ABSTRACT
TH9 cells have been implicated in triggering antitumor immunity. We have identified that GITR co-stimulation inhibits iTreg cell generation but drives TH9 cell differentiation, thereby suppressing tumor growth via enhancing the function of DCs and CTLs in vivo. Our findings provide novel mechanisms by which GITR agonists exert antitumor activity.
KEYWORDS: Antitumor immunity, GITR, iTreg cell, IL-9, TH9 cell
Increasing evidence has indicated the advantages of CD4+ T helper (TH) cells in the treatment of cancer in experimental animals as well as in humans.1,2 The IL-9-producing CD4+ T (TH9) cell population is a newly identified TH subset and has been shown to play roles in mediating parasite expulsion, allergic inflammation and antitumor immunity.3 Notably, TH9 cells have been shown to exert the greatest antitumor activity among the TH subsets in melanoma rejection.4 Hence, the induction of TH9 cell immunity might provide an efficacious strategy for the treatment of tumors in humans.
Glucocorticoid-induced tumor necrosis factor receptor (TNFR)—related protein (GITR) is one of the molecules in the TNFR family that co-stimulates T cells. Treatment with GITR agonists showed strong antitumor effects in various tumor models.5 However, it has been unclear how GITR co-stimulation on T cells generates antitumor activity. In a recent study, we have found that GITR signaling profoundly enhances TH9 cell differentiation and that IL-9 production is required for the antitumor activity mediated by GITR agonists.6
First, we examined whether IL-4Ra signaling plays any role in tumor rejection mediated by the anti-GITR agonistic antibody DTA-1 in a CT26 colon cancer model because DTA-1 treatment increased the expression of IL-4 and IL-13 in tumor-bearing hosts. Although TH2 cell immunity has been suggested as pro-tumorigenic, it is also known to mediate antitumor immunity.7 We found that IL-4Ra signaling was essential for GITR agonist-induced tumor regression by showing that the tumor growth in IL-4Ra knockout (Il4ra−/−) mice was not affected by DTA-1. Notably, we observed that treatment with DTA-1 substantially enhanced IL-9 expression in WT tumor-bearing hosts, while it failed to do so in Il4ra−/− recipients. This result prompted us to analyze the effect of IL-9 on antitumor immunity induced by GITR stimulation. GITR ligation in vivo upregulated IL-9 expression in CD4+ T cells as early as 2 d after DTA-1 treatment. More importantly, the inhibition of tumor growth induced by DTA-1 was markedly reversed by a neutralizing antibody to IL-9. In addition, we employed a mouse model of B16 melanoma expressing ovalbumin (OVA) and compared the antitumor activity of adoptively transferred OVA-specific TH9 cells generated with or without GITR co-stimulation. In this experiment, we observed that GITR engagement endowed donor TH9 cells with superior antitumor efficacy in an IL-9-dependent manner. Based on this finding, we concluded that CD4+ T cells are the main cell type that responds to GITR co-stimulation to induce IL-9-dependent tumor regression.
Due to the lack of IL-9 receptor expression on CT26 and B16 tumor cells, we thought IL-9 acted on the intermediary cells rather than the tumors to exert antitumor activity. When we measured CD8+ cytotoxic T lymphocyte (CTL) responses, we found that tumor-specific cytolytic activity and cytokine and cytolytic marker expression (granzyme B, IFN-γ, TNF-α and CD107a) were all enhanced in CTLs treated with DTA-1 in an IL-9-dependent manner. Interestingly, although IL-9 expression was rapidly upregulated upon GITR ligation as early as day 2, we hardly detected tumor-specific CTL responses and the expression of related effector molecules in tumor-specific CTLs at the early time points. However, these were detected approximately a week after DTA-1 treatment in our experimental setting. In addition, our in vitro study revealed that IL-9 did not directly affect CD8+ T cell cytotoxicity. These observations led us to hypothesize that there must be other mediator(s) that responds to IL-9 and stimulates CTL responses in vivo. In a recent paper, Lu et al. have demonstrated that T cell-derived IL-9 chemoattracted dendritic cells (DCs) into the tumor site, thereby mediating TH9 antitumor immunity through the activation of tumor-specific CTLs.8 We observed that DCs accumulated in the tumor tissue of DTA-1-treated mice, expressed higher levels of CD80, CD86 and MHC class II, and cross-presented tumor antigen (Ag) more efficiently than those of control IgG-treated mice, which were also affected by IL-9. Collectively, we propose that GITR-triggered IL-9 production from CD4+ T cells promotes tumor-specific CTL responses by activating tumor-infiltrating DCs in vivo, which in turn eradicates tumors (Fig. 1).
Figure 1.
