ABSTRACT
Metastatic melanoma is a fatal disease that responds poorly to classical treatments but can be targeted by T cell-based immunotherapy. Cancer vaccines have the potential to generate long-lasting cytotoxic CD8+ T cell responses able to eradicate established and disseminated tumors. Vaccination against antigens expressed by tumor cells with enhanced metastatic potential represents a highly attractive strategy to efficiently target deadly metastatic disease. Cripto-1 is frequently over-expressed in human carcinomas and melanomas, but is expressed only at low levels on normal differentiated tissues. Cripto-1 is particularly upregulated in cancer-initiating cells and is involved in cellular processes such as cell migration, invasion and epithelial–mesenchymal transition, which are hallmarks of aggressive cancer cells able to initiate metastatic disease. Here, we explored the potential of Cripto-1 vaccination to target metastatic melanoma in a preclinical model. Cripto-1 was overexpressed in highly metastatic B16F10 cells as compared to poorly metastatic B16F1 cells. Moreover, B16F10 cells grown in sphere conditions to enrich for cancer stem cells (CSC) progressively upregulated cripto1 expression. Vaccination of C57Bl/6 mice with a DNA vaccine encoding mouse Cripto-1 elicited a readily detectable/strong cytotoxic CD8+ T cell response specific for a H-2 Kb-restricted epitope identified based on its ability to bind H-2b molecules. Remarkably, Cripto-1 vaccination elicited a protective response against lung metastasis and subcutaneous challenges with highly metastatic B16F10 melanoma cells. Our data indicate that vaccination against Cripto-1 represents a novel strategy to be tested in the clinic.
KEYWORDS: Cripto-1, CTLs, DNA vaccination, melanoma, metastasis, T-cell epitope
Introduction
The largest hurdle in the treatment of melanoma patients is to ensure that the disease does not disseminate in the patient, leading to metastasis and eventually death. T cell-based immunotherapy has emerged at the forefront for treating metastatic disease1 and success has been achieved with the treatment of metastatic melanoma and other tumor types using immune-checkpoint blockade and autologous antitumor T cells.2 The former releases the spontaneous antitumor T cell immunity through removal of inhibitory signaling mediated by the PD1 or CTLA4 molecules.3 In the latter case, adoptive transfer of ex vivo expanded and activated tumor infiltrating T cells has successfully cured a proportion of metastatic melanoma patients.4 The efficacy of these strategies relies entirely on the presence of a pre-established antitumor immunity in the patients, which may frequently not be the case.5,6
This limitation can be overcome by educating the immune system through vaccination, which has a long successful history in the prevention of pathogen infections. However, less success has been achieved by vaccines targeting tumor-associated antigens (such as MAGE-A, GP100, NY-ESO-1, Tyrosinase and Her2) to treat cancer, showing limited therapeutic efficacy in clinical trials.7 Vaccines have been shown to elicit both humoral and cellular responses in a substantial proportion of the patients,8 though these responses are typically not potent enough to remove a large tumor burden. In consequence, removing bulky tumors through vaccination-induced antigen-specific T cell immunity remains a challenge that may be overcome by targeting a minor subpopulation of melanoma cells with enhanced potential to establish disseminated tumors, such as CSC.9
Cripto-1 is a glycoprotein that plays a critical role during embryogenesis and is overexpressed in more than 50% of human carcinomas, as well as melanomas, but is expressed only at low levels on normal differentiated tissues.10 Cripto-1 is involved in cellular processes such as cell migration, invasion and epithelial–mesenchymal transition, which are hallmarks of metastatic cancer cells.11,12 Cripto-1 is particularly upregulated in a small subpopulation of cancer-initiating cells, usually referred to as CSC, which has enhanced potential to metastasize and establish new tumor masses at distant sites in melanoma and other types of cancer.13 Specific targeting of cancer-initiating cells has shown to efficiently eradicate established and disseminated melanoma lesions14 and, therefore, it represents a highly attractive strategy to target deadly metastatic disease. Here, we describe that DNA vaccination against the tumor-associated antigen Cripto-1 that elicits specific CD8+ T cell immune responses, leading to decreased tumor burden and reduced metastatic spread in an aggressive syngeneic melanoma model.
