Abstract
Background
Current findings on associations between whole grain (WG) intake and mortality are inconsistent and have not been summarized by meta-analysis.
Methods and Results
We searched for prospective cohort studies reporting associations between WG and mortality from all causes, CVD, and cancer through February 2016 in Medline, Embase, and clinicaltrials.gov, and further included unpublished results from National Health and Nutrition Examination Survey (NHANES) III and NHANES 1999–2004. Fourteen studies were eligible for analysis, which included 786,076 participants, 97,867 total deaths, 23,957 CVD deaths, and 37,492 cancer deaths. Pooled relative risks (RR, 95% confidence intervals [95%CIs]) comparing extreme WG categories (high vs. low) were 0.84 (0.80, 0.88; P<0.001, I2=74%, Pheterogeneity<0.001) for total mortality, 0.82 (0.79, 0.85; P<0.001, I2=0%, Pheterogeneity=0.53) for CVD mortality, and 0.88 (0.83, 0.94; P<0.001, I2=54%, Pheterogeneity=0.02) for cancer mortality. Intakes of WG ingredients in dry weight were estimated among studies reporting RRs for ≥3 quantitative WG categories, which were <50 grams/day among most study populations. The two-stage dose-response random-effects meta-analysis showed monotonic associations between WG intake and mortality (Pnonlinearity>0.05). For each 16 grams/day increase in WG (approximately 1 serving/day), RRs (95%CIs) of total, CVD, and cancer mortality were 0.93 (0.92, 0.94, P<0.001), 0.91 (0.90, 0.93, P<0.001), and 0.95 (0.94, 0.96, P<0.001), respectively.
Conclusions
Our meta-analysis demonstrated inverse associations of WG intake with total and cause-specific mortality, and findings were particularly strong and robust for CVD mortality. These findings further support current Dietary Guidelines for Americans, which recommends at least 3 servings/day of WG intake.
Keywords: whole grain, diet, mortality, meta-analysis
Whole grains (WGs) are rich in dietary fiber, vitamins, minerals, phytochemicals, and other bioactive compounds that may jointly favor long-term health.1, 2 Although dietary guidelines around the world have included WGs as an essential component of healthy eating patterns,3 consumption is largely below recommendations in the U.S. and many European countries.4–7 For example, WG intake among U.S. adults remains below 1 serving/day, despite the long-time recommendation included in the Dietary Guidelines for Americans (DGA) of ≥3 servings/day WG intake.4
Given the lack of large-scale intervention studies investigating the effects of WG intake on chronic disease risk, well-conducted prospective cohort studies provide the most promising evidence regarding the health benefits of WG. Previous meta-analyses of prospective studies have consistently reported inverse associations between WG intake and type 2 diabetes (T2D),8–11 cardiovascular diseases (CVD),8, 12, 13 and certain cancers,14–16 which are among the major global causes of death. However, existing findings on WG intake and mortality remain largely inconclusive. While several studies observed significant inverse associations between WG intake and both total and CVD mortality,17–23 others reported null findings.22, 24 To our knowledge, no studies have quantitatively summarized the published literature on associations of WG intake with total and cause-specific mortality.
Therefore, we performed a meta-analysis of prospective cohort studies on WG intake and risks of total, CVD, and cancer mortality. We estimated WG ingredient intake in dry weight among eligible studies and explored potential dose-response relationships between WG and mortality. In addition, we analyzed associations between WG intake and mortality in two National Health and Nutrition Examination Survey (NHANES) populations and included these original results in this meta-analysis.
Methods
Search Strategy
Our study followed the Meta-analysis Of Observational Studies in Epidemiology guidelines.25 A literature search was performed in Medline, Embase, and clinicaltrials.gov for articles published through February 9, 2015. We combined search terms on exposure (whole grain and its variants, and specific cereal grains, such as oat, barley, brown rice, wheat, etc.) and outcomes (mortality and “cause of death”) without language restriction. Details of the search strategies are shown in Data Supplement. Reference lists of retrieved articles were scanned manually for additional studies.
Eligibility
Candidate papers were included if they met all of the following criteria: (1) they applied a prospective cohort design; (2) the exposure included intakes of WG ingredients (with specified methods for calculation) or WG foods (with specified food items); and (3) the outcome included mortality from all causes, CVD, and/or cancer. For multiple sets of results published from the same study population, we used results based on the longest duration of follow-up.
Search and Data Extraction
Two authors (G.Z. and A.G.) independently screened titles and abstracts of articles retrieved in the initial search, evaluated the eligibility of papers based on full text review, and extracted data. Differences of opinions were resolved by discussion among authors to reach consensus. The following data were extracted from each study: first author’s last name, year of publication, study location, study period, follow-up duration, number of participants, age, amounts of WG ingredient/food intake, methods of diet assessment, number of deaths from all causes, CVD and/or cancer, methods of death confirmation, relative risks (RRs, measured by hazard ratios in all individual studies) and confidence intervals (CIs) for all WG categories, and covariates adjusted in multivariable analysis. For studies reporting WG ingredient intake, we extracted food items and methods for WG ingredient calculation.18, 19, 21–23 For studies reporting WG food intake, we extracted the definitions of and list of specific WG foods.17, 20, 24, 26–28
To evaluate potential dose-response relationships, we further extracted numbers of cases, numbers of participants, and median intakes in each WG category from studies that reported RRs (95% CIs) for three or more quantitative WG categories.18, 20, 21, 23 If numbers of participants and cases were not provided, we used the average number of cases and participants (total participants/cases divided by the number of categories).19, 22 When median intake was missing, we approximated it by using the midpoint of lower and upper bounds. If the highest category was open-ended, we assumed that the highest category had the same WG intake range as the adjacent category. For studies reporting WG as servings/day or analyzing WG foods, we converted medians into WG ingredients in grams by referring to additional publications or databases that provide WG amounts of individual foods (Data Supplement). Study quality was assessed using the Newcastle-Ottawa quality assessment scale (NOS).29 Scores ranged from 0 to 9 points, with higher scores indicating higher study quality (Supplemental Table 1).
