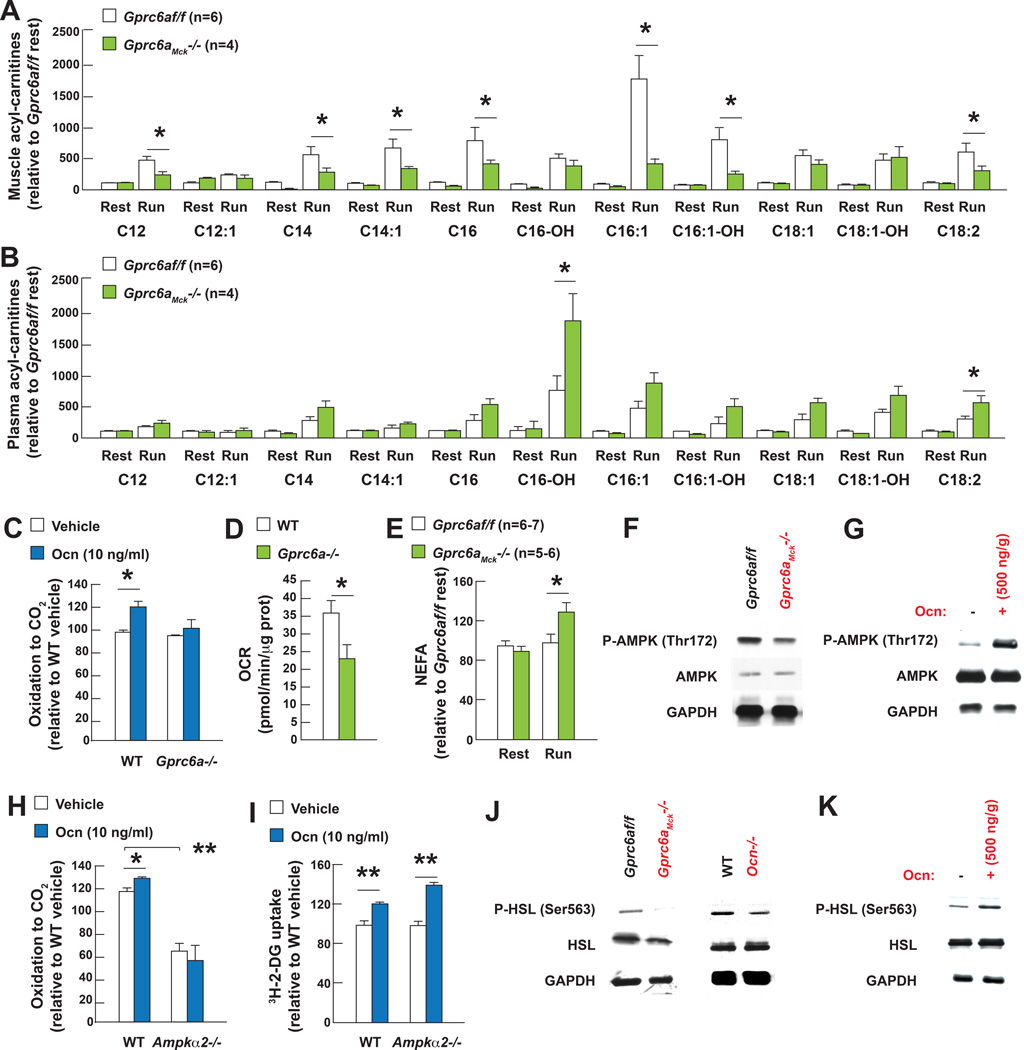
Figure 4. Osteocalcin signaling in myofibers favors FAs utilization during exercise.
A. Acylcarnitine levels in quadriceps muscles and B. plasma of 3 month-old Gprc6af/f and Gprc6aMck−/− mice at rest and after exercise.
C. 14C-oleate oxidation in WT and Gprc6a−/− myotubes treated with osteocalcin (Ocn).
D. Oxygen consumption rate (OCR) in myofibers cultured in Krebs-Ringer HEPES buffer with 3 mM oleic acid.
E. Plasma NEFAs levels in 3 month-old Gprc6af/f and Gprc6aMck−/− mice at rest and after exercise.
F. Western blot analysis after exercise of AMPK phosphorylation (Thr172) in tibialis muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− mice or G. of 15 month-old WT mice injected with Ocn.
H. 14C-oleate oxidation in WT and Ampkα2−/− myotubes treated with Ocn.
I. Uptake of 3H-2-deoxyglucose (3H-2-DG) in WT and Ampkα2−/− myotubes treated with vehicle or Ocn.
J. Western blot analysis after exercise of HSL phosphorylation (Ser563) in tibialis muscles of 3 month-old Gprc6af/f, Gprc6aMck−/−, WT and Ocn−/− mice and K. 15 month-old WT mice injected with Ocn.
