ABSTRACT
The availability of clinical-grade cytokines and artificial antigen-presenting cells has accelerated interest in using natural killer (NK) cells as adoptive cellular therapy (ACT) for cancer. One of the technological shortcomings of translating therapies from animal models to clinical application is the inability to effectively and non-invasively track these cells after infusion in patients. We have optimized the nonradioactive isotope fluorine-19 (19F) as a means to label and track NK cells in preclinical models using magnetic resonance imaging (MRI). Human NK cells were expanded with interleukin (IL)-2 and labeled in vitro with increasing concentrations of 19F. Doses as low as 2 mg/mL 19F were detected by MRI. NK cell viability was only decreased at 8 mg/mL 19F. No effects on NK cell cytotoxicity against K562 leukemia cells were observed with 2, 4 or 8 mg/mL 19F. Higher doses of 19F, 4 mg/mL and 8 mg/mL, led to an improved 19F signal by MRI with 3 × 1011 19F atoms per NK cell. The 4 mg/mL 19F labeling had no effect on NK cell function via secretion of granzyme B or interferon gamma (IFNγ), compared to NK cells exposed to vehicle alone. 19F-labeled NK cells were detectable immediately by MRI after intratumoral injection in NSG mice and up to day 8. When 19F-labeled NK cells were injected subcutaneously, we observed a loss of signal through time at the site of injection suggesting NK cell migration to distant organs. The 19F perfluorocarbon is a safe and effective reagent for monitoring the persistence and trafficking of NK cell infusions in vivo, and may have potential for developing novel imaging techniques to monitor ACT for cancer.
KEYWORDS: Fluorine 19 (19F), in vivo imaging and adoptive cell therapy (ACT), magnetic resonance imaging (MRI), natural killer cells (NKs)
Introduction
The infusion of NK cells as treatment for relapsed solid tumors has been utilized by many centers based on emerging preclinical evidence of antitumor activity. Melanoma, renal cell carcinoma, recurrent breast and ovarian cancer have been treated with NK cells or NK cell lines in adults,1-5 while neuroblastoma, medulloblastoma, osteosarcoma, rhabdomyosarcoma and Ewing sarcoma are being tested in children.6 As NK cells are being more widely used for cancer treatment, understanding where adoptively transferred NK cells traffic after infusion is becoming more critical. It is also not known how long adoptively transferred NK cells persist in vivo, making timing of repeated NK cell infusions difficult to predict. According to the Food and Drug Administration (FDA) Cellular, Tissue and Gene Therapy Advisory Committee, there is an urgent need to track cells in vivo to determine migration patterns and longevity.7 A thorough summary of imaging methods that have been employed to track NK cells has been reviewed previously.8,9 Usage of superparamagnetic iron oxide (SPIO) particles have been previously used to label NK cell lines10-13 for detection in preclinical models by MRI, but have not gained wider clinical use. One limitation is that the hypointense signal produced can make it difficult to discriminate the SPIO-labeled cells from other hypointense signals, like blood, or from signal loss due to susceptibility and field inhomogeneities. 19F is a nonradioactive, 100% naturally abundant isotope of fluorine that can be formulated into perfluorocarbon nanoemulsions and simply incubated with cells in culture.14 Radiofrequency MRI coils can then be tuned to detect and image these fluorine atoms enabling tracking of cellular fate post-infusion.15
In preclinical models, optical imaging using fluorescent and bioluminescent models have provided valuable insight into NK cell trafficking patterns after adoptive transfer,16-18 but these techniques assume murine NK cells traffic similarly to human NK cells, do not give high resolution of the live gross anatomical structures where the NK cells traffic to, and most importantly, are not available in the clinic. Radiolabeling of NK cells with clinically available isotopes, like 18F, 11C or 111In, offers high sensitivity but also lacks high resolution of anatomical structures, exposes the patient to ionizing radiation, and is limited by radioactive decay of the isotope (typically hours to days) preventing detection of long-term NK cell persistence. Plus, the FDA's Center for Devices and Radiological Health has launched an initiative to reduce unnecessary radiation exposure from medical imaging.19
In the past decade, very promising MRI techniques have been developed to monitor and quantify immune cells in vivo using the non-radioactive nucleus 19F as an MRI contrast agent.14,20,21 Because there are trace amounts of fluorine in the body (mainly in the bone matrix and teeth) that exhibit a very short spin–spin relaxation time,15 there is minimal background “noise” and infusions of 19F-labeled cells could be easily identified at a high contrast to noise ratio.22 With a high gyromagnetic ratio similar to that of 1H, 19F has favorable physical properties that improve inherent receptivity and sensitivity, requiring only millimolar quantities per voxel for detection.22 Furthermore, 19F is non-radioactive, non-invasive; depth independent since used by MRI and has no known enzyme for its degradation in vivo (cleared by exhalation or by endoreticulum). Moreover, perfluorocarbon liquids are chemically and biologically inert, and no toxicity has ever been reported using 19F-labeled cells,23 even at large doses. To date, investigators have successfully used this approach to track murine macrophages,24 murine14 and human dendritic cells,25,26 murine T cells,27,28 hematopoietic,29 both murine30 and human neural stem cells31 as wells as murine32 and human mesenchymal stem cells33 in preclinical models. Interestingly, 19F has been FDA approved in a phase I clinical trial to monitor a DC vaccine in colorectal patients. Initial results from three patients have recently been published showing the ability to detect human DCs by 19F-MRI without apparent toxicity.34 However, NK cells have been challenging to label using this approach35 and data is lacking on NK cell trafficking even though they are being infused in clinical trials.
In this study, we will show the impact of labeling human NK cells with a commercially available 19F perfluoropolyether (PFPE) nanoemulsion on viability, cytotoxicity and cell surface marker expression on in vitro human NK cell cultures. We will also show that MRI can detect 19F-labeled human NK cells after subcutaneous and intratumoral injection. Our preliminary results indicate that19F MRI is a promising approach for tracking NK cells in vivo noninvasively without toxicity or ionizing radiation, and has sufficient resolution to detect NK cells within a solid tumor.
Results
Labeling of human NK cells with 19F
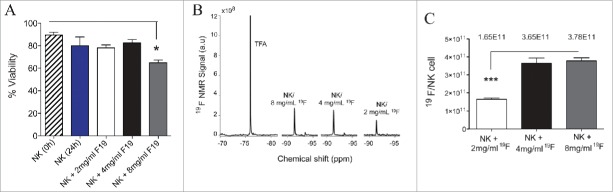
We first determined the impact of labeling IL-2 expanded NK cells ex vivo with 19F by analyzing NK cell viability with increasing concentrations of the tracer. We found no impact on cell viability after 24 h incubation with 2 or 4 mg/mL 19F, compared to unlabeled NK cells (Fig. 1A). Viability of NK cells was only decreased at 8 mg/mL 19F (Fig. 1A). Using NMR, we detected up to 3.78 × 1011 atoms of 19F per NK cell on average, which plateaued between labeling with 4 to 8 mg/mL 19F (Fig. 1B–C). Based on the similar efficiency of internalization of 19F using 4 mg/mL or 8 mg/mL, and since NK cell viability is decreased with 8 mg/mL 19F, we decided to use 4mg/mL 19F for all subsequent experiments.
Figure 1.
High uptake of 19F nanoemulsions by human NK cells do not affect their viability. Human NK cells were expanded for 12 d from PBMCs of a healthy donor and sorted on day 12 of expansion by magnetic bead isolation. Sorted NK cells (CD3− CD56+) were co-incubated with or without 19F (Cell Sense) for 24 h then analyzed for (A) Percent viability using Trypan blue and determined before (0 hour) and after (24 h) co-culture of NK cells with 2 mg/mL, 4 mg/mL or 8 mg/mL 19F. Data presented for 11 compiled experiments. (B) Representative NMR spectra of TFA (control) or NK cells labeled with 2 mg/mL, 4 mg/mL or 8 mg/mL PFPE show 19F signal increasing with the concentration of PFPE in cell media. (C) Concentration of 19F/NK cell determined by NMR for NK cells exposed to different concentration of PFPE for 24 h. Three replicates were set up per group. Bar graph values represent the mean ± SEM tested by a one-way ANOVA. Data representative of at least three experiments with reproducible results.