To utilize all available data in this meta-analysis, we also examined WG intake in relation to mortality in NHANES III and continuous NHANES surveys 1999–2004 and included results in the current investigation. NHANES is a series of nationwide surveys among non-institutionalized U.S. residents, with the primary aim to assess the health and nutritional status of adults and children. To achieve nationwide representativeness, a complex multistage probability-sampling design is used in NHANES surveys. The study protocol was approved by the institutional review board at the Centers for Disease Control and Prevention (Atlanta, Georgia), and written informed consents were obtained from all participants. Because mortality follow-up in NHANES surveys was up to the end of 2010, we included participants surveyed before 2005 to allow for sufficient follow-up (>6 years). We analyzed the association between WG intake and mortality in these two cohorts and included original results in this meta-analysis. WG was estimated by linking data from a single 24-hour dietary recall to the MyPyramid Equivalents Database (MPED). Mortality information was obtained through searching the National Death Index. Details of the study population and method are provided in Data Supplement.
Statistical Analysis
Meta-analysis was based on risk estimates from models with the most complete adjustment of potential confounders but not components of WGs, such as cereal fiber. RRs comparing the highest to the lowest WG group were summarized using a random-effects model.30 Statistical heterogeneity was assessed with Cochran’s Q test (P<0.10) and I2 statistic.31 Analysis was stratified by study location (U.S., Scandinavia, other regions), WG assessments (WG ingredients, WG foods), dietary questionnaire (single food frequency questionnaires/FFQ at baseline, repeated FFQ during follow-up, and others), whether WG is the main exposure (yes, no), sample size (<20,000, ≥20,000), median follow-up duration (<10 years, ≥10 years), adjustments of dietary factors (energy intake and other dietary confounding factors: yes, no), NOS score (<8, ≥8), and mean age at baseline (all adults, only >55 years). In light of the limited number of included studies, we performed univariate meta-regressions to explore whether these factors are potential sources of heterogeneity. Publication bias was assessed by funnel plot and Egger’s linear regression test.32
To examine the parametric relationship between WG intake and mortality, we first calculated the covariance of RRs using number of cases and participants in each WG category, and applied the inverse of variance/covariance matrix as study weight in the analysis.33 A two-stage dose-response random-effects meta-analysis was performed.34 In the first stage, a restricted cubic spline model with two spline variables of WG intake was fitted after taking into account the correlation within each set of published RRs. In the second stage, the regression coefficients and the variance/covariance matrices within each study were combined using the multivariate extension of the method of moments in a multivariate random-effects meta-analysis.34 We calculated the overall significance of models by testing the hypothesis that the two regression coefficients equal zero, and calculated the significance for nonlinearity by testing the hypothesis that the coefficient of the second coefficients equals zero.35 STATA command MVMETA and GLST were used for model fitting. All analyses were conducted with STATA version 12.0 (StataCorp LP, College Station, TX).
Population Attributable Fraction (PAF) was calculated using the following formula: PAF=100%×Pe(RR−1)/(Pe[RR−1]+1). Pe is the prevalence of WG consumption below dietary recommendation among U.S. adults, which is 92.7% according to a recent analysis in NHANES 2009–2010.36 RR is risk estimate from the current meta-analysis, comparing average WG consumption among U.S. adults (0.82 serving/day) with recommended intake (3 servings/day).
Results
Of the 4,062 records retrieved from initial search, 3,966 were excluded after title and abstract screening, and 84 were excluded after full text review. We further excluded five multiple reports based on the same populations, three reports among participants with existing CVD or cancer, four reports with no descriptions of WG ingredient calculation or specific WG food criteria, and one report using WG intake as a continuous variable (Figure 1). Twelve studies from eleven publications were eligible for analysis.
Figure 1.
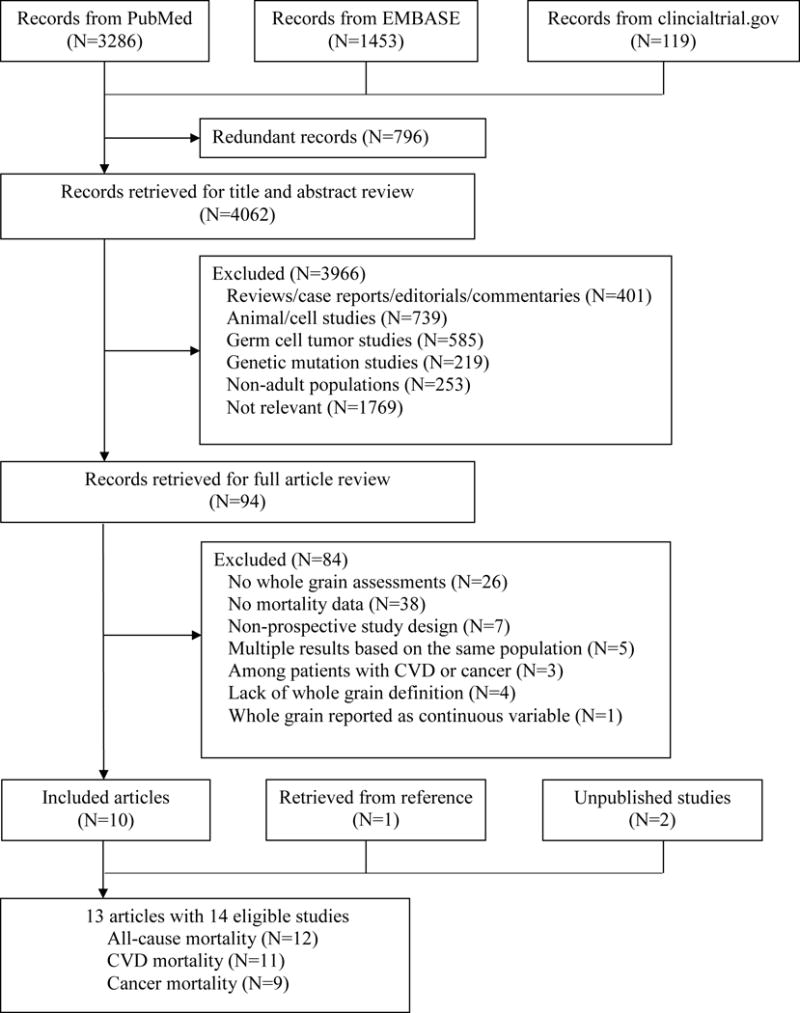
Flow diagram of study screening.