Results
Expression of cripto-1 in melanoma cell lines
We first analyzed the presence of Cripto-1 in different murine tumor models. To this end, tumor cell lines from C57BL/6 (B6) background were screened for Cripto-1 expression by western blot. The Balb/c D2F2 cell line and mouse Cripto-1-transfectant D2F2mCR were used as negative and positive controls for Cripto-1 expression, respectively. Significant expression was detected in B16F10 and RetV melanoma cell lines, the latter derived from the metastatic melanoma ret transgenic mouse model.18 B16F1, a less metastatic B16 melanoma sub-line,19,20 and the MCA205 sarcoma cell line had weaker expression of Cripto-1 (Fig. 1A). Then, B16F10 cells were grown in a sphere culture system (Fig. S1) to expand the proportion of cancer-initiating cells with enhanced metastatic potential.21,22 Interestingly, Cripto-1 expression was progressively increased after each round of sphere culture (Fig. 1B). To evaluate Cripto-1 expression in healthy tissues we screened the healthy mouse tissue gene expression data set obtained by Su et al. (GSE1133:GLP1073) (Fig. 1C).23 As expected, within the data set we found that Cripto-1 is expressed during early embryogenesis, and expression is downregulated beyond day 8.5 into adulthood (Fig. S2). These results indicate that Cripto-1 has the potential to be used as a melanoma-associated antigen in a vaccination context.
Figure 1.
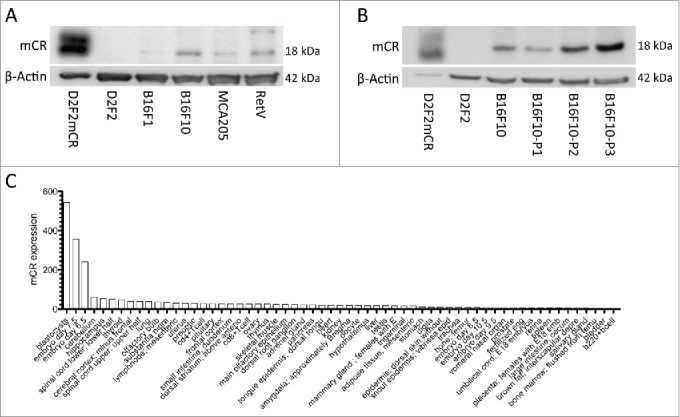
Mouse cancer cells with highly metastatic and aggressive phenotype express Cripto-1. (A) tumor cell lysates from lines on B6 background, RetV, MCA205, B16F10 and B16F1 were checked by protein gel blot for mouse Cripto-1 expression and compared relatively to β-actin. The D2F2 mouse Cripto-1 transfectant (D2F2mCR) was included as positive control. (B) B16F10 tumor cells were grown on low attachment plates for one to three passages (P1, P2 and P3) to enrich for tumor initiating cells and cell lysates were generated from each passage. (C) Expression levels of mouse cripto-1 were assessed from healthy mouse tissue from the GSE1133 data set and represented sorted with highest expression to the left.
Generation of specific CD8+ T cell responses in pmCR immunized mice
To evaluate the potential of Cripto-1 as a melanoma-associated antigen, we first defined the epitopes able to be recognized by CD8+ T cells. A library consisting of 33 long overlapping peptides (15-mers) covering the full-length mouse Cripto-1 amino acid sequence was screened for the ability to bind H-2 molecules using the RMA-S MHC class I stabilization assay. Three of the peptides tested were able to stabilize H-2 Kb at the cell surface of RMA-S cells (mCR1-15, mCR16-30 and mCR46-60), and as expected, the positive control H2 Kb epitopes, Trp2180-18824 and OVA257-264, strongly stabilized H-2 Kb (Fig. 2A). We then identified the presence of MHC class I-restricted epitopes within these 15-mer peptides using an in silico prediction analysis (http://www.cbs.dtu.dk/services/NetMHCpan/) (Fig. 2B). We next evaluated the immunogenicity of the predicted 9-mer epitopes by testing their ability to be processed, presented and recognized by CD8+ T cells in mice vaccinated with plasmid DNA encoding full-length mouse cripto-1 (pmCR). One of these predicted peptides, mCR16-25 (Fig. 3A left panel), elicited readily detectable CD8+ T cells able to produce IFNγ and TNF-α after ex vivo peptide stimulation as acquired by flow cytometry in an ex vivo intracellular cytokine staining (Fig. 3A right panel). None or very low responses specific for mCR1-9 and mCR46-55 were detected (Fig. S3A). We further demonstrated that vaccination-induced mCR16-25-specific CD8+ T cells mediate in vivo cytotoxic killing. Vaccinated mice received spleen cells pulsed with either mCR16-25 or OVA control peptide and stained with high (CFSEhi) or low (CFSElo) concentrations of CFSE, respectively. One day later, specific killing of mCR16-25-pulsed CFSEhi target cells relative to OVA-pulsed CFSElo internal control splenocytes was analyzed by flow cytometry. Killing of mCR16-25 pulsed cells was observed in Cripto-1 vaccinated mice (45% ± 1%) (Fig. 3B). Then, we tested the ability of vaccination-induced mouse Cripto-1-specific CD8+ T cells to recognize mouse melanoma B16F10 melanoma cells in vitro. CD8+ T cells isolated from spleen of mouse Cripto-1-vaccinated animals secreted significant amounts of IFNγ in response to stimulation with B16F10 melanoma cells and mCR16-25 peptide but not after control stimulation with OVA peptide or left unstimulated (Fig. 3C). These results indicate that cripto-1 is an immunogenic melanoma-associated antigen and that vaccination-induced cripto-1-specific cytotoxic CD8+ T cells have the potential to recognize and eliminate highly aggressive metastatic melanoma cells.