NK cell receptor expression upon 19F labeling
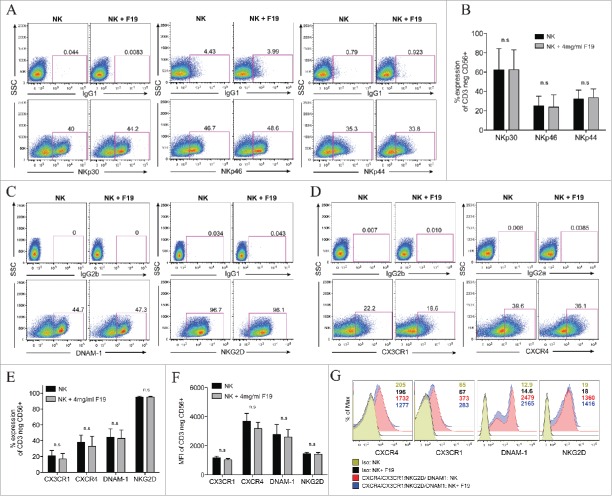
We then evaluated the effects of 19F labeling on CD3−CD56+ NK cell surface receptors expression, particularly those that are involved in NK cell antitumor activity. There were no differences in natural cytotoxicity receptor (NCR) expression in NKp30, NKp46 and NKp44 expression between unlabeled and 19F-labeled NK cells for 24 h (Fig. 2A–B). Additionally, no changes were noted in the activating receptors DNAM-1 or NKG2D and in CX3CR1 and CXCR4 chemokine receptor expression both in percentages (Fig. 2C–E) and in mean fluorescent intensity (MFI) (Fig. 2F–G). NKG2D ligands include MHC class-I-related protein and stress molecules that are upregulated upon DNA damage and heat shock responses in malignant transformations. On the other hand, DNAM-1 receptor, a member of the immunoglobulin superfamily, binds to poliovirus receptor CD155 and CD112 (Nectin-2), both ligands are expressed on some tumor cells. Furthermore, DNAM-1 has been shown to be important in the clearance of tumor cells that lack the expression of ligands to NK-activating receptors.36 Therefore, 19F labeling of NK cells does not appear to affect surface expression of NCRs, receptors to stress ligands, and chemokine receptors suggesting that NK cell migration ability and immunosurveillance against tumors should remain preserved.
Figure 2.
19F labeling of human NK cells does not alter their surface expression of activating natural cytotoxic receptors and chemokine receptors. (A–G) Human NK cells unlabeled or labeled with 4 mg/mL 19F (Cell Sense) for 24 h analyzed by flow cytometry for: (A–B) The percent expression in the NK cytotoxic receptors (NCRs) NKp30, NKp46 and NKp44 vs. IgG1 control stains. (A) Illustrates representative dot plots for each NCR and their isotype controls (IgG1) for unlabeled NK cells or NK cells labeled with 19F. (B) Shows percentage of NKp30, NKp46 and NKp44 on the NK cells (CD3 negative CD56+) in five healthy donors. (C–E) The percent expression or (F–G) mean fluorescent intensity (MFI) in the activating receptors DNAM-1 (DNAX Accessory Molecule-1) and NKG2D and in chemokine receptors CX3CR1 and CXCR4 compared to isotype controls in 19F-labeled or unlabeled NK cells. (D) Percent expression or (G) MFI in the chemokine receptors CX3CR1, CXCR4 or isotype controls after gating on the of CD3neg CD56+ NK cells. MFI numbers are indicated within the histograms with color-coded MFIs indicated in the legend and corresponding to the histograms. (E) Shows the percent and (F) MFI in CX3CR1, CXCR4, DNAM-1 and NKG2D on NK cells from five healthy donors labeled or not with 19F. All gates and histograms are pre-gated on CD3− CD56+ NK cells. Bar graph values represent the mean ± SEM tested by two-way ANOVA. Data representative of at least four independent experiments with reproducible results. n.s.= not significant.
In vitro cytotoxicity of 19F-labeled NK cells
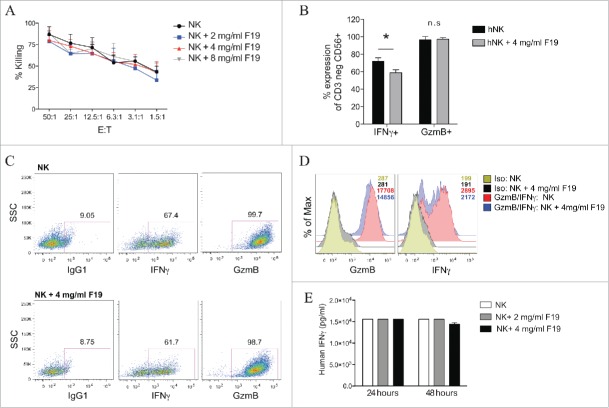
While our flow cytometry data demonstrates that 19F does not negatively impact the expression of NK cell cytotoxicity receptors, we next tested the impact of 19F labeling on NK cell tumor cytotoxicity against K562 cells. There were no differences in NK cell cytotoxicity between unlabeled and NK cells labeled with 2 mg/mL, 4 mg/mL or 8 mg/mL 19F PFPE (Fig. 3A). We next evaluated NK cell function after in vitro stimulation by measuring intracellular production of IFNγ and granzyme B, which are both essential mediators in tumor lysis. By comparing unlabeled versus 19F-labeled NK cells, we found no difference in granzyme B production in percentage and MFI (Fig. 3B–D), whereas a mild increase in the percent and MFI of NK cells secreting IFNγ was observed in 19F-labeled NK cells (Fig. 3B–D). To verify this observation, we quantified IFNγ secretion by ELISA and found no differences after 24 or 48 h of 19F labeling at both 2 mg/mL and 4 mg/mL concentrations (Fig. 3E). Preservation of NK cell production of granzyme B and IFNγ after 19F uptake correlates with their in vitro ability to mediate antitumor responses.
Figure 3.
Human NK cells labeled with 19F maintain their cytotoxic function in vitro. (A) Sorted human NK cells were cultured with 19F for 24 h. Extra 19F not taken up by NK cells was washed out, then NK cells (E: effectors) were cultured with 51Cr-labeled K562 tumor (T: target) for 4 h at 37°C at different E:T ratios to determine their percent lysis of tumor cells, n = 5. Values represent the mean ± SEM of one of four independent experiments tested by two-way ANOVA, with no significant difference seen. (B–D) NK cells labeled with 4 mg/mL 19F or unlabeled were stained for flow cytometry intracellularly for IFNγ and granzyme B or isotype control, n = 5 donors. (B) Compiled percentage or (C) representative flow plots or (D) MFI in isotype control, IFNγ and Granzyme B by unlabeled and 19F-labeled NK cells is illustrated. (E) Human IFNγ production was determined by ELISA in NK cells unlabeled or labeled with 19F at 2 mg/mL or 4 mg/mL 19F for 24 h or 48 h, n = 3, no difference was observed. Dot plots are representative of one of at least five experiments with reproducible results. Bar graph values represent the mean ± SEM of one of five independent experiments tested by two-way ANOVA. p < 0.05, n.s = not significant.
In vivo detection of 19F-labeled NK cells by MRI
Next, we wanted to examine if 19F-labeled NK cells could be detected by in vivo MRI using a volumetric 1H/19F quadrature coil. We performed intratumoral injection of 19F-labeled NK cells into two different xenograft tumor models to determine if the NK cells could be detected within a bulky tumor mass. NK cells were detected at both 1 hour and 48 h after injection into immunodeficient mice bearing human neuroblastoma (Fig. 4A) as well as up to 8 d after injection into human lymphoma (Fig. 4C). On day 0, quantification of 19F signal from the NK cells using MRI showed detection of 95% of the NK cells injected intratumor for the neuroblastoma and 84% NK cells detected after 48 h (Fig. 4B). However, only 74% of the NK cells were detected on day 0 for the lymphoma-bearing mouse and the number of NK cells detected by day 8 was 68% (Fig. 4D). Overall, very little to no migration of the NK cells out of the tumor was observed and a high number of NK cells could be detected within a bulky tumor mass over the duration of the experiment (Fig. 4).
Figure 4.