(Exercise refers to 40 min running at 30cm/s on a treadmill)
How does osteocalcin favor FAs catabolism in myofibers? Once phosphorylated at Thr172, the cellular energy sensor AMPK promotes FAs utilization in muscle by increasing the activity of CPT1B, a transporter of long-chains FAs into the mitochondria (O'Neill et al., 2014). AMPK phosphorylation at Thr172 was reduced in muscles of Gprc6aMck−/− compared to those of control mice after exercise and conversely exogenous osteocalcin increased AMPK phosphorylation in muscles of WT mice during exercise (Figure 4F–G). Likewise, phosphorylation of ACC (Ser79) was decreased and the accumulation of malonyl-CoA was increased in Gprc6aMck−/− muscles after exercise (Figure S4D–E). Osteocalcin promotes FAs oxidation in muscle in an AMPK-dependent manner, since it induces FAs oxidation in WT but not Ampkα2−/− myotubes (Figure 4H). In contrast, osteocalcin favored glucose uptake in an AMPK-independent manner (Figure 4I). Hormone sensitive lipase (HSL) favors hydrolysis of intracellular triglycerides into free FAs in muscle when phosphorylated at Ser563 (Watt and Spriet, 2010). HSL phosphorylation was reduced in muscles of Gprc6aMck−/− and Ocn−/− compared to those of control mice, whereas Hsl expression was unchanged. Conversely HSL phosphorylation was increased in muscles of WT mice receiving osteocalcin prior to exercise (Figure 4J–K and S4F).
Furthermore, the expression of Cd36 and Fatp1 that facilitate the uptake of long-chain FAs into cells, and of Cpt1b that promotes their transport across the mitochondrial membrane (Stahl et al., 2001), was decreased in Gprc6a−/− myotubes while osteocalcin increased the expression of Fatp1, Cpt1b and to a lesser extent of Cd36 in WT myotubes (Figure S5A–B). This explains why the expression of Fatp1, Cpt1b and Cd36 did not increase in muscles of Ocn−/−, Gprc6aMck−/− and of Ocn+/−; Gprc6aMck+/− as they did in those of control mice after exercise and why Fatp1 expression increased in muscles of WT mice receiving osteocalcin prior to exercise (Figure 5A–C).
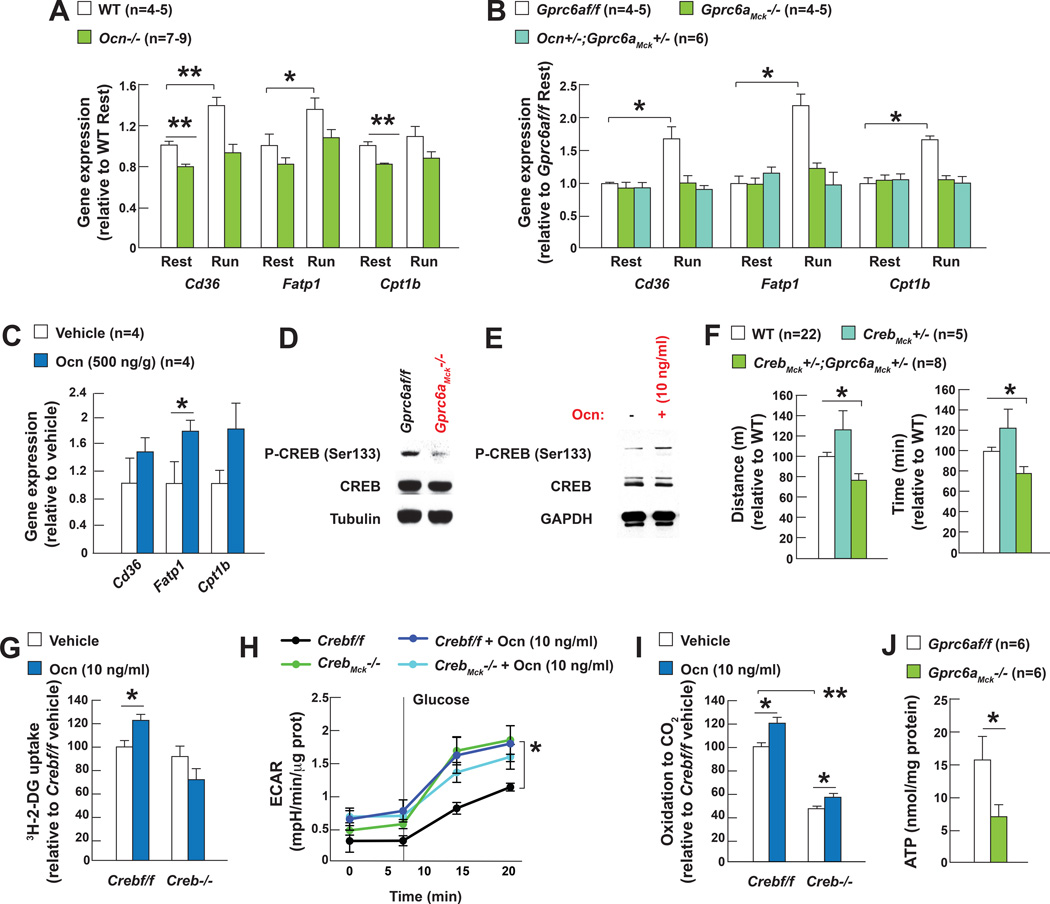
Figure 5. Osteocalcin signaling in myofibers favors expression of FAs transporters during exercise.
A. Cd36, Fatp1 and Cpt1b expression at rest and after exercise in gastrocnemius muscles of 3 month-old WT and Ocn−/− mice.