Figure 2.
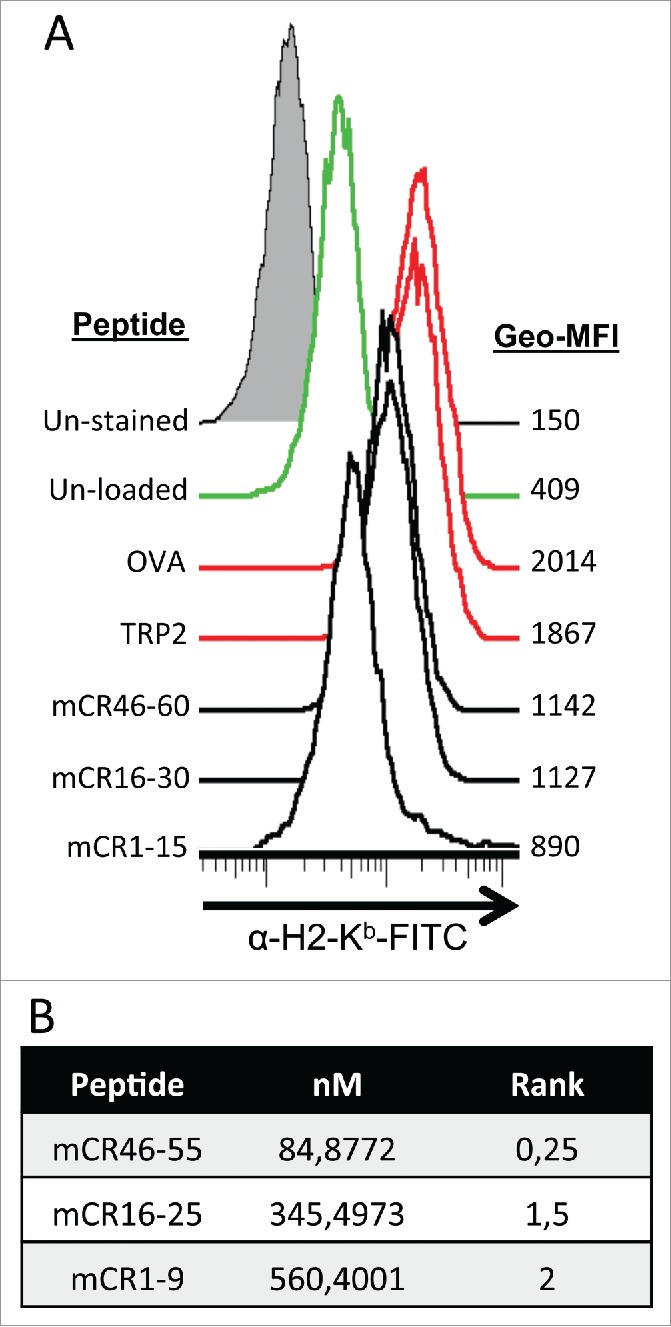
Mouse cripto-1 peptides bind to H2-Kb in vitro and in silico. (A) RMA-s cells were used to measure surface stabilization of H2-Kb using an overlapping 15-mer peptides library. H2-Kb was analyzed by flow cytometery after loading with 100 µg/mL of peptide for 2 h, with negative controls (un-stained, filled gray, and un-load, green), positive controls (OVA and TRP2, red) as well as the peptides mCR46-60, mCR16-30, mCR1-15 (solid black). (B) 9mers within Cripto-1 were identified using in silico prediction analysis with NetMHC.
Figure 3.