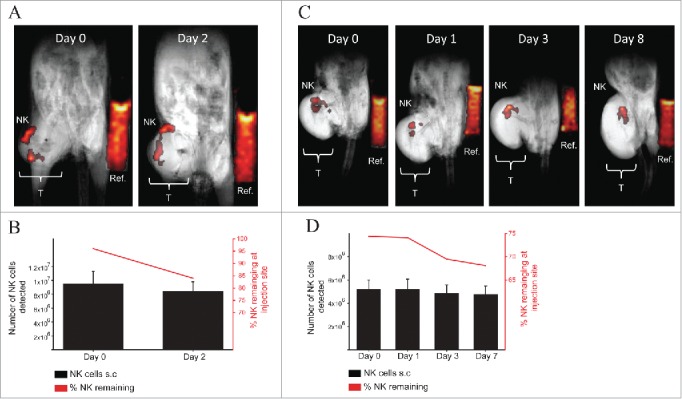
MRI in vivo detection of 19F-labeled human NK cells in NSG mice bearing xenograft human tumors. (A–B) 10 × 106 19F-labeled (4 mg/mL PFPE for 24 h) human NK cells were injected intratumor into one NSG mouse bearing a human neuroblastoma (CHLA-20) on the right flank (T: Tumor). 19F intensity from NK cells or from the reference (Ref: Reference vial of 19F) vial is displayed on a “hot-iron” scale. Mouse was imaged for 1H and 19F by MRI using a volumetric coil and anesthetized using ketamine and xylazine. (A) Composite 19F/1H images at 0 or 2 d post NK cell injection are shown with 32 min and 42 min of scan time for each day respectively. (B) The number (black bars corresponds to left y-axis) and percentage (red line graph corresponds to the right y-axis) of NK detected in the tumor is denoted and was determined based on the efficiency of 19F uptake by NK cells from NMR analysis. (C–D) 7 × 106 19F-labeled (4 mg/mL PFPE for 24 h) NK cells were injected intratumor into one NSG mouse bearing a human mantle cell lymphoma (Z138) on the right flank. (C) Imaging for 1H and 19F was established as described in (A) with scan time of 42 min for each time point. (D) Number (black bars corresponds to left y-axis) and percentage (red line graph corresponds to the right y-axis) of NK cells detected at the tumor site is denoted for each imaging time point. Values represent the mean ± SEM of one single experiment. The uncertainty in the 19F reference mean signal () was estimated as the standard deviation of the ROI drawn on the reference (see methods for quantification of 19F signal).
Migration of 19F-labeled NK cells in vivo
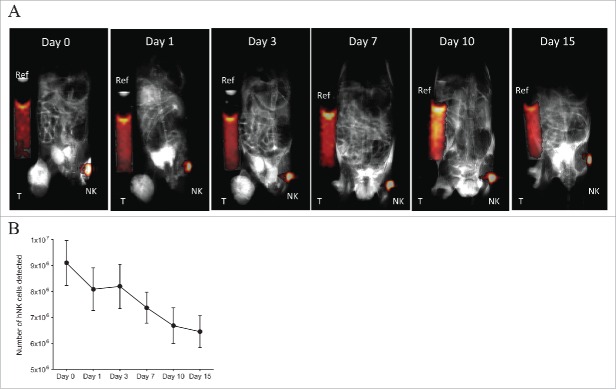
Finally, we sought to determine the ability of 19F-labeled NK cells to migrate to a distant tumor. To address this, 19F-labeled NK cells were injected subcutaneously into the contralateral flank of mice bearing human melanoma (M21). In order to aid in the migration of NK cells to the tumor site, the hu14.18-IL-2 immunocytokine was injected intratumorally (50 ug/50 uL) on days 0–2 and 7–9 post NK cell infusion in addition to intraperitoneal injection of recombinant human IL-2 (1 × 106 IU/0.2 mL) on days 0 and 7 post NK cell infusion. M21 tumors express the disialoganglioside GD2, while the hu14.18-IL18 immunocytokine consists of a GD2-specific monoclonal antibody (mAB) fused to human IL-2 able to recognize and specifically bind to GD2+ tumors. The immunocytokine has shown antitumor effects in vivo in mouse models37-40 in a phase I/II clinical trial in pediatric patients with relapsed/refractory neuroblastoma41,42 and in patients with metastatic melanoma.43 Therefore, we expected that GD2+ tumor and the treatment with hu.14.18-IL2 would recruit NK cells to mediate ADCC at the tumor. At day 0, 10 × 106 19F-NK cells were implanted subcutaneously (flank opposite to the tumor) with on average 9.1 × 106 NK cells detected by MRI on day 0 (Fig. 5A–B). Over time we observed a progressive decease in 19F signal from the NK cells with about 65% of the NK cells or 19F signal remaining at the site of injection by day 15 (Figs. 5A–B). The decrease in 19F signal is indicative of the migration of the NK cells out of the inoculation site to distant organs. However, the 19F signal from the NK cells was not detected at the tumor site or at other organs by MRI (Fig. 5A).
Figure 5.
19F-labeled NK cell migration in NSG mice bearing human GD2+ melanoma and hu14.18-IL-2 treatment. 10 × 106 19F-labeled (4 mg/mL PFPE for 24 h) human NK cells were injected on the left flank subcutaneously into NSG mice (n = 2) bearing human melanoma (M21) tumor on the right flank (T: Tumor). On days 0–2 and 7–9, 50 µg/50 µL hu.14.18-IL-2 immunocytokine was injected i.t. On days 0 and 7, 1 × 106 IU/0.2 mL rh-IL-2 was injected i.p. (A) Mice were imaged for 1H and 19F by MRI for 42 min at different time points. Here, a T2-weighted 1H image was acquired for enhanced tumor visualization. Composite 19F/1H images are depicted for 1 mouse. (B) Illustrates the number of NK cells remaining at the site of injection at each time point for both mice. Day 0 refers to the day of implantation of 19F-labeled human NK cells. Values represent the mean ± SEM of one single experiment, n = 2.
Discussion
Infusions of NK cells for cancer immunotherapy represent an evolving approach both for solid and hematologic cancers and are already occurring at centers around the world.44 Above all, few toxicities have been reported following adoptive transfer of NK cells. However, very little is known about the migration, persistence and biodistribution of NK cells post infusion. In this paper, we show for the first time that ex vivo expanded human NK cells can be efficiently labeled with 19F, enabling their in vivo detection and quantitation by MRI in immunodeficient mice and within the dense architecture of a solid tumor. Importantly, 19F labeling of human NK cells does not alter their viability, their expression of NCRs, cytokines and granzymes necessary for their cytotoxic functions.
Although NK cells have shown antitumor effects in preclinical models, their full therapeutic potential in the clinic has yet to be optimized. Typically, efficacy of cellular therapy is evaluated on the basis of survival rate and tumor regression assessed for weeks and months post treatment. However, lack of response cannot be positively attributed to the inefficacy of NK cells without direct measurement of the location of NK cells in respect to the tumor site and also their persistence post adoptive transfer. The lack of response to NK cell immunotherapy could simply be due to their inability to migrate to the tumor, to a low concentration of transferred NK cells or to a need of multiple adoptive transfers, all of which are dependent on the tumor type (solid vs. blood-borne), the stage of cancer and resistance of the tumor. Presently, we rely on non-specific and insensitive blood draws and bone marrow biopsies to locate infused NK cells by flow cytometry and immunohistochemistry, even though the NK cells may be in secondary lymphoid tissues or in the tumor. Moreover, biopsy of tumors is a difficult and invasive procedure that may lead to the potential of sampling error and risk to the patient. Thus, the field of cellular immunotherapy is in need of a means by which to non-invasively track infused NK cells in both normal organs and tumors.
Usage of 19F is an appealing labeling approach for in vivo imaging of NK cells (or other cell therapies) because (1) it is the only naturally occurring isotope of fluorine and does not decay, (2) does not require phagocytosis to enter the cell, (3) is a component of the chemical structure of over a dozen FDA-approved drugs and thus has a long safety record in humans,15 and (4) perfluorocarbons have already been successfully used in clinical trials for decades in artificial blood substitutes.45 Moreover, using 19F-MRI is an attractive technique as it provides positive contrast, and the high-resolution anatomical 1H clinical images can be registered and superimposed with the highly sensitive 19F hotspot images to create a precise mapping of where the 19F-labeled NK cells exist in relation to the patient's tumor. The ability for repeated imaging over time with 19F-MRI will also provide a sense of trafficking patterns as well as NK cell persistence after infusion. Longer lived PET isotopes such as 89Zr have recently been used in combination with more specific cell labeling strategies such as so-called “click chemistry” to enhance signal half-life and specificity with promising results.46-48 However, prolonged exposure to ionizing radiation remains problematic for cell-trafficking approaches in the clinic.
