Abstract
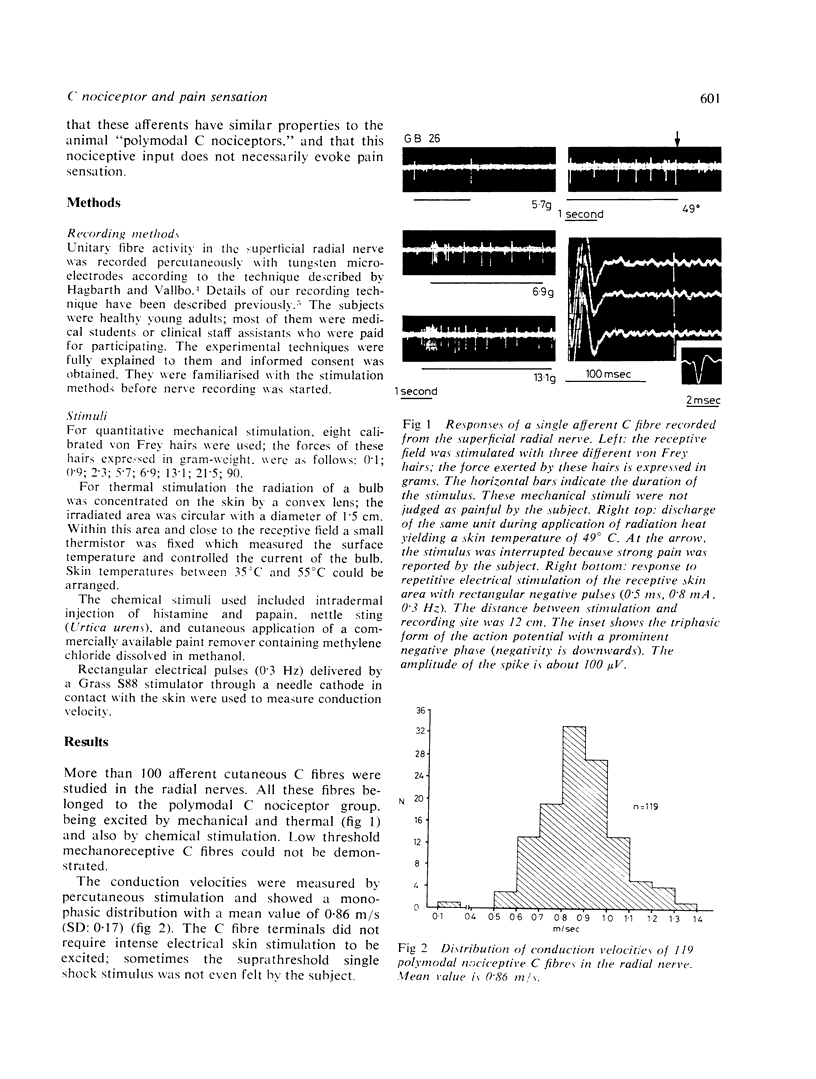
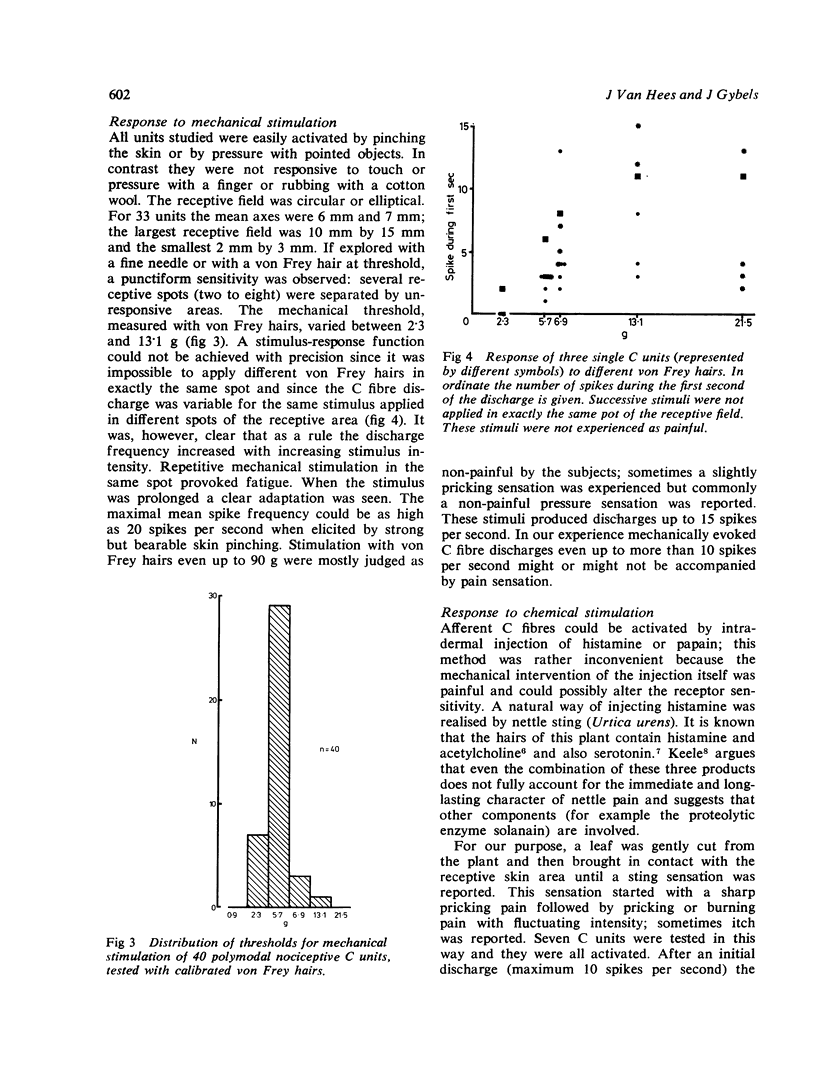
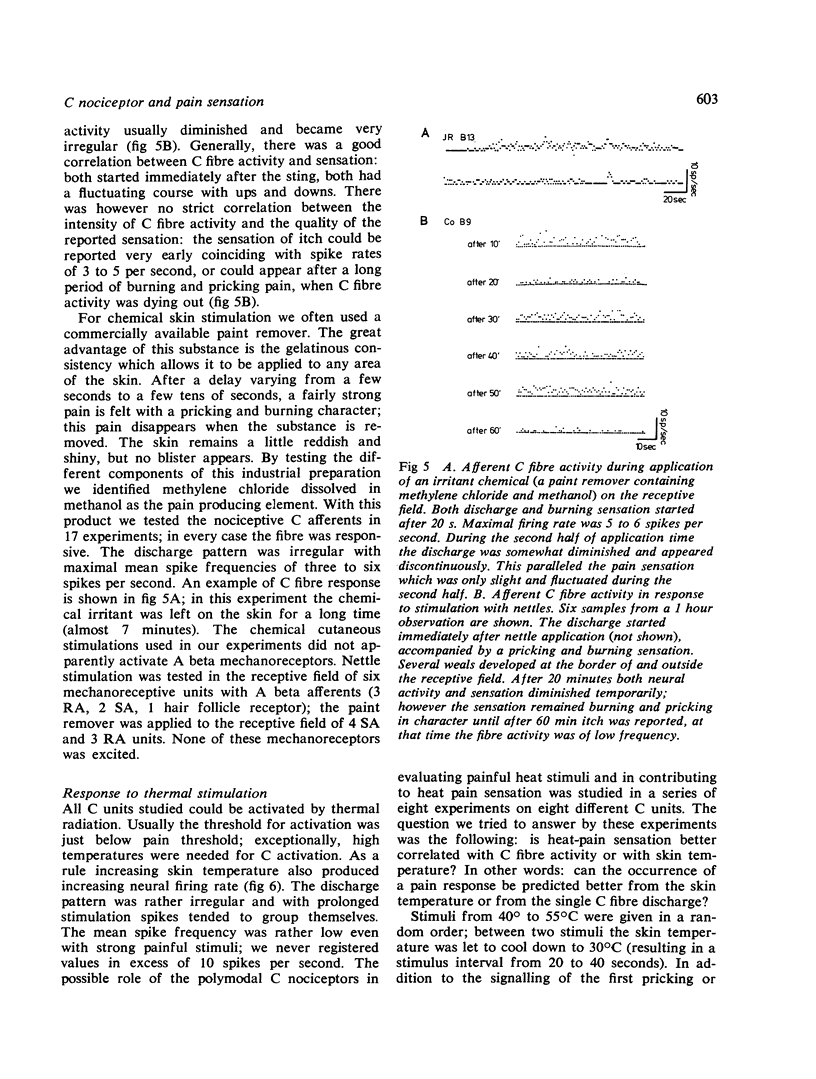
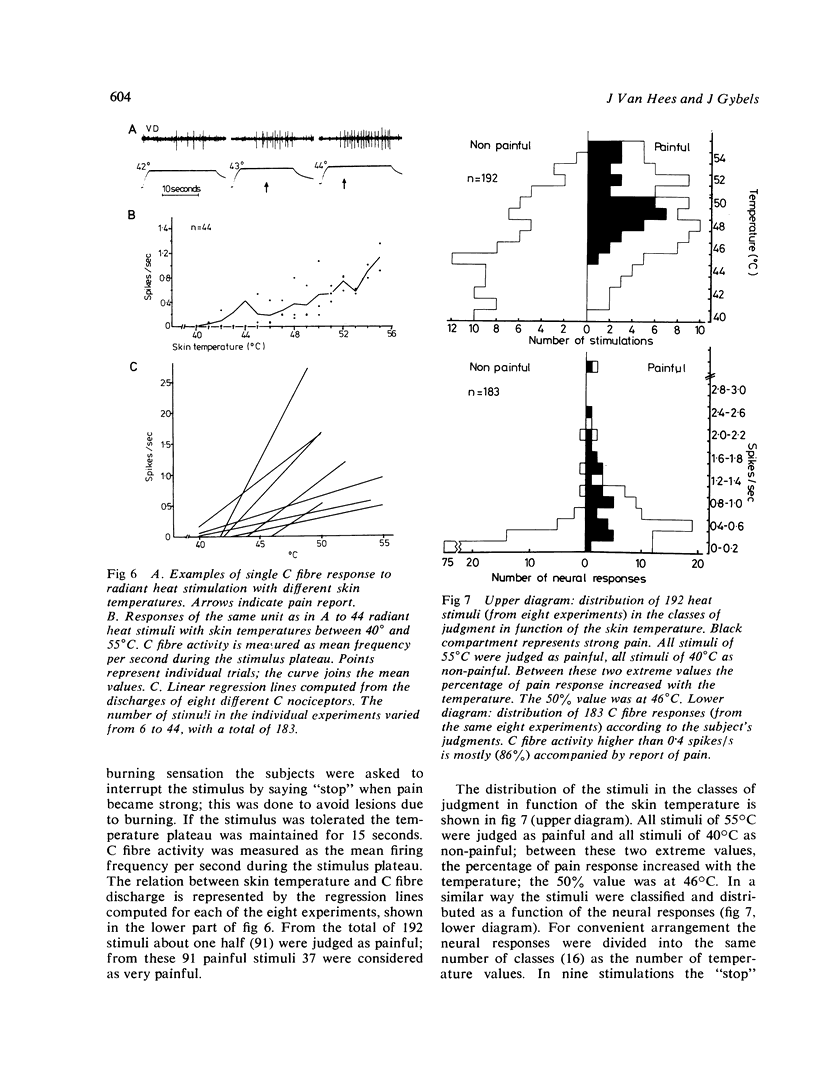
Percutaneous recordings from more than one hundred single C fibres have been performed in the radial nerve of conscious human subjects. All these fibres belong to the poly-modal C nociceptor group, being excited by mechanical and thermal and also by chemical stimulation. Conduction velocities showed a monophasic distribution with a mean value of 0.86 m/s (SD: 0.17). The mechanical threshold, measured with von Frey hairs, varied between 2.3 and 13.1 g. The receptive field was circular or elliptical; for 33 units the mean axes were 6 mm and 7 mm. Mechanically evoked C fibre discharge even up to more than 10 spikes/s was not necessarily accompanied by pain sensation. Nettle sting evoked an irregular C fibre discharge (maximum 10 spikes/s) accompanied by a pricking and burning sensation; the sensation of itch which was sometimes reported, was not correlated with the discharge frequency. C fibre activation by a chemical irritant (paint remover) also evoked an irregular discharge (maximum 3 to 6 spikes/s), accompanied by pricking and burning pain sensation. The C threshold for radiant heat usually lay below the subject's pain threshold. Increasing skin temperature produced increasing neural firing rate. The mean spike frequency rarely exceeded two spikes/s even with stimuli evoking strong heat pain. The occurrence of subjective heat pain response could be as well predicted from th C fibre spike frequency as from the skin temperature. It is concluded that nociceptive C input provoked by thermal or chemical stimuli correlates well with pain sensation. However, similar C input provided by mechanical stimulation which activates also A beta mechanoreceptors, did not necessarily produce pain sensation.