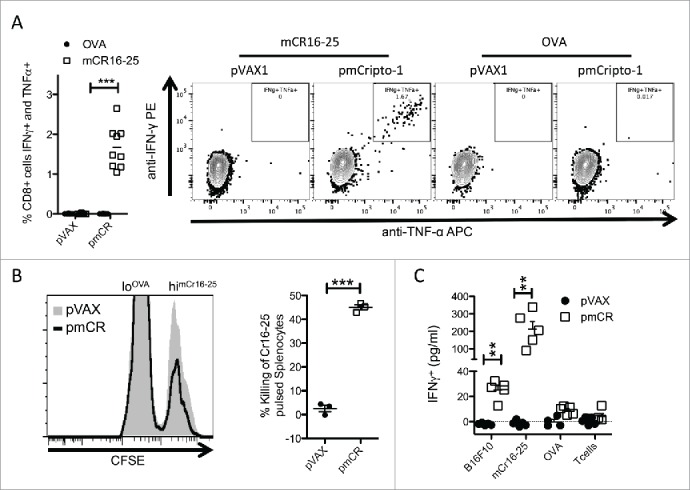
Vaccination with pmCR elicits an epitope specific functional CD8+ T cell response. (A) Peripheral blood lymphocytes from vaccinated mice (n = 9) were stimulated with mCR16-25 specifically for 8 h prior to ICS and significant increase in IFNγ/TNF-α producing cells were found; *** p < 0.001 using Mann–Whitney t-test and a representative dot-plot is shown. (B) Splenocytes from naïve mice were harvested and labeled with lo and hi CFSE prior to loading with OVA and mCR16-25, respectively, and transferred into pmCR or pVAX vaccinated mice. Specific killing was analyzed by FACS after 20 h. mCR16-25 specific killing of splenocytes in vivo was found to be significantly greater in pmCR vaccinated mice; *** p < 0.001 using Student's t-test. (C) CD8+ T cells were isolated using MACs bead positive selection from B6 (n = 5) mice vaccinated with either pmCR or pVAX and stimulated with CR expressing tumor cell line B16F10, mCR16-25 peptide, OVA peptide or with T cells alone; ** p < 0.01 using Mann–Whitney t-test.
Vaccination against mouse Cripto-1 reduces tumor burden and lung metastasis in the B16F10 melanoma model
We next evaluated if vaccination with mouse Cripto-1-encoding DNA vaccines could elicit protective immunity in mice challenged with B16F10 melanoma cells. Mice were vaccinated twice and 2 weeks later received a s.c. challenge with B16F10 melanoma cells and tumor growth was monitored. A significant delay in tumor growth was observed in pmCR-vaccinated mice, as compared to the control empty vector (pVAX) immunized mice (Fig. 4A). The delayed tumor growth led to significantly extended survival in pmCR-vaccinated mice (Fig. 4B). We then evaluated the ability of pmCR vaccination to protect against metastatic lung colonization of i.v. injected B16F10 cells. A significant decrease in the number of metastatic lung foci (Fig. 4C, D) was observed in mice vaccinated with mouse Cripto-1. These results indicate that vaccines encoding Cripto-1 induce immunity with the potential to target highly metastatic melanoma cells. Altogether, these results led us to conclude that pmCR vaccination efficiently induces mouse Cripto-1-specific CD8+ T cell responses able to target tumor burden and metastatic spreading of melanoma.
Figure 4.

Vaccination against mouse Cripto-1 increases survival and reduces metastatic lung burden in B6 mice challenged with mouse Cripto-1 expressing B16F10. (A) Tumor growth was compared in B6 mice (n = 10 per group) vaccinated prophylactically with either pmCR or pVAX with two doses of 20 µg plasmid DNA delivered i.d. with electroporation prior to s.c. challenge with 5×104 B16F10 cells with 2-way ANOVA test; **p < 0.01. (B) Survival of B6 mice vaccinated with pmCR or pVAX was compared after challenge with s.c. B16F10 with Mantel–Cox test; **p < 0.01. The results shown here are representative of two experiments. (C) Representative photos of B16F10 metastatic lung burden in vaccinated mice harvested 14 d post i.v. challenge of 2×105 tumor cells. D, reduction in lung metastasis was evaluated by enumerating foci of tumors in lungs of B16F10 i.v. challenged mice and compared with non-parametric t-test; *p < 0.05. The results shown here are representative of three experiments.
Discussion
Our current understanding of the complex nature of tumors has recently established that a small population of cancer cells within the heterogeneous tumor mass is particularly efficient in initiating the formation of disseminated cancerous lesions. These cells, referred to as cancer initiating cells or CSCs, have unlimited self-renewal potential and are resistant to classical therapies.25 The development of therapies selectively targeting this highly aggressive population to prevent metastatic disease therefore is of considerable importance. Cripto-1 has been shown to be expressed on many different tumors,11 including uveal and cutaneous melanomas.26,27 Interestingly, CR is particularly upregulated in CSC populations found in human melanoma13 and contributes to a more tumorigenic phenotype,28 resembling many of the pathways upregulated in metastatic malignant melanoma. Here, we identified that Cripto-1 is expressed in mouse metastatic melanoma models, including highly metastatic B16F10 cell line (Fig. 1A). Moreover, B16F10 cells grown in sphere culture conditions to enrich for CSC progressively upregulated cripto1 expression, indicating a direct correlation between CR expression and aggressiveness. We further demonstrate that CR can be used to target highly metastatic melanoma in the setting of a cancer vaccine. In mice that were vaccinated with DNA plasmids encoding mouse Cripto-1, we found that growth of the highly aggressive B16F10 cell line was significantly inhibited (Fig. 4A, B). Of particular interest, vaccination against mouse Cripto-1 led to a marked reduction in the metastatic spread of B16F10 tumor cells to lungs (Fig. 4C, D).