Perfluorcarbon 19F nanoemulsions have gained interest in the past decade as an imaging modality to ex vivo label immune cells and monitor their biodistribution in vivo using MRI.20 Ahrens et al. were first to initially demonstrate the ability to efficiently label murine DCs ex vivo with perfluoropolyether (PFPE) agents. In their study, they showed that PFPE does not affect DC function in vitro and were able to track DC migration in mice from the foot pad to the popliteal lymph node 6 h post injection.14 Intravenous injection of PFPE-labeled murine DCs were effectively visualized using an 11.7 Tesla vertical bore MRI and accumulated mainly in the liver and spleen.14 The same group has subsequently employed 19F-MRI to track and quantify: autoreactive mouse T cells,27 antigen specific T cells,28 monocyte and macrophages in two rat models of renal and cardiac allograft rejection,24 and PFPE-labeled human primary DCs in immunodeficient mice.25,26 Other investigators have also reported on the ability to ex vivo label other potential cell therapies with 19F, including murine30 and human neural stem cells,31 human CD34+ hematopoietic stem cells (HSCs) and mouse bone marrow derived HSCs as well as murine32 and human33 mesenchymal stem cells. All of the aforementioned groups have demonstrated that 19F labeling of immune and non-immune cells does not alter their cell viability, phenotype, proliferation or function in vitro compared to unlabeled cells.
Furthermore, Ahrens et al. were first to prove the feasibility of labeling human immune cells and monitor their migration in mice, although duration of imaging was reported only for 18 h post injection of the human DCs.25 This has led to usage of 19F in a phase I clinical trial to monitor an autologous DC vaccine in colorectal patients. Initial results from three patients have recently been published showing the ability to detect human DCs by 19F-MRI without apparent toxicity.34 About 10 million DCs were injected intradermally into quadriceps and patients were imaged for about 9.5 min. DCs were detected 4 h post inoculation with a 50% decrease in 19F signal at the site of injection by 24 h post inoculation in two patients.34 These data demonstrate the feasibility to visualize human immune cells using 19F-MRI, however long-term studies in patients are needed to confirm persistence of 19F signal from the adoptively transferred cells, toxicity and potential false positive signal from death of transferred cells leading to transfer of 19F signal to other host cells. To our knowledge no report has applied 19F-MRI to track human NK cells in vivo.
Several other imaging techniques have been used in pre-clinical or clinical scenarios to monitor NK cells in recipients and monitor the tumor response. These techniques include positron emission tomography (PET), single photon emission computed tomography (SPECT), and optical imaging (OI) (bioluminescence and fluorescence imaging).8,9 In vivo studies using immunocompetent mice or xenogeneic mouse models, mouse NK cells or human NK cell lines (NK92) have been labeled with the radionuclides 18FDG or 11C-methyl iodide for detection by PET. Allogeneic or autologous human NK cells were also labeled ex vivo with the gamma emitting radioisotope indium-111 oxine (111In) for detection by SPECT in patients with renal cell carcinoma49,50 and in patients with colon cancer with liver metastasis.51 In the renal cell carcinoma patients, the NK cells initially accumulated to the lungs, after intravenous injection, and redistributed to the liver and metastatic tumors (lungs and livers) within 24 h.49,50 Conversely, in the study with the colon cancer patients, NK cells were only detected within the tumor after intra-arterial delivery of the NK cells.51 However, the inability to distinguish from cell-bound versus free 111In might give false positive for the true localization of NK cells.50
PET/SPECT imaging provides high sensitivity and specificity, and could be immediately translated to the clinic through the use of FDA-approved radioisotopes (18FDG and 111In). However, several limitations to PET/SPECT still exist. These include mainly ionizing radiation exposure to the patients and fast decay of the tracers (t1/2 = 2.8 d for 11In, t1/2 = 109 min for 18FDG, and t1/2 = 20 min for 11C) which would limit the ability for longitudinal studies to monitor the persistence and biodistribution of the adoptively transferred cells.9,52 Moreover, these radionuclide-based methods have low anatomical resolution (PET/SPECT) and have to be combined with other modalities such as CT (CT) or MRI, which increases the complexity of the methodology and the costs. Using optical imaging (OI), NK cells or NK cell lines (NK92) can be detected in vivo after direct ex vivo labeling with organic fluorochromes (i.e., quantum dots, Cy5.5)23,53,54 or indirectly via transfection of a reporter gene for bioluminescence imaging (BLI) (i.e., luciferase or green fluorescent protein (GFP)).55,56 The advantages of OI methods over MRI or PET/SPECT include cheaper cost of instrumentation, fast imaging, and radiation-free imaging. However, OI is also limited by poor resolution (2–3 mm), lower sensitivity in the case of GFP due to auto-fluorescence and toxicity for certain organic fluorochromes (i.e., quantum dots contain cadmium). Importantly, the main disadvantage to optical methods for cell tracking in vivo is its limited tissue penetration of light (1 mm for fluorescence; 3 mm for bioluminescence), which restricts its application to only small animal imaging and currently there are no available instrumentation for clinical application of optical imaging.8,9,52,57
Compared to PET or OI, MRI is unique as it allows for higher resolution images (100 µm) regardless of tissue depth, does not use ionizing radiation, and allows for high specificity of signal from the transferred cells. However, it is very difficult to generate the contrast necessary to visualize the transplanted cells due to the high 1H background signal from water in vivo. Different images acquired before and after the transfer of the labeled cells can improve contrast but are problematic due to variations in the patient's positioning and due to motion. Additionally, if using gadolinium (Gd3+), it has to be chelated to limit its toxicity and has finite stability so may be problematic for extended residence times in vivo due to eventual release in tissues as free ion. While MRI offers the most beneficial methodology to track immune cells in vivo, conventional contrast agents used clinically do not allow for quantitative and longitudinal assessment of the transferred cells.
Labeling of NK cells with 19F has been reported to be challenging.35 Our data shows for the first time that 19F labeling of human NK cells is feasible, nontoxic and does not appear to affect viability, cytotoxicity or cytokine secretion by NK cells. We also observed no impact on expression of chemokine or activating receptor involved in NK cell cytotoxicity. Since 19F is passively taken up by NK cells, labeling can be performed in a relatively short period of time (24 to 48 h) at the end of an ex vivo expansion, and does not require extensive manipulation of NK cell cultures. Using subcutaneous and intratumoral injection, we were able to detect infused 19F-labeled NK cells in vivo. In human tumor bearing mice, we are able to show a loss of 19F-signal from the NK cell injection site, which is indicative of the migration of the NK cells from the primary injection site to distant organs and perhaps to the tumor site. Also for the first time, we show that an immune effector cell can be visualized within a tumor, via intratumoral injection, using 19F-MRI. Intratumoral injection of cytokines and ACT has been used previously as an effective route of cancer immunotherapy,58,59, and provides “proof of concept” evidence that NK cells can be imaged within a bulky tumor microenvironment.
All previously published data on 19F-MRI imaging of mouse or human immune cells in vitro or implanted in xenogeneic mice have used MRI scanners with field of strength ranging between 7.0T and 11.7T with the shortest imaging session of 1.5 h and up to 3 h per animal.14,25-27,30-32 Conversely, we used a 4.7T MRI scanner with the total imaging session per animal not exceeding 1 h of scan time. The strength of our MRI is closer to the field strength of MRIs in clinical practice today, making our imaging methodology more relevant for clinical imaging of ACT.34 One obvious limitation of our MRI coil was in the inability to detect 19F-labeled NK cells after intravenous (i.v.) infusion (data not shown), the most common route of therapy. Typically, cells infused i.v. first accumulate in the lungs which has a low water content (and thus low 1H signal) and is difficult to image with MR due to susceptibility related signal loss via multiple air-tissue interfaces. Improved imaging protocols that use pulse sequences with shorter echo times,60-63 or surface coils that provide high sensitivity in a localized region may improve sensitivity in the lungs to allow imaging of 19F labeled NK cells after i.v. injection.