B. Cd36, Fatp1 and Cpt1b expression at rest and after exercise in gastrocnemius muscles of 3 month-old Gprc6af/f, Gprc6aMck−/− and Ocn+/−;Gprc6aMck+/− mice and C. in gastrocnemius muscles of 15 month-old WT mice injected with vehicle or osteocalcin (Ocn).
D. Western blot analysis after exercise of CREB phosphorylation (Ser133) in tibialis muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− mice and E. in WT myotubes treated with vehicle or Ocn.
F. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 3 month-old Gprc6aMck+/−;CrebMck+/−, CrebMck+/− and control mice.
G. Uptake of 3H-2-deoxyglucose (3H-2-DG) in Crebf/f and Creb−/− myotubes treated with vehicle or Ocn.
H. Glycolysis determined by the extracellular acidification of the media (ECAR), in Crebf/f and CrebMck−/− myofibers treated with vehicle or Ocn.
I. 14C-oleate oxidation in Crebf/f and Creb−/− myotubes treated with Ocn.
J. ATP accumulation in quadriceps muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− mice after exercise.
(Exercise refers to 40 min running at 30cm/s on a treadmill)
Two observations identify CREB as one mediator of osteocalcin signaling in myofibers. First, CREB phosphorylation was weaker in muscles of Gprc6aMck−/− that in those of control mice after exercise, and stronger in myotubes of WT mice treated with osteocalcin. Second, the exercise capacity of Gprc6aMck+/− ;CrebMck+/− mice is as decreased as the one of Gprc6aMck−/− mice (Figure 5D–F). Accordingly, CREB mediates osteocalcin regulation of glucose uptake in myotubes and glycolysis in myofibers (Figure 5G–H). However, the fact that osteocalcin induces FAs oxidation equally well in control and Creb−/− myotubes indicates that osteocalcin uses other transcriptional mediators to favor FAs oxidation (Figure 5I).
Taken together these results indicate that osteocalcin signaling in myofibers favors uptake and catabolism of both glucose and FAs in a balanced manner during exercise. These findings imply that osteocalcin signaling should be necessary to generate the ATP required for optimum muscle performance during exercise. In agreement with this hypothesis ATP levels were significantly lower in muscles of Gprc6aMck−/− mice than in those of control ones after exercise (Figure 5J). This low ATP content along with the decreased phosphorylation of AMPK suggest that osteocalcin signaling in myofibers regulates AMPK phosphorylation in part in an AMP-independent manner (Jensen et al., 2007).
Osteocalcin is necessary for the increase in Interleukin-6 expression in muscle occurring during exercise
To determine if osteocalcin signaling in myofibers favors adaptation to exercise through additional mechanisms we performed a transcriptomic analysis in muscles of control and Gprc6aMck−/− mice after exercise.
The gene whose expression was the most decreased (80%) in Gprc6aMck−/− muscle after exercise was the one encoding IL-6, a myokine whose circulating levels markedly rise during exercise through previously unknown mechanisms (Whitham et al., 2012) (Figure 6A). A more modest decrease of the expression of the soluble IL-6 receptor was also observed whereas no other myokines known to influence exercise (Bostrom et al., 2012; Egan and Zierath, 2013; Heinemeier et al., 2007) were affected by the absence of osteocalcin signaling in myofibers during exercise (Figure S6A). We verified that Il6 and Il6rα expression was significantly lower in muscles of Gprc6aMck−/− and Ocn−/− than in those of control mice after exercise (Figure 6B–C). We also observed that the rise in circulating IL-6 levels induced by exercise in control mice was blunted in Gprc6aMck−/− and Ocn−/− mice. Of note, IL-6 circulating levels also rise in young woman during exercise (Figure 6D–F). Moreover, the decrease in circulating osteocalcin levels observed in 15 month-old WT mice provide an explanation for the modest increase in circulating IL-6 levels observed during exercise, indeed exogenous osteocalcin partially restored circulating IL-6 levels in these mice during exercise (Figure 6G). These observations identify osteocalcin as a major regulator of Il6 expression in muscle and suggest that the majority of the increase in IL-6 circulating levels observed during exercise originates from muscle.