Vaccination has been a success story in protecting us from a plethora of pathogens, but unfortunately the success seen in this context has not been translated well to therapeutic vaccination against cancer. Cancer vaccines strongly rely on the generation of cytotoxic CD8+ T lymphocytes (CTLs) to mediate effective antitumor immune response.29 Perhaps it should not be expected that vaccine-induced antitumor CTL responses would be capable of removing bulky tumors, particularly when they consist of a heterogeneous cancer cell population that mediate a strong immunosuppression.30 Instead, vaccines should enable the immune system to reach distant sites of disease that evade the capability of traditional therapies. It is therefore essential that anticancer vaccines focus on the elimination of highly metastatic populations, such as CSC. It has been shown that adoptive transfer of CTL redirected against CSC via a CD20-specific chimeric antigen receptor efficiently eliminates melanoma tumors.31 In this work, elimination of less than 2% of the tumor cells was able to eradicate established melanoma lesions in mice. The same principle was successfully translated into humans, where rituximab (an anti-CD20 monoclonal antibody) produced the regression of chemotherapy-refractory metastatic melanoma.14
This study shows the potential of targeting the tumor-associated antigen cripto-1 with a plasmid DNA-based vaccination approach capable of inhibiting aggressive tumor growth and metastatic spread. The key to the potential of DNA vaccines lies in their ability to elicit CTL responses specific for the plasmid-encoded antigens. Herein, we are the first to describe a CTL response to mouse cripto-1 (Fig. 2). Cripto-1, as well as being a GPI anchored extracellular protein, interacts with Notch1 in the ER/golgi apparatus.32 This lends itself to MHC class I immunoproteasome processing. By identifying strong stabilizing partners for H-2 Kb with the help of the TAP-1 deficient RMA-S cell line33 we were able to identify potential CTL epitopes and confirm them in silico (Fig 2B). CD8+ T cells from vaccinated mice were able to generate IFNγ and TNF-α in response to one of the predicted epitopes (Fig. 3A). We further validated that mCR16-25 is an epitope that can be processed and presented to CD8+ T cells during vaccination. CD8+ T cells from mouse Cripto-1-vaccinated mice could indeed recognize mCR16-25 and display epitope specific killing in vivo.
In summary, our findings show that targeting cripto-1 using DNA vaccination elicits CD8+ T cell immunity capable of significantly reducing aggressive metastatic melanoma tumor growth. We identify, for the first time, CTL epitopes specific to mouse cripto-1, and have in ongoing work also several human CTL epitopes defined. The possibility of targeting cripto-1 expressed on the small CSC population is attractive, particularly in an adjuvant setting to avoid metastatic spread.
Material and methods
Mice and cell lines
C57BL/6 mice were bred, maintained at the Microbiology and Tumor Biology Center (Karolinska Institutet, Stockholm, Sweden) and were handled by strict adherence to the European guidelines and University Ethical Committee. Animal studies performed were reviewed and approved by the Regional Animal ethics committees; Stockholms Norra Djurförsoksetiska Nämnd Avdelning 2, Sweden with ethical permit number N426/11. RetV (generously donated by Prof. V. Umansky, DKFZ Heidelberg), MCA205, B16F10, B16F1, D2F2 and RMA-s were maintained with GlutaMAX-RPMI supplemented with 10% heat-inactivated FCS, 50 IU/mL penicillin, and 50 µg/mL streptomycin (Life Technologies). Cell lines were maintained at 37°C with 5% CO2 at 95% humidity and were split as was necessary using 0.05% Trypsine/EDTA (Life Technologies). To generate Cripto-1 overexpressing cell lines, mouse Cripto-1 lentiviral particles were acquired (Amsbio, Abindon, UK) and were used to transduce D2F2 with mouse Cripto-1. Followed by cell sorting for Cripto-1 positive cells using FACS.