Concurrent with the promise of 19F MRI for tracking NK cells in vivo, there are several remaining limitations that must be recognized and/or overcome when translating into patients. Field strengths of clinical MRI systems limit detection in vivo to about 104 cells, which may preclude detection of cells after trafficking to tumors from ancillary delivery intravenously or percutaneously. Nonetheless, optimal field strength, imaging sequence, and scan time needed to obtain sufficient SNR has not yet been fully assessed and could improve sensitivity. In addition, if the 19F-labeled NK cells were to undergo cell death, macrophages or dendritic cells could phagocytose them, also leading to false positive signals. Verification of 19F-labeled NK cells will therefore still need to be done pre-clinically with fluorescent or optical methods until better alternatives are available.
Methods
Animals
Female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and used between 8–16 weeks of age. All animals were bred and housed in a pathogen-free facility throughout the study. The Animal Care and Use Committee at the University of Wisconsin approved all experimental protocols.
Reagents and tumor cell lines
Peripheral blood mononuclear cells (PBMCs) and NK cells were cultured in NK media containing X-VIVO-10 (Lonza, cat#: 04-380Q) supplemented with 10% human AB serum (Corning Cellgro Inc., cat#: 35-060-Cl), 1% penicillin-strepomycin-glutamine (HyClone, cat#: SV30082.01), and 100 IU/mL recombinant human Interleukin-2 (National Cancer Institute BRB Preclinical Repository). The human chronic myelogenous leukemia cell lines K562 (American Type Culture Collection) and K562-41BBL-IL15-GFP (Waisman Biomanufacturing University of Wisconsin Madison) were maintained in RPMI-1640 (Corning Cellgro Inc., cat#: 15-040-CV) medium supplemented with 10% heat-inactivated FBS (Gibco, cat#: 10437-028) and 1% penicillin-streptomycin-glutamine (HyClone, cat#: SV30082.01) at 37°C in a humidified 5% CO2 atmosphere. CHLA-20 human neuroblastoma was a gift from Dr Mario Otto (University of Wisconsin), initially obtained from the Children Oncology Group (COG) cell line repository. M21 human melanoma was obtained from the laboratory of Dr Paul Sondel (University of Wisconsin) and gifted by Dr Ralph Reisfeld (La Jolla, CA). Z138 human mantle cell lymphoma was purchased from the American Tissue Culture Collection. CHLA-20, M21 and Z138 cell lines were grown in RPMI-1640 medium containing 10% heat inactivated FBS and 1% penicillin-streptomycin-glutamine, as formulated above, and cultured at 37°C in a humidified 5% CO2 atmosphere.
In vivo tumor models and hu14.18-IL-12 immunocytokine
Tumor cell viability was checked by trypan blue (Thermo Scientific, cat#: SV300084.01) and counted with a hemocytometer prior to in vivo implantation. CHLA-20, Z138 and M21 live cells at 4 × 106 were suspended in 0.1 mL PBS and injected subcutaneously into the right flanks of NSG mice. Once tumor sizes reached on average 8 × 8 cm (diameter × length), human NK cells were labeled with 4 mg/mL 19F for 24 h, then extra 19F was washed away from the NK cell cultures and 19F-labeled NK cells were resuspended in 0.1 mL PBS and injected intratumorally at 10 × 106 or 7 × 106 cells into CHLA-20 or Z138 tumors, respectively.
For mice bearing the M21 tumors (right flank), 10 × 106 19F-labeled NK cells were resuspended in 0.1 mL PBS and injected subcutaneously in the left flank opposite to the tumor. For NSG mice bearing M21 tumors, mice were treated intraperitoneal (1 × 106 IU/0.2 mL) with recombinant human IL-2 (National Cancer Institute BRB Preclinical Repository) on days 0 and 7 and treated intratumorally (50 µg/50 µL) with hu14.18-IL2 (EMD 272063) on days 0–2 and 7–9 of 1H/19F imaging by MRI. The immunocytokine hu14.18-IL2 (EMD 272063), a GD2-specific monoclonal antibody (mAB) fused to human IL-2 and recognizes GD2, was gifted by Dr Paul Sondel (University of Wisconsin) and supplied by the Biological Resources NCI (Frederick, MD), EMD Pharmaceuticals (Durham, NC), Merck Serono (Darmstadt, Germany) and Apeiron Biologics (Vienna, Austria).
Isolation of NK cells
Healthy donor peripheral whole blood was obtained through an IRB-approved protocol (2012-0130-CR002). PBMCs were isolated from the buffy coats by density-gradient separation, and the lymphocyte fraction was isolated from PBMCs using Ficoll (Stem Cell Technologies, cat# 07861). For NK expansion from PBMCs, K562-41BBL-IL15 feeder cells were gamma-irradiated at 100 Gy (JL Shepherd model109 Cesium irradiator) and co-cultured with PBMCs at a 1:1 ratio of feeder cells to PBMCs in NK media. PBMCs and feeder cells were cultured at 37°C in 5% CO2 on a shaker for 12 d. Fresh media was supplemented on days 4 and 8. On day 12, human NK cells were isolated by negative selection using magnetic cell separation beads (Miltenyi Biotec, cat#: 130-092-657) and sorted using auto-MACS® Separator (Miltenyi Biotec Inc., San Diego CA). NK cell purity was determined by flow cytometry staining for anti-CD3 and anti-CD56 and purity was >95% (CD3− CD56+).
19F labeling and injection of NK cells
Human NK cells were cultured for 24 or 48 h in a commercially available emulsified PFPE tracer agent CS-ATM-1000 (Celsense, Pittsburgh, PA) in NK media. After 24 or 48 h, NK cells were harvested and washed three times to remove excess PFPE before in vitro and in vivo testing. NK cell viability before and after 19F labeling was assayed using a standard trypan blue (Thermo Scientific, cat#: SV300084.01) exclusion method. For in vivo studies, 19F-labeled NK cells were injected into NSG mice subcutaneously or intratumorally.
NK cell cytotoxicity
Human NK cell cytotoxic function was determined by a standard 4-h [51Cr]-release assay against the NK cell–sensitive tumor cell line K562 using 19F-labeled or unlabeled NK cells as effector cells. K562 cells (targets) were labeled with 50 µCi 51Cr (NEZ030S; Perkin Elmer) per 106 cells and incubated for 60 min at 37°C, then cells were washed to remove extra 51Cr and target cells were resuspended in media. K562 targets (5 × 103) were added to each well at different effector to target (E:T) ratio and incubated at 37°C for 4 h. The γ-scintillation of supernatant was measured by a γ-counter (Perkin Elmer). Maximum release was determined by adding 100 µL of 1X-Triton X-100 detergent (Sigma-Aldrich, cat#: 9002-93-1) to target cells. Spontaneous release was determined by adding 100 µL of media to 100 µL of target cells. Specific 51Cr release was calculated as: % lysis = 100% × (Experimental–Spontaneous)/(Maximum–Spontaneous).
NMR
To determine labeling efficiency and quantify 19F uptake by human NK cells, 19F nuclear magnetic resonance (NMR) spectra were acquired on 19F-labeled NK cell pellets. Human NK cells (3 × 106 or as indicated in the figure legends) were labeled with 19F-PFPE (Celsense, CS-ATM-1000), pelleted to discard any 19F remaining in the culture medium, and the dry cell pellets were lysed in a lysis buffer containing 1% Triton X-100 (Sigma Aldrich, cat#: 9002-93-1) and 0.1% Trifluoroacetic acid (TFA) (Sigma Aldrich, cat#: T6508) diluted in deuterium oxide (Sigma Aldrich, cat#: 7789-20-0). NK cells in lysis buffer were placed in a capillary tube. The added amount of TFA acted as both a chemical shift (−76 ppm) and quantification (three 19F atoms per TFA molecule) reference for analysis. Samples were transferred into NMR vials and further diluted with D2O such that the cell lysate solution fit the entire detection region of the NMR coil. One-dimensional (1D) 19F NMR spectra were obtained on the NK cells using a 9.0 s recycle delay (TR), 90° flip angle, and 128 averages with the primary 19F NMR peak from the NK cells identified around −91 ppm. All spectra were acquired using a 9.4T vertical bore Varian Unity-Inova NMR spectrometer (Agilent, Santa Clara, CA, USA). The following equation was used to determine the 19F-labeling density,
| (1) |
where is the number of 19F atoms per cell, is the integrated area of major peak of the NK cell pellet, is moles of TFA reference added, is Avogardro's number, is the integrated area under the TFA reference peak, and is the number of cells in pellet. The average was taken as the mean from three separate samples and its uncertainty () was estimated as the standard deviation. NMR peak integration was performed in VNMR 6.1C (Agilent, CA, USA) with plotting performed in MATLAB R2014b (The Mathworks Inc. MA, USA).