Baseline characteristics of NHANES III (n=11,666) and NHANES 1999–2004 (n=12,488) are provided in Supplemental Tables 2 and 3. As shown in Supplemental Table 4, WG intake was inversely associated with total and cause-specific mortality, although none of the associations reached statistical significance.
Thus, 14 unique sets of results were included in the meta-analysis, which included 786,076 participants, 97,867 total deaths (n=12), 23,957 CVD deaths (n=11), and 37,492 cancer deaths (n=9, Table 1). Two studies reported findings for WG bread and WG cereals separately,24, 28 and one study performed only sex-specific analysis.22 Most studies (n=10) were conducted in U.S. populations, with the remaining studies in either Scandinavian countries (n=3) or the UK (n=1). Median follow-up durations ranged from 6 to 28 years, and study periods were between 1971 and 2010. Six studies analyzed WG foods, and eight estimated intakes of WG ingredients (four used the MPED database and four used self-developed methods). Foods accounting for WG intake ranged from a single item (such as WG bread, dark bread, or WG cereals) in FFQ to a comprehensive list of grain-based foods available from 24-hour dietary recall (Table 1 and Data Supplement). The association between WG intake and mortality was the primary study aim of 10 investigations, and the rest considered WGs a component of healthy diets or a source of dietary fiber. Most studies applied FFQs to assess dietary exposure (three used repeated FFQ, seven used baseline FFQ, two used 24-hour dietary recall, and one used diet history questionnaire). NOS scores ranged from 6 to 9, with nine studies scoring 8 or above (Supplemental Table 4).
Table 1.
Characteristics of studies included in meta-analysis.
| Author publication year, location, reference number, |
Sample age in years |
Duration period |
Exposure | Dietary assessment |
Whole grain item† | Cause of death: number |
Risk estimate | Death confirmation/ death code |
Covariates |
|---|---|---|---|---|---|---|---|---|---|
| Fraser 1992, US, (17) | 26,473 Adventists ≥25 years | 6 years 1977–1982 | Whole grain food | Baseline FFQ | Whole grain bread 100% whole-wheat bread used most often vs. white bread used most often. | CVD: 463 | CVD: reference 0.77(0.54–1.08) 0.74(0.56–0.99) |
death certificates, registry and records ICD | Age, sex, smoking, exercise, relative weight, high blood pressure, nuts, beef, cheese, fish, coffee, legumes, and fruits. |
| Key 1996, UK, (24) | 10,771 vegetarians 16–79 years |
17 years 1973–1995 |
Whole grain food | Baseline FFQ | Whole grain bread Having whole grain daily vs. not having whole grain daily. |
All-cause: 2562 CHD: 350 stroke: 147 Cancer: 451 |
Whole grain bread: All-cause: 0.88(0.78, 0.98) CHD: 0.85(0.68, 1.06); Stroke: 1.08(0.75, 1.54) Cancer: 0.91(0.75, 1.11) |
death certificates, ICD8, ICD9 | Age, sex, and smoking |
| Jacobs 2001, Norway, (18) | 33,848 35–56 years | 14 years 1977–1994 |
Whole grain from bread Self-developed |
Baseline FFQ | Whole grain bread Women (median bread score): 0.38/0.83/1.13/1.65/2.7 Men (median bread score): 0.45/0.83/0.90/1.65/3.30 |
All-cause: 2058 CVD: 758 Cancer: 870 |
All-cause: reference 0.87(0.75–1.01) 0.8(0.71–0.92) 0.85(0.74–0.98) 0.75(0.65–0.88) CVD: reference 0.93(0.73–1.18) 0.84(0.68–1.05) 0.84(0.66–1.05) 0.77(0.6–0.98) Cancer: reference 0.96(0.76–1.2) 0.89(0.73–1.08) 0.9(0.73–1.11) 0.79(0.62–1.02) |
Registry ICD8, ICD9 | Age, energy intake, sex, current smoking, past smoking, physical activity during leisure, physical activity during work, customary use of cod liver oil, customary use of multivitamins, saturated fat intake, systolic blood pressure, serum total cholesterol, and body mass index |
| Steffen 2003,US,(26) | 11,940 45–64 years | 11 years 1987–1999 | Whole grain food | Repeated FFQ | Dark bread and Whole grain cold breakfast cereal. Whole grain cold cereals contained ≥ 25% whole grain or bran by weight | All-cause: 867 |
All-cause: reference 0.96(0.79–1.17) 0.8(0.65–0.99) 0.87(0.7–1.08) 0.77(0.61–0.97) |
Family report, death record, registry N.A. | Age, race, sex, time-dependent energy intake, smoking status, pack-years of smoking, physical activity, alcohol intake, and hormone replacement in women |
| Sahyoun 2006, US, (19) | 535 60–98 years | 12–15 years 1981–1995 | Whole grain MPED |
3-day food record | All Whole grain foods from 3-day food records. Whole grain (serving/day): 0.31/0.86/1.49/2.9 |
CVD:89 | CVD: reference 0.76(0.41–1.43) 0.76(0.41–1.41) 0.48(0.25–0.96) |
Registry ICD9 | Age, sex, race, educational attainment, marital status, smoking status, BMI, waist-to-hip ratio, systolic blood pressure, exercise, calories, percentage calories from saturated fatty acids, use of antihypertensive medication, and use of lipid-lowering medication |