Western blots
Cell lysates were prepared with 1M RIPA buffer (50nM Tris-HCl, pH 7.4, 1% Triton-X, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM NaF) with 1x protease inhibitor (Roche, Cat. No. 04693159001) at 1×106 cells/mL directly after collection from cell culture. Prior to loading on the gel protein concentrations were determined with BCA protein assay (Thermo Scientific, Rockford, IL) according to the manufacturers protocol. Twenty µg protein per sample was loaded on 10% NuPAGE Bis-Tris acrylamide gels (Invitrogen) and run at 200 V for 45 min with MOPS SDS running buffer (Invitrogen) followed by transfer onto PVDF membrane (Immobilon-P; Millipore, Bedford, MA) for 3 h at 40V. Blocking of the membrane was done using TBS-0.5% Tween 20 (Sigma-Aldrich), 2.5% milk powder or 2.5% BSA, followed by wash in TBS-0.5% Tween 20 and incubation with primary antibodies: rabbit α-human Cripto antibody, cross reactive to mouse 1:1,000 (Rockland, cat.no. 600-401-997) and mouse α-β-Actin antibody 1:25,000 (Sigma-Aldrich) overnight at 4°C. Secondary staining was done using α-rabbit IgG, HRP-linked (Cell Signaling Technology) and α-mouse IgG, HRP-linked (Cell Signaling Technology) for 1 h at room temperature. Development was done using Amersham ECl Prime Western Blotting Detection Reagent (GE Healthcare). Using a LAS-1000 CCD camera system (Fujifilm, Tokyo, Japan) luminescence was detected.
Vaccination and plasmids
Mice were treated on week 8 and week 10 either by intradermal injection of 40 µg of plasmid injected in PBS followed by electroporation protocol as described previously15 using IGEA plate electrodes. Cripto-1 encoding plasmids were generously provided by Bianco C et al. and cloned into the pVAX vector (Invitrogen, Carlsbad, CA, USAP).16 Plasmid based vaccines were produced by transformation of E. coli (TOP10, Invitrogen) with pVAX plasmid and grown in Luria-Bertani medium containing Kanamycin (50µg/mL). To generate endotoxin-free vaccine, plasmids were purified using GigaPrep Endofree Kit (Qiagen GMBH, Hilden, Germany).
Tumor models
B16F10 tumor cells were transplanted into the C57BL/6 mice to model melanoma. B16F10 was injected s.c (50,000) or i.v. (200,000) in 100µL PBS after being harvested when in vitro growth was logarithmic and at 80% confluence. Tumor size was monitored by palpation with calipers and mice were sacrificed when they became moribund or when the tumor reached a volume of 1,000 mm3. Mice that were injected i.v. were sacrificed at day 14 and lungs were excised and B16F10 foci were enumerated.
Immunological assays
Overlapping 15 amino acid Cripto-1 peptides were generated to cover the whole protein. RMA-s cells were washed with RPMI medium without FCS and kept at room temperature for 2 h. 2×105 cells were seeded into 96-well plates containing complete medium as well as peptides at a concentration of 100 µg/mL for 6 h. Cells were washed and stained with α-mouse-H2-Kb-FITC (BioLegend, 116505) to detect cell surface MHC class I molecules by flow cytometry. Mouse derived peptides; mCR16-25 (SAFEFGPVA), mCR46-55 (RSFQFVPSV), mCR1-9 (MGYFSSSVVL), Surv20-28 (ATFKNWPFL), TRP2180-188 (SVYDFFVWL), OVA257-264 (SIINFEKL) were acquired from China Peptides (ChinaPeptides Co. Ltd. Shanghai, China) at >95% purity. Peptides were used to stimulate mouse lymphocytes in peripheral blood harvested from immunized mice. Cells were seeded into 96-well plates and stimulated with 10µg/mL of MHC class I-restricted peptide. After 2 h, GolgiPlug (Becton, Dickinson and Company) was added for the last 6 h of stimulation. Cells were stained with α-mouse-CD8+, α-mouse-IFNγ, α-mouse-TNF-α using the Cytofix/Cytoperm Fixation/Permeabilization Solution (Becton, Dickinson and Company) according to manufacturer's instruction prior to acquisition of cells on LSRII FACS (Becton, Dickson and Company). Data were analyzed using FlowJo (Tree Star). CD8+ cells were isolated from immunized mice splenocytes using MACS Beads CD8+ positive selection (Miltenyi Biotec). 105 lymphocytes were seeded into 96-well plates and either co-cultured with 5×104 B16F10 cells or peptides (10µg/mL) or non-stimulated overnight and supernatants were harvested after 18 h. IFNγ was evaluated using Mouse IFNγ ELISA development kit (MabTech, Nacka Strand, Sweden) following manufactures instruction. In vivo cytotoxicity was evaluated as previously described.17 Splenocytes were harvested from naïve C57BL/6 mice and labeled with 0.2, lo, or 2 µM, hi, of CFSE in PBS for 5 min. Ten percent FCS containing PBS was used to halt labeling of the cells. Hi naïve target splenocytes were pulsed with target peptide (10µg/mL) and lo naïve splenocytes were pulsed with control peptide (10µg/mL). Labeled splenocytes were mixed and injected i.v. into naïve, antigen immunized (AI) or control immunized (CI) mice. After 20 h spleens were harvested from the mice and acquired by flow cytometery. In vivo cytotoxicity was calculated as follows: 100 − ((percentage of hi mCR16-25 peptide-pulsed targets in AI or CI mice/percentage of lo control targets in AI or CI mice)/(percentage of hi mCR16-25 peptide-pulsed targets in naïve mice/lo control targets in naïve mice) × 100). Samples were acquired on BD LSRII and analyzed on FlowJo.