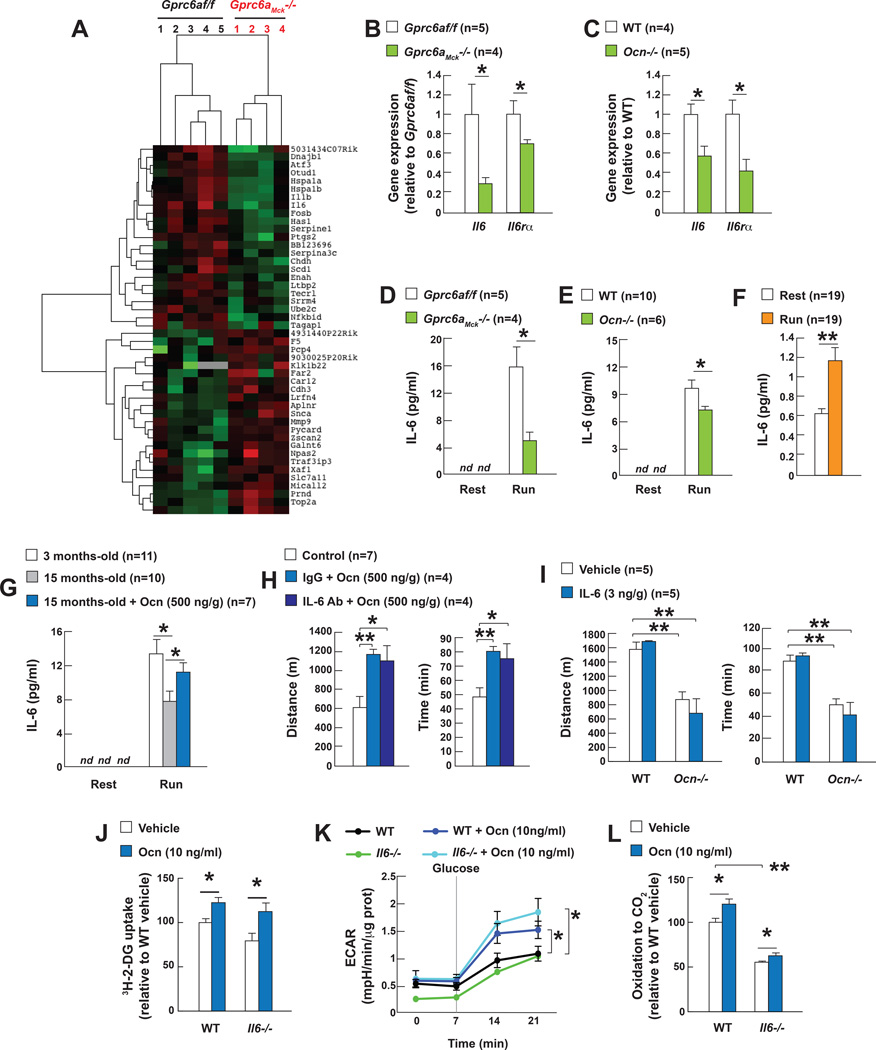
Figure 6. Osteocalcin is necessary for the increase in Interleukin-6 expression in muscle during exercise.
A. RNASeq analyses in gastrocnemius muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− mice after exercise.
B. Expression after exercise of Il6 and Il6rα in gastrocnemius muscles of 3 month-old Gprc6af/f and Gprc6aMck−/− and C. WT and Ocn−/−, mice.
D. Circulating IL-6 at rest and after exercise in 3 month-old Gprc6af/f and Gprc6aMck−/− mice, E. WT and Ocn−/− mice and F. in women (45 min run on a treadmill (6.5km/h)).
G. Circulating IL-6 at rest and after exercise in 3 and 15 month-old mice and 15 month-old mice treated with vehicle or osteocalcin (Ocn).
H. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 15 month-old mice treated with vehicle or Ocn and an antibody against IL-6 or a control IgG (control group include mice treated with vehicle alone, vehicle and IL-6 antibody, vehicle and IgG).
I. Performance during an endurance test (running on a treadmill at 30cm/s until exhausted) of 6 month-old WT and Ocn−/− mice treated with IL-6.
J. Uptake of 3H-2-deoxyglucose (3H-2-DG), K. Glycolysis, ad determined by the extracellular acidification of the media (ECAR) and L. 14C-Oleate oxidation in WT and Il6−/− myotubes treated with vehicle or osteocalcin.
(Exercise refers to 40 min running at 30cm/s on a treadmill, nd = non-detected)
The results presented above raise the important question of whether or not osteocalcin signaling in myofibers during exercise requires the presence of IL-6 in muscle or in blood. In vivo, injection of a neutralizing antibody against IL-6 prior to exercise did not prevent the increase in exercise capacity induced by exogenous osteocalcin in WT mice and exogenous IL-6 (3ng/g of body weight) did not improve the exercise capacity of WT or Ocn−/− mice (Figure 6H–I and S6B). Ex vivo, osteocalcin increased glucose uptake (3H-2-DG uptake), glycolysis (ECAR) and FAs oxidation (14C-oleate oxidation) equally well in WT and Il6−/− myotubes (Figure 6J–L). Taken together these results show that osteocalcin signals in muscle regardless of the presence or absence of IL-6 in the general circulation or in myofibers. However, IL-6 favors adaptation to exercise through previously described mechanisms and a novel one presented below.