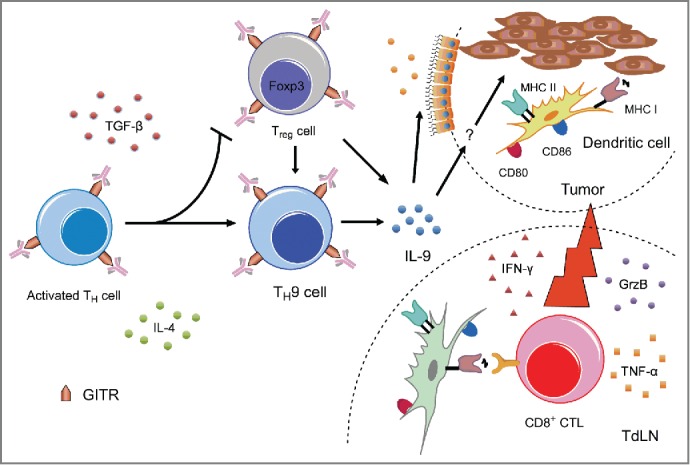
A schematic illustration of TH9 cell-mediated antitumor immunity induced by GITR co-stimulation. GITR triggering inhibits iTreg cell generation and promotes TH9 cell differentiation. IL-9 production triggers epithelial cells to chemoattract DCs into the tumor and enhances the cross-presentation and costimulatory capacity of the tumor-infiltrating DCs. These tumor-Ag-crosspresenting DCs then potentiate tumor-specific CD8+ CTL responses, thereby facilitating tumor regression.
Next, by using an in vitro culture system, we demonstrated that GITR co-stimulation preferentially enhanced mouse TH9 differentiation in a cell-intrinsic manner. Additionally, we observed an increase in human TH9 differentiation when human GITR was triggered by a stimulatory antibody, suggesting that GITR stimulation might be capable of inducing TH9 responses in humans. Notably, under induced Treg (iTreg) cell-polarizing conditions, GITR co-stimulation inhibited iTreg cell generation and diverted the differentiation of CD4+ T cells toward TH9 effector cells (Fig. 1). Hence, GITR co-stimulation might have two advantages: (i) eliminating potential immune suppressors by inhibiting iTreg cell generation in tumor sites and (ii) promoting antitumor TH9 effectors that repress tumor growth. A recent study by Xiao et al. supports this notion by showing that this phenomenon could take place in vivo in lymphopenic tumor-bearing hosts.9 Moreover, we demonstrated that reprogramming established Treg cells into IL-9-producing cells is possible when they are simulated with a GITR agonist in the presence of IL-4.
In summary, our study demonstrated a role for GITR co-stimulation in TH9 cell development and its functional cascade in the tumor microenvironment. However, it remains unanswered which cell type is the direct target of IL-9 to facilitate tumor-Ag cross-presentation by DCs. We speculate that cytolytic innate cells such as eosinophils,10 macrophages, NK cells and other cell types might be involved in this process. It will be important to investigate whether the antitumor activity of the GITR-agonistic antibody in human cancer patients is also IL-9-dependent. A combination of IL-9-inducing GITR agonists with immune checkpoint blockers likely further improves antitumor immunity in vivo.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the National R&D Program for Cancer Control, Ministry of Health and Welfare (No. 0720500, C.-Y. Kang) and the Mid-career Researcher Program (No. 2015R1A2A1A10055844, C.-Y. Kang) of the National Research Foundation of Korea (NRF).
References
- 1.Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol 2001; 22:269-76; PMID:11323286; http://dx.doi.org/ 10.1016/S1471-4906(01)01896-8 [DOI] [PubMed] [Google Scholar]
- 2.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol 2009; 21:200-8; PMID:19285848; http://dx.doi.org/ 10.1016/j.coi.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol 2014; 35:61-8; PMID:24215739; http://dx.doi.org/ 10.1016/j.it.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK et al.. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med 2012; 18:1248-53; PMID:22772464; http://dx.doi.org/ 10.1038/nm.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol 2012; 24:217-24; PMID:22245556; http://dx.doi.org/ 10.1016/j.coi.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim IK, Kim BS, Koh CH, Seok JW, Park JS, Shin KS, Bae EA, Lee GE, Jeon H, Cho J et al.. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med 2015; 21:1010-7; PMID:26280119; http://dx.doi.org/ 10.1038/nm.3922 [DOI] [PubMed] [Google Scholar]
- 7.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens 2007; 70:1-11; PMID:17559575; http://dx.doi.org/ 10.1111/j.1399-0039.2007.00869.x [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J et al.. Th9 cells promote antitumor immune responses in vivo. J Clin Invest 2012; 122:4160-71; PMID:23064366; http://dx.doi.org/ 10.1172/JCI65459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao X, Shi X, Fan Y, Zhang X, Wu M, Lan P, Minze L, Fu YX, Ghobrial RM, Liu W et al.. GITR subverts Foxp3(+) Tregs to boost Th9 immunity through regulation of histone acetylation. Nat Commun 2015; 6:8266; PMID:26365427; http://dx.doi.org/ 10.1038/ncomms9266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling G. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol 2015; 16:609-17; PMID:25915731; http://dx.doi.org/ 10.1038/ni.3159 [DOI] [PubMed] [Google Scholar]