In vivo MRI
Prior to MRI, mice were anesthetized with 1 mg ketamine (Ketaject, NDC#: 57319-542-02) and 0.1 mg xylazine (LLOYD Inc., NADA#: 139-236) per 10 g body weight, monitored with a respiration pad, and maintained at 36°C using a warm-air blower. An external phantom vial containing PFPE with known 19F spin density (ρR) of 2.3 × 1016 19F atoms/mm3 was placed in the field-of-view (FOV) and used as a 19F reference during image acquisition. MRI was performed on a 4.7 T small animal MRI system (Agilent Technologies, Santa Clara, CA) using a volume quadrature 19F coil tunable to 19F (187.9 MHz) and to 1H (200 MHz). Multiple coronal 19F 2 mm slice images were acquired using a multi-slice spin echo acquisition with 2000/9.0 ms TR/TE, 16 echoes, 16 kHz receiver bandwidth, 72 × 36 mm FOV, 64 × 32 matrix size, 1.1 × 1.1 mm2 in-plane resolution, and 40 averages resulting in a 42.6 min total scan time for 19F imaging. The same coil was used to acquire anatomical 1H images using either a T1-weighted gradient echo acquisition with an 80.4/3.4 ms TR/TE, 20° flip angle, and 0.28 × 0.28 mm2 in-plane resolution or a T2-weighted fast spin echo with a 3500/16.5 ms TR/TE, 8 echo train length and 0.28 × 0.28 mm2 in-plane resolution. The FOV and slice thickness (2 mm) were identical for both 19F and 1H images to allow for easy co-registration between nuclei. One mouse bearing the human neuroblastoma CHLA-20, one mouse bearing the human mantle cell lymphoma Z138 and two mice bearing the human melanoma M21 were imaged by MRI for quantification of the number of 19F-labeled NK cells and their localization in vivo at different time points (indicated in the figure legends for each tumor model).
Quantitative analysis of 19F signal in vivo
All image analysis was performed in MATLAB R2014b (Mathworks; Natick, MA). Quantitation of the number of cells based on 19F signal was performed similar to the previously published algorithm.27 Briefly, low-SNR magnitude image voxels were corrected for their Rician distribution by generating a look-up table between measured and expected signal intensity. Using a region-of-interest (ROI) drawn in the noise/background, the standard deviation of either the real or imaginary signal of the complex 19F MR data was calculated () on a per-slice basis. This value was used to generate the look-up table and voxels with signal below a threshold of 8× were corrected to lower signal values. Signal levels above 8 were not corrected as they approach a Gaussian distribution. Further, a minimum signal threshold of 5 was applied to select voxels with 19F signal above the noise. For visualization, 19F magnitude images were interpolated to match the 1H image matrix size. 19F images were overlaid on 1H images for anatomical positioning of 19F signal with the above signal threshold applied. All the voxels with 19F signal above the signal threshold () were summed for all slices. The average signal in the reference vial was measured with a drawn ROI (). The spin density of the reference () was normalized to the imaging voxel size in units of 19F atoms per voxel. The apparent number of cells () in the MR image could then be computed using the previously determined Fc (see Equation 1 under NMR methods) and Equation 2 below.
| (2) |
To determine the uncertainty in (), Equation 3 below was used.
| (3) |
The uncertainty in the 19F reference mean signal () was estimated as the standard deviation of the ROI drawn on the reference. The uncertainty in the summed 19F voxel signal () was estimated by Equation 4 below,27
| (4) |
where is the standard deviation of the noise in the MR image and is the number of voxels identified as having 19F signal above the noise threshold. No uncertainty was assumed in the spin density in the 19F reference .
Flow cytometry
Human NK cells were cultured for 24 h with different concentrations of 19F PFPE or cultured without label as a control. After labeling, 1 × 106 NK cells were incubated with Fc block clone 3G8 (BD PharMingen, cat#: 564220) and stained at 4°C for 20 min with anti-human antibodies including FITC-CD3 (OKT3, cat#: 317306), PE-IgG1k (MOPC-21, cat#: 400114), PE-NKp44 (P44-8, cat#: 325108), PE-DNAM-1 (11A8, cat#: 338306), PeCy7-NKG2D (1D11, cat#: 320812), PeCy7-IgG1k (MOPC-21, cat#: 400126), PeCy7-NKp46 (9E2, cat#: 331916), AF647-NKp30 (P30-15, cat#: 325212), AF647-IgG1k (MOPC-21, cat#: 400130 and 400136), AF647-IFNγ (4S.B3, cat#: 502516), AF647-Granzyme B (GB11, cat#: 515405), APC-CX3CR1 (2A9-1, cat#: 341609), APC-CXCR4 (12G5, cat#: 306510), BV421-CD56 (HCD56, cat#: 318328), and BV510-CD45 (HI30, cat#: 304036). All antibodies were purchased from BioLegend (San Diego, CA).
For intracellular detection of IFNγ, NK cells were stimulated with media containing 1 µg/mL PMA (Sigma Aldrich, cat#: P-8139), 10 µg/mL ionomycin (Sigma Aldrich, cat#: I0634) plus Golgi Stop (BD Biosciences, cat#: 554724) and Golgi Plug (BD Biosciences, cat#: 555029) for 4 h at 37°C or in the presence of media containing only Golgi Stop and Golgi Plug. After the 4 h, cell surface staining was performed followed by intracellular staining using the BD kit Cytofix/Cytoperm Plus fixation/permeabilization kit (BD Biosciences, cat#: 555028). Flow cytometry data was acquired on a MACSQuant analyzer 10 (Miltenyi Biotec Inc., San Diego CA) and mqd files were converted to fcs files using The MACSQuantify™ Software. Listmode data were analyzed using FlowJo software (TreeStar).
ELISA
Human IFNγ levels in supernatants from unlabeled human NK cells or 19F-labeled NK cells were quantified using Legend Max ELISA kit with pre-coated plates with human IFNγ according to manufacturer's instructions (BioLegend, Inc., cat#: 430107). IFNγ cytokine concentration was extrapolated relative to a standard curve created by serial dilution of human IFNγ standards run in parallel. ELISA plates were at 450 nm on a VERSAmax Tunable Plate Reader (Molecular Devices, Sunnyvale, CA) and data were collected using SOFTmax PRO software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Statistics were performed using GraphPad Prism version 6.0 for the Macintosh OS (GraphPad Software, San Diego, CA). Data were expressed as mean ± SEM. For analysis of three or more groups, the non-parametric ANOVA test was performed with the Bonferroni or Sidak's multiple comparisons post-test. Analysis of differences between two normally distributed test groups was performed using the Student's t-test. Welch's correction was applied to Student's t-test data sets with significant differences in variance. A p value less than 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Michael Martinez, Mallery Olsen, Erbay Salievski, and Lauren Reil for valuable assistance with this study. Thank you to Beth Rauch and the Department of Medical Physics for core scan time to allow methods development and to the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) Flow Cytometry core. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work is supported in part by the UWCCC, NIH/NCI K08 CA174750, NIH/NCATS UL1TR000427 and TL1TR000429, the Stand Up To Cancer – St. Baldrick's Pediatric Dream Team Translational Research Grant SU2C-AACR-DT1113 (C.M.C.), NCI P30 CA014520 – UWCCC (B.B., S.B.F. and C.M.C.), American Cancer Society (B.B., S.B.F. and C.M.C.), Alex's Lemonade Stand Foundation (C.M.C.), The American Association of Immunologists Careers in Immunology Fellowship (M.N.B) and the St. Baldrick's Foundation (M.P.K. and C.M.C).