Interleukin-6 favors the production of bioactive osteocalcin during exercise
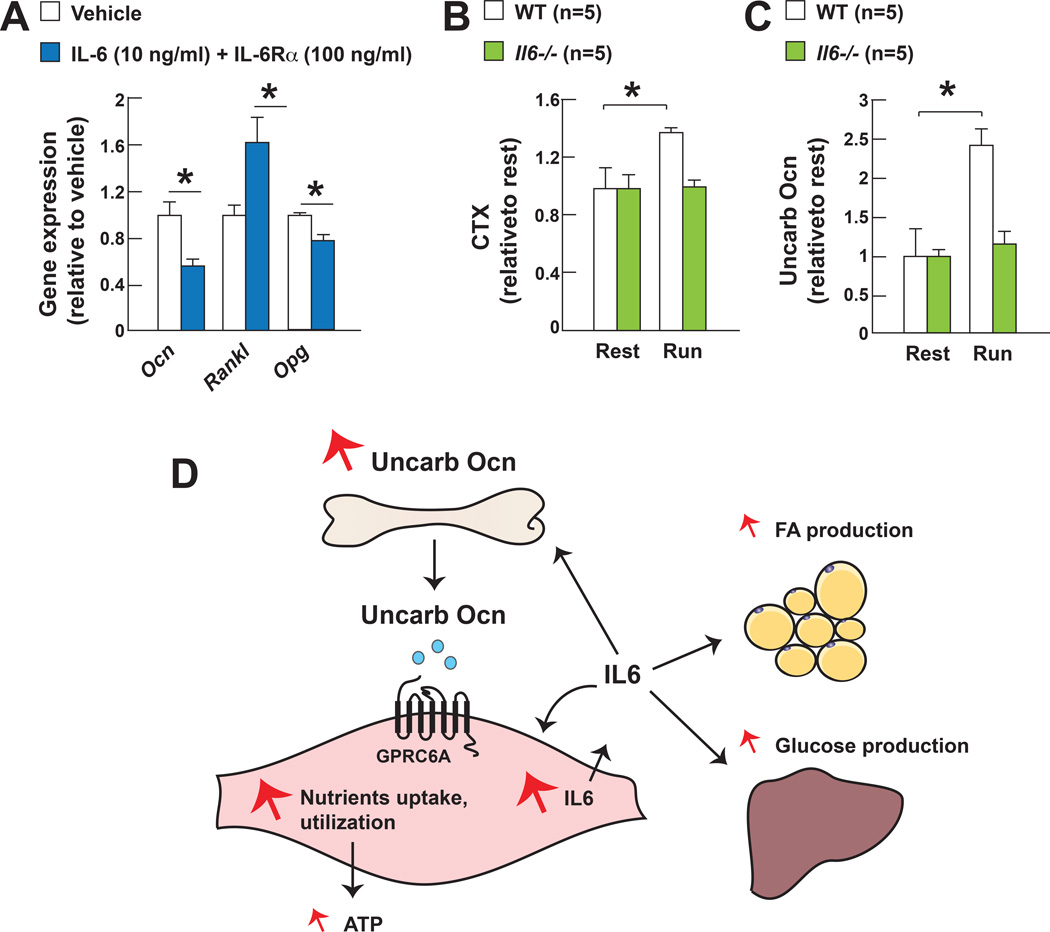
Indeed, since IL-6 has been shown to signal in bone cells (Tamura et al., 1993), we asked whether one mechanism whereby IL-6 favors adaptation to exercise may be by favoring the production of osteocalcin by osteoblasts and/or its activation by bone resorption (Ferron et al., 2010). In cell culture, IL-6 decreased the expression of Osteocalcin in osteoblasts, but more importantly, increased the expression of Rankl, a cytokine that favors osteoclast differentiation, and decreased the one of Osteoprotegerin (Opg), a decoy receptor for Rankl and an inhibitor of bone resorption (Teitelbaum and Ross, 2003) (Figure 7A). These results provide an explanation for why bone resorption and circulating levels of bioactive osteocalcin significantly increase during exercise in WT mice that have high circulating levels of IL-6 at that time, but do not do so in Il6−/− mice (Figure 1A–C and Figure 7B–C). This set of experiment reveals the existence of a feed-forward regulatory loop linking the production of osteocalcin in bone and the synthesis of IL-6 in muscle that appears to be necessary for the increase in muscle function during exercise.
Figure 7. IL-6 favors the production of active osteocalcin during exercise.
A. Ocn, Rankl and Opg expression in osteoblasts treated with IL-6 and soluble IL-6Rα.
B. Serum CTX levels in 2 month-old WT and Il6−/− mice after exercise.
C. Serum undercarboxylated osteocalcin (uncarb Ocn) levels in 2 month-old WT and Il6−/− mice at rest and after exercise.
D. Schematic representation of how osteocalcin signaling in myofibers and muscle-derived IL-6 cooperate to favor adaptation to exercise.
(Exercise refers to 40 min running at 30cm/s on a treadmill).
Discussion
This study reveals that osteocalcin signaling in myofibers is necessary to increase adaptation to exercise in part because it promotes the uptake and catabolism of glucose and FAs in myofibers, and in part because it increases the secretion by muscle during exercise of IL-6, a myokine that favors the production of bioactive osteocalcin (Figure 7D). This study also shows that osteocalcin is sufficient to reverse the decline in muscle function occurring during aging.
Osteocalcin signaling in myofibers is necessary for adaptation to exercise
During exercise the uptake and catabolism of glucose and FAs in myofibers increase. The fact that insulin exerts mostly anabolic functions in muscle and that its circulating levels decrease during exercise (Saltiel and Kahn, 2001) implies that muscle function and thereby adaptation to exercise are regulated by other secreted factors that would stimulate glucose uptake into muscle, glycogen breakdown, and/or FAs uptake and catabolism. Conceivably, the circulating levels of these factors would rise during exercise. It is precisely because its circulating levels double during exercise that we tested if osteocalcin is implicated in the regulation of adaptation to exercise. Analyzing mutant mouse strains lacking Osteocalcin and/or its receptor Gprc6a, in a cell-specific manner showed that osteocalcin signaling in myofibers through Gprc6a is needed for muscle function during exercise. The fact that exogenous osteocalcin could not correct the poor exercise capacity of Gprc6aMck−/− mice and that Ocn+/−; Gprc6aMck+/− mice display the same decrease in exercise capacity than Ocn−/− or Gprc6aMck−/− mice identify osteocalcin as the main ligand of Gprc6a responsible of its regulation of muscle function and adaptation to exercise. Cell-specific and inducible deletion of Ocn established that this function of osteocalcin reflects an influence of bone on muscle that is not of developmental origin. This function of osteocalcin is also not secondary to its signaling in the heart. Hence, by signaling through Gprc6a in myofibers, osteocalcin is a systemic regulator of the adaptation to exercise in adult mice.