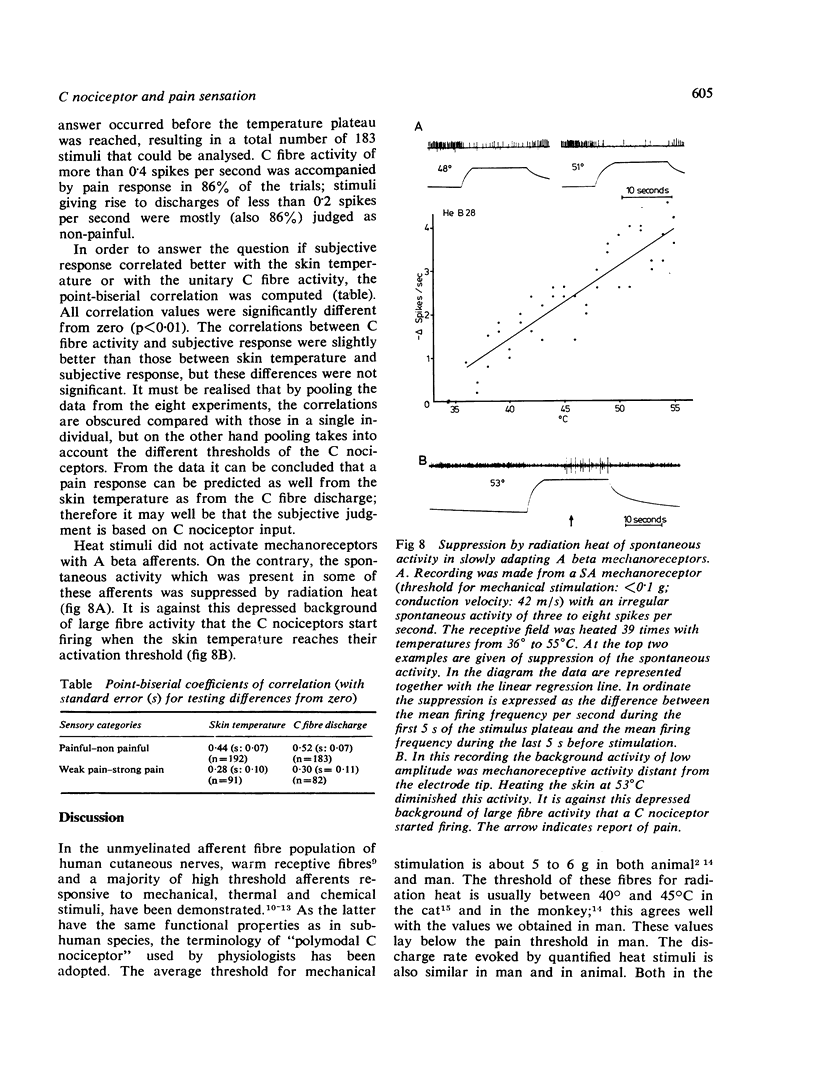
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- COLLIER H. O., CHESHER G. B. Identification of 5-hydroxytryptamine in the sting of the nettle (urtica dioica). Br J Pharmacol Chemother. 1956 Jun;11(2):186–189. doi: 10.1111/j.1476-5381.1956.tb01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelin N., Feldberg W. The mechanism of the sting of the common nettle (urtica urens). J Physiol. 1947 Oct 15;106(4):440–455. doi: 10.1113/jphysiol.1947.sp004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gybels J., Handwerker H. O., Van Hees J. A comparison between the discharges of human nociceptive nerve fibres and the subject's ratings of his sensations. J Physiol. 1979 Jul;292:193–206. doi: 10.1113/jphysiol.1979.sp012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Vallbo A. B. Mechanoreceptor activity recorded percutaneously with semi-microelectrodes in human peripheral nerves. Acta Physiol Scand. 1967 Jan-Feb;69(1):121–122. doi: 10.1111/j.1748-1716.1967.tb03498.x. [DOI] [PubMed] [Google Scholar]
- Handwerker H. O., Neher K. D. Characteristics of C-fibre receptors in the cat's foot responding to stepwise increase of skin temperature ot noxious levels. Pflugers Arch. 1976 Sep 30;365(2-3):221–229. doi: 10.1007/BF01067022. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous and subcutaneous sense organs. Br Med Bull. 1977 May;33(2):97–102. doi: 10.1093/oxfordjournals.bmb.a071432. [DOI] [PubMed] [Google Scholar]
- KEELE C. A. Chemical causes of pain & itch. Proc R Soc Med. 1957 Jul;50(7):477–484. [PMC free article] [PubMed] [Google Scholar]
- Konietzny F., Hensel H. Letters and notes: Warm fiber activity in human skin nerves. Pflugers Arch. 1975 Sep 9;359(3):265–267. doi: 10.1007/BF00587384. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977 Nov;40(6):1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Campbell J. N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978 Mar;41(2):509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E. Afferent C units responding to mechanical, thermal and chemical stimuli in human non-glabrous skin. Acta Physiol Scand. 1974 Nov;92(3):374–390. doi: 10.1111/j.1748-1716.1974.tb05755.x. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Identification of afferent C units in intact human skin nerves. Brain Res. 1974 Mar 8;67(3):387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Van Hees J., Gybels J. M. Pain related to single afferent C fibers from human skin. Brain Res. 1972 Dec 24;48:397–400. doi: 10.1016/0006-8993(72)90198-9. [DOI] [PubMed] [Google Scholar]