| Jacobs 2007, US, (20) | 27,312 women 55–69 years | 17 years 1986–2001 | Whole grain food | Baseline FFQ | Dark bread, cold breakfast cereal, brown rice, popcorn, wheat germ, bran, cooked oatmeal, other grains (e.g., bulgur, kasha, and couscous). Breakfast cereals containing ≥25% whole grain or bran were considered whole grains. Whole grain range (serving/week): 0–3.5(median 1.75) 4–7(median: 5.5) 7.5–10.5(median: 9) 11–18.5(median: 14.75) ≥19(median: 22.75) |
All-cause: 5552 CVD: 1,900 Cancer: 2,099 |
All-cause: reference 0.88(0.81–0.96) 0.88(0.81–0.96) 0.8(0.73–0.87) 0.79(0.72–0.87) CVD: reference 0.96(0.84–1.1) 0.83(0.72–0.96) 0.83(0.71–0.96) 0.73(0.62–0.86) Cancer: reference 0.86(0.75–0.99) 0.95(0.83–1.09) 0.83(0.72–0.96) 0.89(0.77–1.04) |
death record ICD9, ICD10 | Age, energy intake, BMI, waist-hip ratio, smoking, education, physical activity, estrogen use, multivitamin supplement use, and intakes of alcohol, refined grain, coffee, red meat, fish and seafood, and total fruit and vegetables |
| Tognon 2011, Sweden, (27) | 1,037 70 years |
N.A. 1971–2009 |
Whole grain food | Dietary history questionnaire | Whole grain cereal Above vs. below medians. Medians were 74.2g in women and 92.8g in men. |
All-cause: 630 |
All-cause: 0.85(0.73–1) |
Registry N.A. |
Sex, baseline body mass index (BMI), waist circumference, smoking status, physical activity level, marital status, education, and birth cohort. |
| Huang 2015, US, (21) | 367,442 50–71 years | 14 years 1995–2009 | Whole grain MPED |
Baseline FFQ |
Ready-to-eat cereals, high-fiber cereals, other fiber cereals, Whole grain bread or dinner rolls, cooked cereal, popcorn, pancakes, waffles, French toast or crepes, rice or other cooked grains, bagels, English muffins, tortillas, pasta, crackers, chips, cookies/brownies, sweet pastries, and pies Median (serving/day): 0.13/0.3/0.47/0.69/1.2 |
All-cause: 46,067 CVD: 11283 Cancer: 19,043 |
All-cause: reference 0.93(0.9–0.95) 0.89(0.87–0.92) 0.85(0.82–0.87) 0.83(0.81–0.86) CVD: reference 0.93(0.88–0.98) 0.88(0.83–0.93) 0.81(0.77–0.86) 0.83(0.78–0.88) Cancer: reference 0.94(0.9–0.98) 0.91(0.87–0.95) 0.88(0.84–0.92) 0.85(0.81–0.89) |
Registry ICD9, ICD10 | Age, gender, number of cigarettes smoked per day, time of smoking cessation, race or ethnicity group, alcohol intake, education level, marital status, health status, obesity, physical activity, consumption of red meat, total fruit and total vegetables, total energy intake, and hormone usage |
| Roswall 2015, US, (28) |
44,961 women 29–49 years |
21 years 1992–2013 |
Whole grain food | Baseline FFQ | Whole grain bread, oatmeal Whole grain consumers vs. non-consumers |
All-cause: 1,855 CVD: 270 Cancer: 1,113 |
Bread All-cause: reference 0.83(0.76–0.92) Oatmeal All-cause: reference 0.99(0.9–1.09) |
Registry ICD9, ICD10 | Age, smoking status, duration, current tobacco consumption, time since smoking cessation, school education, BMI, alcohol intake, red meat intake, processed meat intake, and energy intake |
| Johnsen 2015, Scandinavian, (22) |
119,518 30–64 years |
12.1 years 1992–2009 |
Whole grain Self-developed |
Baseline FFQ | Breakfast cereals, non-white bread, crisp bread Median (g/d): Men, all-cause mortality: 21/37/54/80 Women, all-cause mortality: 20/33/49/74/ Men, CHD mortality: 21/36/54/82 Women, CHD mortality: 21/33/51/70 Men, stroke mortality: 20/37/50/79 Women, stroke mortality: 20/31/53/76 Men, cancer mortality: 21/37/54/80 Women, cancer mortality: 21/33/49/76 |
All-cause: 7,839 CHD: 1,156 stroke: 280 Cancer: 3,150 |
Men All-cause: reference 0.82(0.75–0.9) 0.72(0.66–0.78) 0.75(0.68–0.82) CHD: reference 0.79(0.65–0.96) 0.8(0.66–0.97) 0.74(0.61–0.91) Stroke: reference 0.7(0.44–1.1) 0.69(0.43–1.11) 0.71(0.44–1.15) Cancer: reference 0.85(0.73–0.99) 0.68(0.58–0.79) 0.74(0.63–0.87) Women All-cause: reference 0.8(0.73–0.87) 0.74(0.67–0.81) 0.74(0.67–0.81) CHD: reference 0.83(0.6–1.14) 0.59(0.42–0.82) 0.65(0.46–0.91) Stroke: reference 0.55(0.34–0.88) 0.5(0.31–0.82) 0.8(0.48–1.33) Cancer: reference 0.99(0.87–1.13) 0.94(0.82–1.07) 0.88(0.77–1.02) |
Registry N.A. |
Age, follow-up time, education, smoking intensity, alcohol, BMI, total energy intake and alcohol intake, with Whole grain products and Whole grain types mutually adjusted |
| Wu 2015, US, (23) | 74,341 female nurses 38–63 years | 26 years 1984–2010 | Whole grain Self-developed |
Repeated FFQ | Whole wheat and whole-wheat flour, whole oats and whole-oat flour, whole cornmeal and whole-corn flour, whole rye and whole-rye flour, whole barley, bulgur, buckwheat, brown rice and brown rice flour, popcorn, amaranth, and psyllium Whole grain (g/d): 4.2/9.7/14.7/21.1/33.0 |
All-cause: 15106 CVD: 2,989 Cancer: 5,964 |
All-cause: reference 0.98(0.93–1.03) 1.0001(0.95–1.05) 0.94(0.89–0.99) 0.88(0.84–0.93) CVD: reference 0.97(0.87–1.08) 0.96(0.86–1.08) 0.82(0.73–0.92) 0.86(0.76–0.96) Cancer: reference 1.02(0.94–1.1) 1.1(1.02–1.19) 1.06(0.98–1.15) 0.99(0.91–1.07) |