Osteocalcin favors uptake and catabolism of nutrients in muscle during exercise
While osteocalcin does not affect in any appreciable manner muscle contractility or mitochondrial biogenesis, it favors the uptake and utilization of nutrients in myofibers during exercise in several ways. First, osteocalcin signaling in myofibers favors the breakdown of glycogen, a major source of glucose for contracting muscles during exercise. Second, it promotes the translocation of the glucose transporter GLUT4 to the plasma membrane and thereby enhances glucose uptake and glycolysis. None of the other proposed ligands of Gprc6a increased glucose uptake and glycolysis in myofibers. Third, osteocalcin signaling in myofibers increases FAs uptake and catabolism. Through these combined functions osteocalcin signaling in myofibers provides the carbon atoms necessary to promote the activity of the TCA cycle and thereby to produce the ATP that is needed to increase muscle function. That osteocalcin signaling in myofibers is important mainly during exercise is consistent with the notion that the endocrine regulation of nutrients uptake and utilization in muscle differs at rest and during exercise (Egan and Zierath, 2013). These findings do not exclude in any way the likely possibility that other molecules may contribute to the regulation of adaptation to exercise. The fact that osteocalcin signaling in myofibers increases uptake and catabolism of both glucose and FAs provides an explanation for why RER that reflects the respective utilization of both nutrients, is not affected in Gprc6Mck−/− mice during exercise.
A crosstalk between bone and muscle determines adaptation to exercise
Our study also identified IL-6, a myokine whose circulating levels rise during exercise and that enhances exercise capacity (Pedersen and Febbraio, 2012), as an osteocalcin target gene in muscle. In turn, IL-6 favors adaptation to exercise in part by signaling in bone to increase osteoclast differentiation and the generation of bioactive osteocalcin. Thus a feed-forward loop between bone and muscle promotes adaptation to exercise through at least three synergistic mechanisms: First, osteocalcin enhances the uptake and catabolism of glucose and FAs in myofibers. Second, the rise in IL-6 secretion from muscle during exercise triggered by osteocalcin, allows for the generation of glucose and FAs (Febbraio et al., 2004; van Hall et al., 2003). Third, IL-6 through its regulation of bone resorption increases the production of bioactive osteocalcin (Figure 7D). This model does not exclude the likely possibility that osteocalcin and IL-6 favor adaptation to exercise through additional mechanisms.
Osteocalcin signaling in muscle can revers the age-related decline in muscle function
The fact that osteocalcin is necessary for adaptation to exercise together with the precipitous decline of its circulating levels before midlife in all species tested, raised the question of whether osteocalcin could also be sufficient to reverse the decline in muscle function that is caused by aging. Whether administered acutely or chronically, exogenous osteocalcin increased the exercise capacity of 3 month-old WT mice and restored the exercise capacity of 9, 12 and even 15 month-old mice to that of 3 month-old ones. This ability of osteocalcin to reverse the age-related decline in exercise capacity in mice requires that this hormone signals in myofibers. These results identify osteocalcin signaling in myofibers as a novel and powerful means to fight the age-related decline in muscle function. Given the growing number of molecules proposed to affect muscle functions (Baskin et al., 2015) it will be important to determine if osteocalcin synergizes with some of them to favor adaptation to exercise.
Experimental Procedures
Animal and human studies
Ocn−/− mice were maintained on a 129-Sv genetic background, OcnOsb−/−, Gprc6aMck−/−, Gprc6aMyh6−/−, CrebMck−/−, Ocn+/−;Gprc6aMck+/− and Gprc6aMck+/−; CrebMck+/− mice on a 129-Sv/C57/BL6 mixed genetic background and Il6−/− (Jackson Laboratories) mice on a C57/BL6 genetic background. Littermates were used as controls in all experiments. Mice genotypes were determined by PCR. Generations of Ocn and Gprc6a conditional alleles have been described (Oury et al., 2013; Oury et al., 2011). For exercise studies, all mice were trained to run on a treadmill for 3 days (10 minutes/day, increasing speed from 10 to 17cm/s, an electric shock at 1mA to trigger running). Exercise tests were performed on mice fed ad libitum at 2–6 pm. The day of the test, mice were acclimated to the treadmill for 5 minutes followed by 10 minutes running at a constant speed (17cm/s) followed by a gradual speed increase up to 30cm/s. Then mice run either until exhaustion to determine endurance capacity or for 40 minutes. A gradual speed increase test was performed to determine the maximal VO2 during exercise: mice were acclimated to the treadmill for 5 minutes followed by 1 minute running at 5cm/s followed by a gradual speed increase (3cm/s every minute) until exhaustion, defined as a number of times a mouse fall off to the electric grid during one minute over 15. For all biochemical and metabolic analyses, blood/tissues were collected and processed either at rest or at the end of a 40 min run (30cm/s). Intraperitoneal (i.p.) injections of exogenous osteocalcin or IL-6 (Sigma) were performed immediately before exercise. To neutralize IL-6, 500 µg of a neutralizing antibody (R &D, #MAB206) (Pedersen et al., 2016) were administered i.p. 1 hour before exercise. Osteocalcin was synthetized as previously described (Oury et al., 2013).