References
- 1.Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008; 10:625-32; PMID:18836917; http://dx.doi.org/ 10.1080/14653240802301872 [DOI] [PubMed] [Google Scholar]
- 2.deMagalhaes-Silverman M, Donnenberg A, Lembersky B, Elder E, Lister J, Rybka W, Whiteside T, Ball E. Posttransplant adoptive immunotherapy with activated natural killer cells in patients with metastatic breast cancer. J Immunother 2000; 23:154-60; PMID:10687148; http://dx.doi.org/ 10.1097/00002371-200001000-00018 [DOI] [PubMed] [Google Scholar]
- 3.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D et al.. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011; 13:98-107; PMID:20849361; http://dx.doi.org/ 10.3109/14653249.2010.515582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lister J, Rybka WB, Donnenberg AD, deMagalhaes-Silverman M, Pincus SM, Bloom EJ, Elder EM, Ball ED, Whiteside TL. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with activated natural killer cells in the immediate posttransplant period. Clin Cancer Res 1995; 1:607-14; PMID:9816022 [PubMed] [Google Scholar]
- 5.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ et al.. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005 Apr 15; 105(8):3051-; PMID:15632206; http://dx.doi.org/10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 6.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013; 15:1563-70; PMID:24094496; http://dx.doi.org/ 10.1016/j.jcyt.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 7.Minutes Meeting #45. Food and Drug Administration Center for Biologics Evaluation and Research April 10 and 11, 2008; Cell, Tissue and Gene Therapies Advisory Committee. [Google Scholar]
- 8.Jha P, Golovko D, Bains S, Hostetter D, Meier R, Wendland MF, Daldrup-Link HE. Monitoring of natural killer cell immunotherapy using noninvasive imaging modalities. Cancer Res 2010; 70:6109-13; PMID:20631071; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sta Maria NS, Barnes SR, Jacobs RE. In vivo monitoring of natural killer cell trafficking during tumor immunotherapy. Magn Reson Insights 2014; 7:15-21; PMID:25114550; http://dx.doi.org/10.4137/MRI.S13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daldrup-Link HE, Meier R, Rudelius M, Piontek G, Piert M, Metz S, Settles M, Uherek C, Wels W, Schlegel J et al.. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur Radiol 2005; 15:4-13; PMID:15616814; http://dx.doi.org/ 10.1007/s00330-004-2526-7 [DOI] [PubMed] [Google Scholar]
- 11.Mallett CL, McFadden C, Chen Y, Foster PJ. Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy 2012; 14:743-51; PMID:22443465; http://dx.doi.org/ 10.3109/14653249.2012.667874 [DOI] [PubMed] [Google Scholar]
- 12.Meier R, Golovko D, Tavri S, Henning TD, Knopp C, Piontek G, Rudelius M, Heinrich P, Wels WS, Daldrup-Link H. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn Reson Med: Off J Soc Magnet Resonance Med / Soc Magnet Resonance Med 2011; 65:756-63; PMID:20928869; http://dx.doi.org/ 10.1002/mrm.22652 [DOI] [PubMed] [Google Scholar]
- 13.Sheu AY, Zhang Z, Omary RA, Larson AC. MRI-monitored transcatheter intra-arterial delivery of SPIO-labeled natural killer cells to hepatocellular carcinoma: preclinical studies in a rodent model. Invest Radiol 2013; 48:492-9; PMID:23249649; http://dx.doi.org/ 10.1097/RLI.0b013e31827994e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahrens ET, Flores R, Xu H, Morel PA. In vivo imaging platform for tracking immunotherapeutic cells. Nat Biotechnol 2005; 23:983-7; PMID:16041364; http://dx.doi.org/ 10.1038/nbt1121 [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed 2011; 24:114-29; PMID:20842758; http://dx.doi.org/ 10.1002/nbm.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grégoire C, Cognet C, Chasson L, Coupet C-A, Dalod M, Reboldi A, Marvel J, Sallusto F, Vivier E, Walzer T. Intrasplenic trafficking of natural killer cells is redirected by chemokines upon inflammation. Euro J Immunol 2008; 38:2076-84; PMID:18624307; http://dx.doi.org/ 10.1002/eji.200838550 [DOI] [PubMed] [Google Scholar]
- 17.Liou HLR, Myers JT, Barkauskas DS, Huang AY. Intravital imaging of the mouse popliteal lymph node. 2012:e3720 J Vis Exp. 2012 Feb 8; (60). pii: 3720. PMID: 22349264; http://dx.doi.org/10.3791/3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson JA, Zeiser R, Beilhack A, Goldman JJ, Negrin RS. Tissue-specific homing and expansion of donor NK cells in allogeneic one marrow transplantation. J Immunol 2009; 183:3219-28; PMID:19657090; http://dx.doi.org/ 10.4049/jimmunol.0804268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.F.D.A. US. White paper: initiative to reduce unnecessary radiation exposure from medical imaging. [Google Scholar]
- 20.Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol 2013; 13:755-63; PMID:24013185; http://dx.doi.org/ 10.1038/nri3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed 2013; 26:860-71; PMID:23606473; http://dx.doi.org/ 10.1002/nbm.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu L, Hockett FD, Chen J, Zhang L, Caruthers SD, Lanza GM, Wickline SA. A generalized strategy for designing (19)F/(1)H dual-frequency MRI coil for small animal imaging at 4.7 Tesla. J Magn Reson Imaging: JMRI 2011; 34:245-52; PMID:21698714; http://dx.doi.org/ 10.1002/jmri.22516 [DOI] [PubMed] [Google Scholar]
- 23.Lim YT, Cho MY, Noh YW, Chung JW, Chung BH. Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy. Nanotechnology 2009; 20:475102; PMID:19875875; http://dx.doi.org/ 10.1088/0957-4484/20/47/475102 [DOI] [PubMed] [Google Scholar]
- 24.Hitchens TK, Ye Q, Eytan DF, Janjic JM, Ahrens ET, Ho C. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2011; 65:1144-53; PMID:21305593; http://dx.doi.org/ 10.1002/mrm.22702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helfer BM, Balducci A, Nelson AD, Janjic JM, Gil RR, Kalinski P, de Vries IJ, Ahrens ET, Mailliard RB. Functional assessment of human dendritic cells labeled for in vivo (19)F magnetic resonance imaging cell tracking. Cytotherapy 2010; 12:238-50; PMID:20053146; http://dx.doi.org/ 10.3109/14653240903446902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonetto F, Srinivas M, Heerschap A, Mailliard R, Ahrens ET, Figdor CG, de Vries IJ. A novel (19)F agent for detection and quantification of human dendritic cells using magnetic resonance imaging. Int J Cancer J Int du Cancer 2011; 129:365-73; PMID:20839261; http://dx.doi.org/ 10.1002/ijc.25672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas M, Morel PA, Ernst LA, Laidlaw DH, Ahrens ET. Fluorine-19 MRI for visualization and quantification of cell migration in a diabetes model. Magn Reson Med: Off J Soc Mag Reson Med / Soc Magn Reson Med 2007; 58:725-34; PMID:17899609; http://dx.doi.org/ 10.1002/mrm.21352 [DOI] [PubMed] [Google Scholar]
- 28.Srinivas M, Turner MS, Janjic JM, Morel PA, Laidlaw DH, Ahrens ET. In vivo cytometry of antigen-specific t cells using 19F MRI. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2009; 62:747-53; PMID:19585593; http://dx.doi.org/ 10.1002/mrm.22063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helfer BM, Balducci A, Sadeghi Z, O'Hanlon C, Hijaz A, Flask CA, Wesa A. (1)(9)F MRI tracer preserves in vitro and in vivo properties of hematopoietic stem cells. Cell Transplant 2013; 22:87-97; PMID:22862925; http://dx.doi.org/ 10.3727/096368912X653174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Cabello J, Walczak P, Kedziorek DA, Chacko VP, Schmieder AH, Wickline SA, Lanza GM, Bulte JW. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med: Off J Soc Magn Reson Med/ Soc Magn Reson Med 2008; 60:1506-11; PMID:19025893; http://dx.doi.org/ 10.1002/mrm.21783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PloS One 2011; 6:e29040; PMID:22216163; http://dx.doi.org/ 10.1371/journal.pone.0029040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PloS One 2015; 10:e0118544; PMID:25767871; http://dx.doi.org/ 10.1371/journal.pone.0118544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribot EJ, Gaudet JM, Chen Y, Gilbert KM, Foster PJ. In vivo MR detection of fluorine-labeled human MSC using the bSSFP sequence. Int J Nanomed 2014; 9:1731-9; PMID:24748787; http://dx.doi.org/ 10.2147/IJN.S59127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahrens ET, Helfer BM, O'Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2014; 72:1696-701; PMID:25241945; http://dx.doi.org/ 10.1002/mrm.25454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim YT, Cho MY, Kang JH, Noh YW, Cho JH, Hong KS, Chung JW, Chung BH. Perfluorodecalin/[InGaP/ZnS quantum dots] nanoemulsions as 19F MR/optical imaging nanoprobes for the labeling of phagocytic and nonphagocytic immune cells. Biomaterials 2010; 31:4964-71; PMID:20346494; http://dx.doi.org/ 10.1016/j.biomaterials.2010.02.065 [DOI] [PubMed] [Google Scholar]