family report or registry ICD8 | Age, ethnicity; body mass index; smoking status; alcohol intake; physical activity; family history of diabetes mellitus, cancer, and heart disease; multivitamin use; aspirin use at least once per week; history of hypertension; high cholesterol level or diabetes at baseline; and total energy, postmenopausal status and postmenopausal hormone use (women), and alternative healthy eating index). |
| Wu 2015, US, (23) | 43,744 male health professionals 32–87 years | 24 years 1986–2010 | Whole grain Self-developed |
Repeated FFQ | Whole wheat and whole-wheat flour, whole oats and whole-oat flour, whole cornmeal and whole-corn flour, whole rye and whole-rye flour, whole barley, bulgur, buckwheat, brown rice and brown rice flour, popcorn, amaranth, and psyllium Whole grain (g/day): 5.9/14.4/22.1/31.3/47.8 |
All-cause: 11,814 CVD: 3,621 Cancer: 3,921 |
All-cause: 1(1, 1); 1.00(0.94, 1.05) 0.97(0.91, 1.02) 1.01(0.95, 1.07) 0.95(0.89, 1.00) CVD:1(1, 1) 0.92(0.84, 1.02) 0.92(0.83, 1.02) 0.91(0.82, 1.01) 0.84(0.75, 0.93) Cancer: (1, 1) 1.01(0.92, 1.11) 0.98(0.88, 1.08) 1.01(0.91, 1.12) 0.95(0.86, 1.05) |
family report or registry ICD8 | Age, ethnicity; body mass index; smoking status; alcohol intake; physical activity; family history of diabetes mellitus, cancer, and heart disease; multivitamin use; aspirin use at least once per week; history of hypertension; high cholesterol level or diabetes at baseline; and total energy, and alternative healthy eating index. |
| NHANES III unpublished, US |
11,666 >20 years |
20 years 1988–2010 |
Whole grain MPED |
24-hour dietary recall | All foods containing whole grains in 24-hour dietary recall |
All-cause: 2,545 CVD: 702 Cancer: 636 |
All-cause: reference 0.98(0.86, 1.12) 0.88(0.77, 1.02) CVD: reference 1.11(0.86, 1.44) 0.99(0.71, 1.39) Cancer: reference 1.08(0.82, 1.42) 0.84(0.66, 1.07) |
Registry ICD9, ICD10 | Age, ethnicity, education, physical activity, smoking status, alcohol consumption, BMI, energy intake, and HEI score. |
| NHANES 1999–2004 unpublished, US | 12,488 >20 years |
20 years 1999–2010 |
Whole grain MPED |
24-hour dietary recall | All foods containing whole grains in 24-hour dietary recall | All-cause: 972 CVD: 229 Cancer: 245 |
All-cause: reference 0.90(0.73, 1.11) 0.83(0.65, 1.07) CVD: reference 0.98(0.53, 1.80) 0.74(0.41, 1.32) Cancer: reference 0.72(0.48, 1.07) 0.63(0.37, 1.09) |
Registry ICD9, ICD10 | Age, ethnicity, education, physical activity, smoking status, alcohol consumption, BMI, fruit, vegetable, meat, energy intake, and added sugar |
Abbreviations: N.A., Not available; MPED, MyPyramid Equivalents Database; FFQ, food frequency questionnaire; CVD, Cardiovascular disease; ICD, International Statistical Classification of Diseases and Related Health Problems; BMI, body mass index; NHANES, National Health and Nutrition Examination Survey; HEI, health eating index.
Details of whole grain calculation are provided in Supplemental Methods
Main analysis
Figure 2 shows forest plots on mortality, comparing the highest to the lowest groups of WG intake. Pooled RR (95%CI) for total mortality was 0.84 (0.80, 0.88; P<0.001), with significant heterogeneity detected among studies (I2=74%; Pheterogeneity<0.001). For CVD mortality, two studies provided results for coronary heart disease (CHD) and stroke separately, and one study reported only CHD mortality, yielding 15 individual estimates. Pooled RR (95%CI) was 0.82 (0.79, 0.85; P<0.001), without apparent heterogeneity (I2=0.0%; Pheterogeneity=0.53). RR (95%CI) of cancer mortality was 0.88 (0.83, 0.94; P<0.001), pooling 11 individual estimates from nine studies (I2=54%; Pheterogeneity=0.02).
Figure 2.
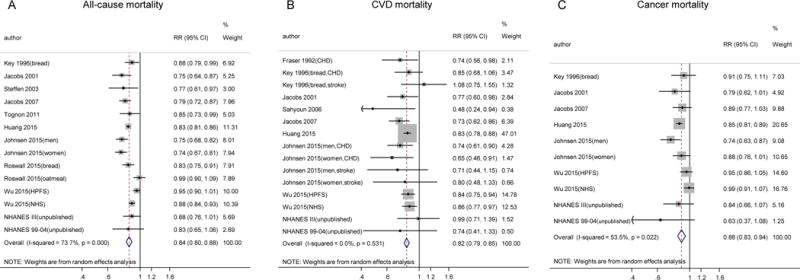
Meta-analysis of the association between whole grain intake and mortality. All-cause (A), CVD (B), and cancer mortality (C) in prospective cohort studies. Relative risks correspond to comparisons of extreme categories of exposure within each study. The area of each square is proportional to the inverse of the variance of the log relative risks. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from an inverse variance–weighted random-effects model. NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; CHD, coronary heart disease; RR, relative risk.
Results of stratified analysis are shown in Supplemental Tables 5, 6 and 7, with significantly lower RRs observed in most subgroups. In univariate meta-regression, heterogeneities of all-cause mortality and cancer mortality were not explained by study location, WG assessments, dietary questionnaire, study aim, sample size, median follow-up duration, adjustment of dietary factors, NOS score, or ages at baseline. Funnel plots for publication bias are shown in Supplemental Figure 1. Egger’s test did not suggest publication bias for total mortality (P=0.72), CVD mortality (P=0.21), or cancer mortality (P=0.64).