Rhesus monkeys (Macaca mulatta) were housed as described in supplemental information at the NIH Animal Center, Poolesville, MD. Human healthy volunteers were used to assay osteocalcin (Elecsys, Roche Diagnosis) across lifespan (see supplemental information).
Energy metabolism studies
Glucose and insulin tolerance tests were performed as described (Lee et al., 2007). Ex vivo glucose uptake in EDL and soleus muscles was measured as described (Bruning et al., 1998) with minor modifications (see supplemental information). For in vivo glucose uptake, a bolus of 2-deoxy-d-[3H]glucose (3H-2-DG) (10µCi) was administered before running (40 min at 30cm/s). 3H-2-DG and 3H-2-DG-6-P content in muscle was determined by liquid scintillation counter and normalized to muscle weight (see supplemental information). Glucose uptake and FAs oxidation in myotubes were assayed as described (Sebastian et al., 2007). GLUT4 translocation was determined by optic microscopy as described (Zeigerer et al., 2002) (see supplemental information). Oxygen consumption rate (OCR) and extracellular acidification of the media (ECAR) in myofibers were measured using a XF24 Seahorse analyzer® (Seahorse Biosciences) (see supplemental information). To analyze mitochondrial function, myofibers were treated sequentially with oligomycin (10µg/ml), FCCP (200µM), rotenone (0.2µM). OCR was recorded following administration of each of them.
Metabolite profiling was done at the Einstein Stable Isotope and Metabolomics Core Facility (Bronx, NY). Metabolites from freeze-clamped skeletal muscles were extracted, derivatized, and run for GC-TOFMS analysis (Qiu et al., 2014). LC/MS/MS analysis was used to quantify glycolytic and TCA cycle metabolites (Serasinghe et al., 2015). The Biocrates Absolute IDQ p180 kit (Wang-Sattler et al., 2012) was used to quantify acylcarnitines by LC/MS/MS in plasma and muscle (see supplemental information). 13C-glucose tracer analyses were done at CASE Mouse Metabolic Phenotyping Center (Cleveland, OH) (see supplemental information). ATP was measured using commercial kit (Abcam). Ex vivo analyses of muscle contractility and resistance to fatigue were performed as detailed in supplemental information.
Biochemistry and Molecular Biology
Serum osteocalcin, PINP, CTX, IL-6 and insulin levels were measured using ELISA assays. Blood glucose level was measured using an Accu-Check glucometer. Cyclic AMP accumulation was measured using a commercial kit (R&D). For gene expression, 1µg of total RNA was reverse transcribed into cDNA. qPCR analyses were performed using a SYBER green master mix (Applied Biosciences) and a CFX-Connect real time PCR (Bio-Rad). Relative expression levels of each gene were normalized to the one of Hprt or Gapdh. The genome-wide differential expression of Gprc6aMck−/− vs. Gprc6af/f in muscle was measured by RNASeq (see supplemental information). For western blot analyses, protein extracts were separated in 8% acrylamide/Bis-acrylamide (Bio-Rad) gels and transferred to nitrocellulose membranes that were blotted using specific antibodies. For in situ hybridization, muscles were frozen in liquid N2-cooled methylbutane. Samples were sectioned at 10μm using a cryostat. In situ hybridization was performed with a DIG-labeled riboprobe. Mitochondria histomorphometry and enzyme histochemistry were performed following standard protocols (see supplemental information).
Cell culture
Culture of skeletal muscle myoblasts was performed as described (Gharaibeh et al., 2008), using 15–20 days-old mice. Myoblasts were differentiated into myotubes for 3–4 days in a medium containing 5% horse serum. For in vitro gene inactivation of Gprc6a and Il6, myoblasts from either WT or mutant littermate mice were isolated and differentiated into myotubes. For in vitro gene inactivation of Creb and Ampkα2, floxed myoblasts were isolated from mice then divided into two groups and infected with either empty or Cre-expressing adenovirus for 2 days (University of Iowa). Muscle fibers from flexor digitorum brevis muscle were isolated from 2–3 month-old WT or mutant mice. Muscles were dissociated with DMEM 2% collagenase for 2 hours at 37 °C, in 5% CO2 incubator. Muscle fibers were disaggregated from the tissue using a wide bore pipet and plated on matrigel-coated plates at approximately 50% confluence (see supplemental information). Myofibers were used after overnight incubation. Osteoblasts culture was performed as described (Ducy and Karsenty, 1995).
Statistics
All data are presented as mean ± standard error of mean. Statistical analyses were performed using unpaired, two-tailed Student’s t test for comparison between two groups and ANOVA test for experiments involving more than two groups. For all experiments, * denotes P ≤ 0.05, ** P ≤ 0.005.