Cancer cell spheroid culture
B16F10 were used in the generation of spheroid cultured tumor cells. B16F10 cells were seeded into Ultra-Low Cluster Plate (Costar) with 50,000 cells in 3 mL of melanoma spheroid culture medium. B16F10 melanoma spheroid culture medium consisted of MBM-4 (Lonza) containing the following: CaCl2, bovine pituitary extract (BPE), recombinant human Fibroblast Growth Factor (rhFGF), recombinant human Insulin, hydrocortisone, PMA, GA-1000 and 10% FBS (Lonza, CC-3249). Cells were monitored daily and split, using enzymatic and mechanical dissociation, every third day for melanoma or depending on sphere aggregate cluster size. Cells were collected prior to sphere culturing and at every consecutive passage, which were denoted as P1, P2 and P3 as their passage number indicated.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
R.K. is supported by grants from The Swedish Cancer Society, The Cancer Society of Stockholm, The Swedish Medical Research Council, ALF-Project grant from Stockholm City Council, from the “Knut and Alice Wallenberg Foundations” and from the Karolinska sponsored Center for Immune Modulatory Therapies for Autoimmunity and Cancer (IMTAC). A.L. has been supported by CONICYT Program PFB-16, CONICYT 791100038, FONDECYT 11110525, CORFO-Innova 12IDL2-13348, Millennium Institute on Immunology and Immunotherapy P09/016-F.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/ 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr Opin Immunol 2015; 33:23-35; PMID:25621841; http://dx.doi.org/ 10.1016/j.coi.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol 2012; 13:1129-32; PMID:23160205; http://dx.doi.org/ 10.1038/ni.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21:233-40; PMID:19304471; http://dx.doi.org/ 10.1016/j.coi.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS et al.. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med 2014; 371:2189-99; PMID:25409260; http://dx.doi.org/ 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124-8; PMID:25765070; http://dx.doi.org/ 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009; 27:83-117; PMID:19007331; http://dx.doi.org/ 10.1146/annurev.immunol.021908.132544 [DOI] [PubMed] [Google Scholar]
- 8.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol 2003; 3:630-41; PMID:12974478; http://dx.doi.org/ 10.1038/nri1150 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt P, Abken H. The beating heart of melanomas: a minor subset of cancer cells sustains tumor growth. Oncotarget 2011; 2:313-20; PMID:21487158; http://dx.doi.org/ 10.18632/oncotarget.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianco C, Salomon DS. Targeting the embryonic gene Cripto-1 in cancer and beyond. Expert Opin Ther Pat 2010; 20:1739-49; PMID:21073352; http://dx.doi.org/ 10.1517/13543776.2010.530659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: An oncofetal gene with many faces. Curr Top Dev Biol 2005; 67:85-133; PMID:15949532; http://dx.doi.org/ 10.1016/S0070-2153(05)67003-2 [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 13.Strizzi L, Margaryan NV, Gilgur A, Hardy KM, Normanno N, Salomon DS, Hendrix MJC. The significance of a Cripto-1 positive subpopulation of human melanoma cells exhibiting stem cell-like characteristics. Cell Cycle 2013; 12:1450-6; PMID:23574716; http://dx.doi.org/ 10.4161/cc.24601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlaak M, Schmidt P, Bangard C, Kurschat P, Mauch C, Abken H. Regression of metastatic melanoma in a patient by antibody targeting of cancer stem cells. Oncotarget 2012; 3:22-30; PMID:22289880; http://dx.doi.org/ 10.18632/oncotarget.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos A, Roos A-K, Quest AFG, Kiessling R. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother 2009; 59:81-92; PMID:19526360; http://dx.doi.org/ 10.1007/s00262-009-0725-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechselberger C, Wechselberger C, Ebert A, Ebert AD, Bianco C, Bianco C, Khan NI, Khan NI, Sun Y, Sun Y et al.. Cripto-1 Enhances Migration and Branching Morphogenesis of Mouse Mammary Epithelial Cells. Exp Cell Res 2001; 266:95-105; PMID:11339828; http://dx.doi.org/ 10.1006/excr.2001.5195 [DOI] [PubMed] [Google Scholar]