Dose-response analysis
Among 10 studies that provided adequate data for dose-response analysis, estimated median intakes across WG categories ranged from 0.7–21 grams/day (in a U.S. study) to 20–80 grams/day (in a Scandinavian study). The dose-response relationship between WG ingredient intake and mortality is shown in Figure 3. Using no WG consumption as the reference, RRs (95%CIs) of total mortality were 0.93 (0.89, 0.97; P<0.001; Figure 3A) at 10 grams/day, 0.84 (0.77, 0.91; P<0.001) at 30 grams/day, 0.80 (0.75, 0.85; P<0.001) at 50 grams/day, and 0.78 (0.74, 0.82; P<0.001; P trend<0.001; P nonlinearity=0.06) at 70 grams/day. For CVD mortality (Figure 3B), RRs (95%CIs) were 0.92 (0.88, 0.96; P<0.001) at 10 grams/day, 0.81 (0.75, 0.89; P<0.001) at 30 grams/day, 0.78 (0.72, 0.84; P<0.001) at 50 grams/day, and 0.77 (0.68, 0.87; P<0.001; P trend<0.001; P nonlinearity=0.10) at 70 grams/day compared with no WG consumption. For cancer mortality (Figure 3C), RRs (95%CIs) were 0.96 (0.91, 1.01; P=0.12) at 10 grams/day, 0.89 (0.89, 0.99; P=0.04) at 30 grams/day, 0.85 (0.76, 0.94; P=0.001) at 50 grams/day, and 0.80 (0.72, 0.89; P<0.001; P trend<0.001, P nonlinearity=0.67) at 70 grams/day, compared with no WG consumption. Since tests for non-linear trends were not rejected (P>0.05 for all outcomes), we further estimated RRs (95%CIs) for each 16 grams/day increase in WG ingredient consumption (approximately one serving/day), which were 0.93 (0.92, 0.94) for total mortality, 0.91 (0.90, 0.93) for CVD mortality, and 0.95 (0.94, 0.96) for cancer mortality.
Figure 3.
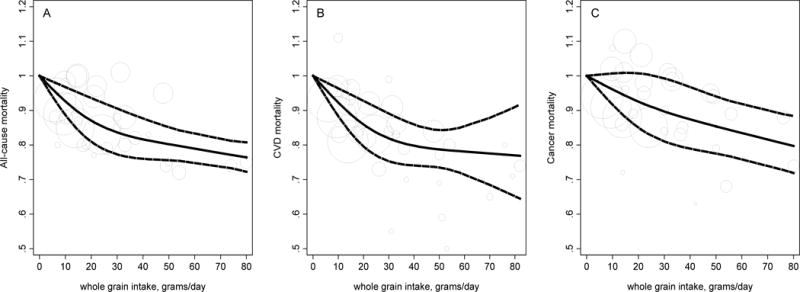
Dose-response analysis for associations between whole grain intake and mortality. All-cause (A; Ptrend<0.001, Pnonlinearity=0.06), CVD (B; Ptrend<0.001, Pnonlinearity=0.10), and cancer (C; Ptrend<0.001, P nonlinearity=0.67) mortality in prospective cohort studies. The pooled linear risk trend (thick solid line) and its 95% CI (thick dashed lines) were obtained by a random-effects dose-response meta-analysis. Circles are inversely proportional to the variance of log relative risks.
Sensitivity analysis
Results did not change in the following sensitivity analyses (Supplemental Table 8): 1) restricting the analysis in studies eligible for dose-response meta-analysis; 2) excluding studies that used only cereal as WG source;24, 27, 28 3) excluding studies that used only bread as WG source;17, 18, 24, 28 4) excluding the largest study by Huang et al;21 and 5) for CVD mortality, excluding studies that analyzed only stroke mortality or CHD mortality. Dose-response curves were similar in the following sensitivity analyses: 1) assuming WG bread was made from 100% WG flour in studies by Steffen et al. and Jacob et al. (Supplemental Figure 2);20, 26 2) assuming one ounce-equivalent of WG in MPED database is 16 grams of dry WG ingredients (Supplemental Figure 3); and 3) excluding the largest study by Huang et al. (Supplemental Figure 4).21
Population Attributable Fraction
Estimated PAFs were 10.4% for total mortality, 11.6% for CVD mortality, and 10.2% for cancer mortality.
Discussion
Our study demonstrated significant inverse associations between WG intake and mortality from all causes, CVD, and cancer. Further dose-response analysis showed a strong monotonic association of WG with total and cause-specific mortality. These findings support current dietary guidelines that recommend ≥3 servings/day WG consumption for long-term health and longevity.
Studies included in this meta-analysis collectively showed strong and robust inverse associations between WG intake and CVD mortality. The results were in line with existing findings linking WG intake with CVD risk, as well as other cardiometabolic conditions such as type 2 diabetes. Meta-analyses of 8 to 14 prospective cohort studies reported a 21% CVD risk reduction comparing the highest WG intake group with the lowest8, 12, 13 For diabetes risk, pooled RR (95%CIs) comparing extreme WG groups was 0.74 (0.69, 0.80),8 with each 2–3 servings/day increment associated with 21–32% lower risk.9, 15 Two meta-analyses of clinical trial results consistently documented that WG intake lowered fasting glucose, LDL-cholesterol, total cholesterol, and body fat percentage.8, 37
The relationship between WG and cancer outcomes is less clear. In our meta-analysis, significantly lower cancer mortality was observed only when daily WG consumption exceeded 30 grams/day. An early meta-analysis of 40 case-control studies found that WG intakes were lower among patients with various types of cancer than controls,14 although the retrospective study design of these early studies hindered causal inference. Subsequent prospective studies consistently found inverse associations between WG intake and colorectal cancer, but not with other cancers.23 For example, Haas et al reported an inverse association between WG intake and colorectal cancer incidence based on a meta-analysis of 25 studies.16 Aune et al further performed a dose-response analysis of six prospective studies and found a linear inverse association between WG food intake and colorectal cancer risk, with each 3-serving increment associated with a 17% lower risk.15 It is possible that the association between WG intake and cancer is dependent on population characteristics and cancer types and is partly explained by other lifestyle and dietary factors,23 which needs to be investigated by further studies.