Supplementary Material
Acknowledgments
We thank Drs. C. Cosentino, P. Ducy, L. Herrero, M. Hussain, R. Sellers, D. Serra and E. Sornay-Rendu for advices or reagents and Dr. M. McKee for electronic microscopy analyses. This work was supported by NIH grants 5RO1AR045548, PO1AG032959 (GK), P60DK020541 (IJK), RO1HL60665 (RNK) and U24DK76174 (MP), the Wilf Family Foundation (RNK), an Ellison Senior Scholar Award (GK), an EMBO post-doctoral fellowship (KL) and the Intramural Research Program of the National Institute on Aging (RdC, SJM, JAM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Contribution
P.M. and G.K. conceived the study; P.M., K.L., M.F., J.W. and M.G.D. performed most experiments; I.K. metabolomics studies; C.C., J.B., and P.S. human studies; A.L analyzed muscle contractility; Y.C. and R.N.K. heart function; S.M., J.M. and R.C. provided mice and monkey serum samples; C.T. and T.E.M. analyzed GLUT4 translocation; M.P. tracer analyses; P.M. and G.K. wrote the manuscript.
References
- Baskin KK, Winders BR, Olson EN. Muscle as a "mediator" of systemic metabolism. Cell Metab. 2015;21:237–248. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Catoire M, Kersten S. The search for exercise factors in humans. FASEB J. 2015;29:1615–1628. doi: 10.1096/fj.14-263699. [DOI] [PubMed] [Google Scholar]
- Coderre L, Kandror KV, Vallega G, Pilch PF. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. J Biol Chem. 1995;270:27584–27588. doi: 10.1074/jbc.270.46.27584. [DOI] [PubMed] [Google Scholar]
- Da Cruz S, Parone PA, Lopes VS, Lillo C, McAlonis-Downes M, Lee SK, Vetto AP, Petrosyan S, Marsala M, Murphy AN, et al. Elevated PGC-1alpha activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab. 2012;15:778–786. doi: 10.1016/j.cmet.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 2006;55:1776–1782. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53:1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, MacLean DA, Graham TE, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol. 1998;275:E235–E242. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol. 2007;102:573–581. doi: 10.1152/japplphysiol.00866.2006. (1985) [DOI] [PubMed] [Google Scholar]
- Jensen TE, Rose AJ, Jorgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007;292:E1308–E1317. doi: 10.1152/ajpendo.00456.2006. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell. 2016;164:1248–1256. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry. 3rd. New York: Worth Publishers; 2000. [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995;92:5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Novotny SA, Warren GL, Hamrick MW. Aging and the Muscle-Bone Relationship. Physiology (Bethesda) 2015;30:8–16. doi: 10.1152/physiol.00033.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill HM, Lally JS, Galic S, Thomas M, Azizi PD, Fullerton MD, Smith BK, Pulinilkunnil T, Chen Z, Samaan MC, et al. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57:1693–1702. doi: 10.1007/s00125-014-3273-1. [DOI] [PubMed] [Google Scholar]
- Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, Huang YY, Lee H, Srinivas P, Gao XB, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer KA, Evans CR, Qi NR, Minogue CE, Carson JJ, Chermside-Scabbo CJ, Koch LG, Britton SL, Pagliarini DJ, Coon JJ, et al. Maximal Oxidative Capacity during Exercise Is Associated with Skeletal Muscle Fuel Selection and Dynamic Changes in Mitochondrial Protein Acetylation. Cell Metab. 2015;21:468–478. doi: 10.1016/j.cmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat Rev Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, Li H, Cai S, Xie D, Huang C, et al. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin Cancer Res. 2014;20:2136–2146. doi: 10.1158/1078-0432.CCR-13-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Katz A, Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol. 1990;259:C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Sebastian D, Herrero L, Serra D, Asins G, Hegardt FG. CPT I overexpression protects L6E9 muscle cells from fatty acid-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;292:E677–E686. doi: 10.1152/ajpendo.00360.2006. [DOI] [PubMed] [Google Scholar]
- Serasinghe MN, Wieder SY, Renault TT, Elkholi R, Asciolla JJ, Yao JL, Jabado O, Hoehn K, Kageyama Y, Sesaki H, et al. Mitochondrial Division Is Requisite to RAS-Induced Transformation and Targeted by Oncogenic MAPK Pathway Inhibitors. Mol Cell. 2015;57:521–536. doi: 10.1016/j.molcel.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab. 2001;12:266–273. doi: 10.1016/s1043-2760(01)00427-1. [DOI] [PubMed] [Google Scholar]
- Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y, Koishihara Y, Ohsugi Y, Kumaki K, Taga T, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A. 1993;90:11924–11928. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005–3010. doi: 10.1210/jc.2002-021687. [DOI] [PubMed] [Google Scholar]
- Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Spriet LL. Triacylglycerol lipases and metabolic control: implications for health and disease. Am J Physiol Endocrinol Metab. 2010;299:E162–E168. doi: 10.1152/ajpendo.00698.2009. [DOI] [PubMed] [Google Scholar]
- Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Bruning JC, et al. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287:10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A, Lampson MA, Karylowski O, Sabatini DD, Adesnik M, Ren M, McGraw TE. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol Biol Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath JR, Wallberg-Henriksson H. Looking Ahead Perspective: Where Will the Future of Exercise Biology Take Us? Cell Metab. 2015;22:25–30. doi: 10.1016/j.cmet.2015.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.