- 17.Ligtenberg MA, Rojas-Colonelli N, Kiessling R, Lladser A. NF-κB activation during intradermal DNA vaccination is essential for eliciting tumor protective antigen-specific CTL responses. Hum Vaccin Immunother 2014; 9:2189-95; PMID:23884215; http://dx.doi.org/9778055 10.4161/hv.25699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato M, Kato M, Takahashi M, Takahashi M, Akhand AA, Akhand AA, Liu W, Liu W, Dai Y, Dai Y et al.. Transgenic mouse model for skin malignant melanoma. Oncogene 1998; 17:1885-8; PMID:9778055; http://dx.doi.org/ 10.1038/sj.onc.1202077 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci 2002; 70:791-8; PMID:11833741; http://dx.doi.org/ 10.1016/S0024-3205(01)01454-0 [DOI] [PubMed] [Google Scholar]
- 20.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977; 197:893-5; PMID:887927; http://dx.doi.org/ 10.1126/science.887927 [DOI] [PubMed] [Google Scholar]
- 21.Ramgolam K, Lauriol J, Lalou C, Lauden L, Michel L, la Grange de P, Khatib A-M, Aoudjit F, Charron D, Alcaide-Loridan C et al.. Melanoma Spheroids Grown Under Neural Crest Cell Conditions Are Highly Plastic Migratory/Invasive Tumor Cells Endowed with Immunomodulator Function. PLoS One 2011; 6:e18784; PMID:21526207; http://dx.doi.org/ 10.1371/journal.pone.0018784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo J, Sun B, Zhao X, Gu Q, Dong X, Liu Z, Ma Y, Zhao N, Liu Y, Chi J et al.. The in-vitro spheroid culture induces a more highly differentiated but tumorigenic population from melanoma cell lines. Melanoma Res 2013; 23(4):254-63 [DOI] [PubMed] [Google Scholar]
- 23.Su AI, Su AI, Wiltshire T, Wiltshire T, Batalov S, Batalov S, Lapp H, Lapp H, Ching KA, Ching KA et al.. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 2004; 101:6062-7; PMID:15075390; http://dx.doi.org/ 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, El-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med 1997; 185:453-9; PMID:9053445; http://dx.doi.org/ 10.1084/jem.185.3.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wicha MS. Cancer Stem Cells: An Old Idea–A Paradigm Shift. Cancer Res 2006; 66:1883-90. Available from; PMID:16488983; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3153 [DOI] [PubMed] [Google Scholar]
- 26.De Luca A, Lamura L, Strizzi L, Roma C, D'Antonio A, Margaryan N, Pirozzi G, Hsu M-Y, Botti G, Mari E et al.. Expression and functional role of CRIPTO-1 in cutaneous melanoma. Br J Cancer 2011; 105:1030-8; PMID:21863025; http://dx.doi.org/ 10.1038/bjc.2011.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallikarjuna K, Vaijayanthi P, Krishnakumar S. Cripto-1 expression in uveal melanoma: An immunohistochemical study. Exp Eye Res 2007; 84:1060-6; PMID:17412323; http://dx.doi.org/ 10.1016/j.exer.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 28.Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, Salomon DS. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol 2010; 177:532-40; PMID:20616345; http://dx.doi.org/ 10.2353/ajpath.2010.100102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagès F, Galon J, Galon J, Dieu-Nosjean M-C, Tartour E, Sautès-Fridman C, Fridman W-H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2009; 29:1093-102; PMID:23884215; http://dx.doi.org/19200836 10.1038/onc.2009.416 [DOI] [PubMed] [Google Scholar]
- 30.Gross S, Geldmacher A, Sharav T, Losch F, Walden P. Immunosuppressive mechanisms in cancer: Consequences for the development of therapeutic vaccines. Vaccine 2009; 27:3398-400; PMID:19200836; http://dx.doi.org/ 10.1016/j.vaccine.2009.01.070 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA 2011; 108:2474-9; PMID:21282657; http://dx.doi.org/ 10.1073/pnas.1009069108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Nagaoka T, Lee JM, Bianco C, Gonzales M, Castro NP, Rangel MC, Sakamoto K, Sun Y, Callahan R et al.. Enhancement of Notch receptor maturation and signaling sensitivity by Cripto-1. J Cell Biol 2009; 187:343-53; PMID:19948478; http://dx.doi.org/ 10.1083/jcb.200905105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ljunggren HG, Ljunggren H-G, Stam NJ, Stam NJ, Ohlén C, Öhlén C, Neefjes JJ, Neefjes JJ, Höglund P, Höglund P et al.. Empty MHC class I molecules come out in the cold. Nature 1990; 346:476-80; PMID:2198471; http://dx.doi.org/ 10.1038/346476a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