Despite limited available data, WG consumption may also contribute to lower mortality from other causes. Among over 30,000 Norwegians, a non-linear inverse association was observed for WG bread consumption and risk of non-cardiovascular and non-cancer deaths.18 In the Iowa Women’s Health Study, intakes of WG foods were inversely associated with risk of death attributed to inflammatory diseases other than CVD and cancer, such as infectious diseases, respiratory system diseases, and digestive diseases.20 Huang et al found an inverse association between WG intake and mortality from respiratory diseases, infections, and other unknown causes.21 Johnsen et al also reported that WG intake was associated with lower mortality due to causes other than CVD, cancer, diabetes, and respiratory diseases.22 Clearly, more data are required to further elucidate the potential benefits of WGs on other health conditions.
Our analysis revealed a largely linear dose-response relationship of WG intake with total and CVD mortality. Although risk estimates were less stable at very high doses due to limited data, the monotonically decreasing curves for WG up to 50 grams/day strongly supported the current DGA recommendations to consume ≥3 ounce-equivalents (48 grams of WG ingredients) of WG foods for the prevention of chronic diseases. It is critical to note that individuals should choose foods that are high in WG ingredients (at least 16 grams WG ingredients in dry weight per serving) to achieve approximately 48 grams/day of WG intake, while maintaining a low consumption of refined carbohydrates, which have established adverse effects.38,39 In this regard, low-carbohydrate diets that ignore the health benefits of WG foods should be adopted with caution, as they have been linked to higher CVD risk and mortality.40–42 Instead, diets that emphasize the quality of carbohydrates, fats, and proteins, such as the Mediterranean diet, Dietary Approaches to Stop Hypertension diet, and Alternate Healthy Eating Index 2010, should be recommended to facilitate disease prevention.43
Multiple bioactive compounds in WGs could contribute to the prevention of obesity, CVD, diabetes, cancer, poor gut health, nervous system disorders, poor skeletal health, and oxidative stress. The high fiber content may lower cholesterol production and glucose response and increase satiety, partially through inducing the production of short-chain fatty acids, which also lower carcinogenic potential. The high magnesium content in WGs may improve insulin sensitivity, suppress glucose update, and lower blood pressure. WGs also contain other minerals and constituents, including Fe, Zn, Cu, Se, polyphenols, carotenoids, and tocopherols, which may suppress the oxidative stress that underlies the pathogenesis of many chronic diseases.2
The strengths of our study include a large number of participants in original studies and careful examination of dose-response relationships. We standardized WG intake to reduce heterogeneity of WG estimates among individual studies and facilitate comparison with the DGA. According to sensitivity analyses, findings were independent of differences in WG assessment methods. Meanwhile, our study has some limitations. Because studies used in our meta-analysis were carried out before a more consistent definition of WG became available,44 lists of WG foods or food sources varied substantially among individual studies. For U.S. adults, over 70% of daily WG intake are from breads and cereals (yeast breads/rolls, quick breads, pastas/cooked cereals/rice, and ready-to-eat cereals),4 which were included by most studies in our dose-response meta-analysis. Because of the small number of studies available, meta-regression has limited capacity to identify potential sources of heterogeneity. As most studies are from U.S. and Scandinavian populations, it is not known whether these findings can be generalized to populations of other ethnicities. Most studies used FFQ to collect dietary data, which may underestimate WG intake when the list of food items was limited. Since WG contents vary both between and within food items, measurement error in WG and misclassification of participants are inevitable. Such random measurement errors may primarily weaken the true associations in these prospective studies. For studies that used short-term dietary assessment tools, such as 3-day food records, day-to-day variations of WG intake may reduce the validity of these assessments to reflect long-term diet. Other potential limitations include unmeasured or residual confounding from healthier lifestyles associated with high WG intake, and the inclusion of participants with prevalent and unmeasured new onset of disease during follow-up, which may substantially change the diet at baseline or during the follow-up period.
In conclusion, WG consumption was inversely associated with mortality in a dose-response manner, and the association with CVD mortality was particularly strong and robust. These observations endorse current dietary guidelines that recommend increasing WG intake to replace refined grains to facilitate long-term health and help prevent premature death.
Supplementary Material
Clinical Perspectives.
Whole grains (WG) provide various essential nutrients for long-term health and have been associated with lower risk of many chronic diseases. However, WG consumption is largely below dietary recommendation in the U.S. and many European countries. We performed the first meta-analysis of prospective cohort studies on WG intake and risk of mortality, which included 14 studies with 786,076 participants and 97,867 total deaths. Accordingly, inverse associations between WG intake and mortality from all-cause, CVD, and cancer were observed, and findings for CVD mortality was robust and strong compared to those for total and cancer mortality. All estimated WG ingredient intakes were <80 grams/day, with most studies <50 grams/day. Within this range, dose-response analysis revealed a largely linear relationship between the amount of WG intake and total, CVD, and cancer mortality. Our findings corroborated current Dietary Guidelines for Americans that recommended ≥3 servings/day WG consumption (48 gram/day WG ingredients) to facilitate the prevention of chronic diseases and premature death.
Acknowledgments
We are grateful to Paul Guttry for professional English editing of the manuscript. Author Contributions: GZ and QS designed the study. GZ and QS analyzed data. GZ wrote the first draft of the manuscript. All authors contributed to the interpretation of data, critical revision of the manuscript, and had final approval of the submitted and published version. GZ and QS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Sources: This research was supported by NIH grants ES022981 and ES021372. Qi Sun was supported by a career development award, R00-HL098459, from the National Heart, Lung, and Blood Institute.
Footnotes
Journal Subject Terms: Diet and Nutrition; Risk Factors; Mortality/Survival
Disclosures: None.