- 36.Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med 2008; 205:2965-73; PMID:19029380; http://dx.doi.org/ 10.1084/jem.20081752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhtoiarov IN, Neal ZC, Gan J, Buhtoiarova TN, Patankar MS, Gubbels JA, Hank JA, Yamane B, Rakhmilevich AL, Reisfeld RA et al.. Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: impact on conjugation, cytotoxicity, and targeting. J Leukocyte Biol 2011; 89:625-38; PMID:21248148; http://dx.doi.org/ 10.1189/jlb.0710422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD et al.. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res: Off J Am Assoc Cancer Res 2004; 10:4839-47; PMID:15269160; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0799 [DOI] [PubMed] [Google Scholar]
- 39.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 1998; 91:1706-15; PMID:9473237 [PubMed] [Google Scholar]
- 40.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, Alderson KL, Gan J, Reisfeld RA, Gillies SD et al.. Intratumoral treatment of smaller mouse neuroblastoma tumors with a recombinant protein consisting of IL-2 linked to the hu14.18 antibody increases intratumoral CD8+ T and NK cells and improves survival. Cancer Immunol, Immunother: CII 2013; 62:1303-13; PMID:23661160; http://dx.doi.org/ 10.1007/s00262-013-1430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B et al.. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol: Off J Am Soc Clin Oncol 2010; 28:4969-75; PMID:20921469; http://dx.doi.org/ 10.1200/JCO.2009.27.8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C et al.. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res: Off J Am Assoc Cancer Res 2006; 12:1750-9; PMID:16551859; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albertini MR, Hank JA, Gadbaw B, Kostlevy J, Haldeman J, Schalch H, Gan J, Kim K, Eickhoff J, Gillies SD et al.. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol, Immunother: CII 2012; 61:2261-71; PMID:22678096; http://dx.doi.org/ 10.1007/s00262-012-1286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013; 10:230-52; PMID:23604045; http://dx.doi.org/ 10.1038/cmi.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spahn DR. Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care 1999; 3:R93-7; PMID:11094488; http://dx.doi.org/ 10.1186/cc364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bansal A, Pandey MK, Demirhan YE, Nesbitt JJ, Crespo-Diaz RJ, Terzic A, Behfar A, DeGrado TR. Novel (89)Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res 2015; 5:19; PMID:25918673; http://dx.doi.org/ 10.1186/s13550-015-0098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Watering FC, Rijpkema M, Perk L, Brinkmann U, Oyen WJ, Boerman OC. Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. Biomed Res Int 2014; 2014:203601; PMID:24991539; http://dx.doi.org/10.1155/2014/203601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato N, Wu H, Asiedu KO, Szajek LP, Griffiths GL, Choyke PL. (89)Zr-oxine complex PET cell imaging in monitoring cell-based therapies. Radiology 2015; 275:490-500; PMID:25706654; http://dx.doi.org/ 10.1148/radiol.15142849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brand JM, Meller B, Von Hof K, Luhm J, Bahre M, Kirchner H, Frohn C. Kinetics and organ distribution of allogeneic natural killer lymphocytes transfused into patients suffering from renal cell carcinoma. Stem Cells Dev 2004; 13:307-14; PMID:15186726; http://dx.doi.org/ 10.1089/154732804323099235 [DOI] [PubMed] [Google Scholar]
- 50.Meller B, Frohn C, Brand JM, Lauer I, Schelper LF, von Hof K, Kirchner H, Richter E, Baehre M. Monitoring of a new approach of immunotherapy with allogenic (111)In-labelled NK cells in patients with renal cell carcinoma. Euro J Nuclear Med Mol Imaging 2004; 31:403-7; PMID:14685783; http://dx.doi.org/ 10.1007/s00259-003-1398-4 [DOI] [PubMed] [Google Scholar]
- 51.Matera L, Galetto A, Bello M, Baiocco C, Chiappino I, Castellano G, Stacchini A, Satolli MA, Mele M, Sandrucci S et al.. In vivo migration of labeled autologous natural killer cells to liver metastases in patients with colon carcinoma. J Transl Med 2006; 4:49; PMID:17105663; http://dx.doi.org/ 10.1186/1479-5876-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kircher MF, Gambhir SS, Grimm J. Noninvasive cell-tracking methods. Nat Rev Clin Oncol 2011; 8:677-88; PMID:21946842; http://dx.doi.org/ 10.1038/nrclinonc.2011.141 [DOI] [PubMed] [Google Scholar]
- 53.Tavri S, Jha P, Meier R, Henning TD, Muller T, Hostetter D, Knopp C, Johansson M, Reinhart V, Boddington S et al.. Optical imaging of cellular immunotherapy against prostate cancer. Mol Imaging 2009; 8:15-26; PMID:19344572; http://dx.doi.org/10.2310/7290.2009.00002 [PubMed] [Google Scholar]
- 54.Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen X, Wu JC, Sunwoo JB, Cheng Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials 2012; 33:5584-92; PMID:22575830; http://dx.doi.org/ 10.1016/j.biomaterials.2012.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olson JA, Zeiser R, Beilhack A, Goldman JJ, Negrin RS. Tissue-specific homing and expansion of donor NK cells in allogeneic bone marrow transplantation. J Immunol 2009; 183:3219-28; PMID:25241945; http://dx.doi.org/ 10.4049/jimmunol.0804268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood 2009; 114:3227-34; PMID:19667398; http://dx.doi.org/ 10.1182/blood-2009-06-228759 [DOI] [PubMed] [Google Scholar]
- 57.Sutton EJ, Henning TD, Pichler BJ, Bremer C, Daldrup-Link HE. Cell tracking with optical imaging. Eur Radiol 2008; 18:2021-32; PMID:18506449; http://dx.doi.org/ 10.1007/s00330-008-0984-z [DOI] [PubMed] [Google Scholar]
- 58.Duval L, Schmidt H, Kaltoft K, Fode K, Jensen JJ, Sorensen SM, Nishimura MI, von der Maase H. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin Cancer Res: Off J Am Assoc Cancer Res 2006; 12:1229-36; PMID:16489078; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1485 [DOI] [PubMed] [Google Scholar]
- 59.Hasumi K, Aoki Y, Wantanabe R, Mann DL. Clinical response of advanced cancer patients to cellular immunotherapy and intensity-modulated radiation therapy. Oncoimmunology 2013; 2:e26381; PMID:24349874; http://dx.doi.org/ 10.4161/onci.26381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmid F, Holtke C, Parker D, Faber C. Boosting (19) F MRI-SNR efficient detection of paramagnetic contrast agents using ultrafast sequences. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2013; 69:1056-62; PMID:19657090; http://dx.doi.org/ 10.1002/mrm.24341 [DOI] [PubMed] [Google Scholar]
- 61.Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2013; 70:1241-50; PMID:23213020; http://dx.doi.org/ 10.1002/mrm.24570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiger M, Pruessmann KP, Hennel F. MRI with zero echo time: hard versus sweep pulse excitation. Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2011; 66:379-89; PMID:21381099; http://dx.doi.org/ 10.1002/mrm.22799 [DOI] [PubMed] [Google Scholar]
- 63.Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med: Off J Soc Magn Reson Med / Soc Magn Reson Med 2012; 67:510-8; http://dx.doi.org/ 10.1002/mrm.23017 [DOI] [PubMed] [Google Scholar]