References
- 1.Slavin J. Why whole grains are protective: Biological mechanisms. Proc Nutr Soc. 2003;62:129–134. doi: 10.1079/PNS2002221. [DOI] [PubMed] [Google Scholar]
- 2.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr Res Rev. 2010;23:65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 3.Ferruzzi MG, Jonnalagadda SS, Liu S, Marquart L, McKeown N, Reicks M, Riccardi G, Seal C, Slavin J, Thielecke F, van der Kamp JW, Webb D. Developing a standard definition of whole-grain foods for dietary recommendations: Summary report of a multidisciplinary expert roundtable discussion. Adv Nutr. 2014;5:164–176. doi: 10.3945/an.113.005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill CR, Fulgoni VL, 3rd, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the united states population: National health and nutrition examination survey 2001–2010. Nutrients. 2015;7:1119–1130. doi: 10.3390/nu7021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann KD, Pearce MS, McKevith B, Thielecke F, Seal CJ. Low whole grain intake in the uk: Results from the national diet and nutrition survey rolling programme 2008–11. Br J Nutr. 2015;113:1643–1651. doi: 10.1017/S0007114515000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins SM, Hébel P, Colin J, Reyé B, Bellisle F. Whole grain consumption – a french perspective. Proc Nutr Soc. 2015;74:E124. (121 pages; Abstract) [Google Scholar]
- 7.Kyro C, Skeie G, Dragsted LO, Christensen J, Overvad K, Hallmans G, Johansson I, Lund E, Slimani N, Johnsen NF, Halkjaer J, Tjonneland A, Olsen A. Intake of whole grain in scandinavia: Intake, sources and compliance with new national recommendations. Scand J Public Health. 2012;40:76–84. doi: 10.1177/1403494811421057. [DOI] [PubMed] [Google Scholar]
- 8.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 11.Chanson-Rolle A, Meynier A, Aubin F, Lappi J, Poutanen K, Vinoy S, Braesco V. Systematic review and meta-analysis of human studies to support a quantitative recommendation for whole grain intake in relation to type 2 diabetes. PloS One. 2015;10:e0131377. doi: 10.1371/journal.pone.0131377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol. 2015;115:625–629. doi: 10.1016/j.amjcard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DR, Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: An expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- 15.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas P, Machado MJ, Anton AA, Silva AS, de Francisco A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: Meta-analysis of cohort studies. Int J Food Sci Nutr. 2009;60(Suppl 6):1–13. doi: 10.1080/09637480802183380. [DOI] [PubMed] [Google Scholar]
- 17.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The adventist health study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 18.Jacobs DR, Jr, Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the norwegian county study. Eur J Clin Nutr. 2001;55:137–143. doi: 10.1038/sj.ejcn.1601133. [DOI] [PubMed] [Google Scholar]
- 19.Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM. Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr. 2006;83:124–131. doi: 10.1093/ajcn/83.1.124. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs DR, Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the iowa women’s health study. Am J Clin Nutr. 2007;85:1606–1614. doi: 10.1093/ajcn/85.6.1606. [DOI] [PubMed] [Google Scholar]
- 21.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: Prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. doi: 10.1186/s12916-015-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen NF, Frederiksen K, Christensen J, Skeie G, Lund E, Landberg R, Johansson I, Nilsson LM, Halkjaer J, Olsen A, Overvad K, Tjonneland A. Whole-grain products and whole-grain types are associated with lower all-cause and cause-specific mortality in the scandinavian helga cohort. Br J Nutr. 2015;114:608–623. doi: 10.1017/S0007114515001701. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: Two large prospective studies in us men and women. JAMA Intern Med. 2015;175:373–384. doi: 10.1001/jamainternmed.2014.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key TJ, Thorogood M, Appleby PN, Burr ML. Dietary habits and mortality in 11,000 vegetarians and health conscious people: Results of a 17 year follow up. BMJ. 1996;313:775–779. doi: 10.1136/bmj.313.7060.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Steffen LM, Jacobs DR, Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: The atherosclerosis risk in communities (aric) study. Am J Clin Nutr. 2003;78:383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- 27.Tognon G, Rothenberg E, Eiben G, Sundh V, Winkvist A, Lissner L. Does the mediterranean diet predict longevity in the elderly? A swedish perspective. Age. 2011;33:439–450. doi: 10.1007/s11357-010-9193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roswall N, Sandin S, Lof M, Skeie G, Olsen A, Adami HO, Weiderpass E. Adherence to the healthy nordic food index and total and cause-specific mortality among swedish women. Eur J Epidemiol. 2015;30:509–517. doi: 10.1007/s10654-015-0021-x. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA, S B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011 www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed August 12, 2013.
- 30.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Cook NR, Bergstr A, Hsieh C-C. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose-response data. Comput Stat Data Anal. 2009;53:4157–4167. [Google Scholar]
- 35.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 36.Reicks M, Jonnalagadda S, Albertson AM, Joshi N. Total dietary fiber intakes in the us population are related to whole grain consumption: Results from the national health and nutrition examination survey 2009 to 2010. Nutr Res. 2014;34:226–234. doi: 10.1016/j.nutres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98:872–884. doi: 10.3945/ajcn.113.064659. [DOI] [PubMed] [Google Scholar]
- 38.AlEssa HB, Bhupathiraju SN, Malik VS, Wedick NM, Campos H, Rosner B, Willett WC, Hu FB. Carbohydrate quality and quantity and risk of type 2 diabetes in us women. Am J Clin Nutr. 2015;102:1543–1553. doi: 10.3945/ajcn.115.116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Hruby A, Bernstein AM, Ley SH, Wang DD, Chiuve SE, Sampson L, Rexrode KM, Rimm EB, Willett WC, Hu FB. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J Am Coll Cardiol. 2015;66:1538–1548. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in swedish women: Prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foo SY, Heller ER, Wykrzykowska J, Sullivan CJ, Manning-Tobin JJ, Moore KJ, Gerszten RE, Rosenzweig A. Vascular effects of a low-carbohydrate high-protein diet. Proc Natl Acad Sci U S A. 2009;106:15418–15423. doi: 10.1073/pnas.0907995106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann Intern Med. 2010;153:289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Kamp JW, Poutanen K, Seal CJ, Richardson DP. The healthgrain definition of ‘whole grain’. Food Nutr Res. 2014;58:221004. doi: 10.3402/fnr.v58.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


