ABSTRACT
To investigate the thymic regenerative potential in adults accepting chemotherapy for lymphoma. The dynamics of thymic activity in 54 adults from baseline to 12 mo post-chemotherapy was analyzed by assessing thymic structural changes with serial computed tomography (CT) scans, and correlating these with measurements of thymic output by concurrent analysis of single-joint (sj) T-cell receptor excision circles (sjTREC) and CD31+ recent thymic emigrants (RTE) in peripheral blood. Furthermore, the consequence of thymic renewal on peripheral CD4+ T cell recovery after chemotherapy was evaluated. Time-dependent changes of thymic size and thymic output assessed by both sjTREC levels and CD31+ RTE counts in peripheral blood were observed during and after chemotherapy. Enlargement of thymus over baseline following chemotherapy regarded as rebound thymic hyperplasia (TH) was identified in 20 patients aged 18−53 y (median 33 y). By general linear models repeated measure analysis, it was found that, patients with TH (n = 20) had a faster recovery of sjTREC levels and CD31+ RTE counts after chemotherapy than patients with comparable age, gender, diagnosis, disease stage, thymic volume and output function at baseline but without TH (n = 18) (p = 0.035, 0.047); besides, patients with TH had a faster repopulation of both naïve CD4+ T cell and natural regulatory CD4+ T cell subsets than those without TH (p = 0.042, 0.038). These data suggested that adult thymus retains the capacity of regeneration after chemotherapy, especially in young adults. The presence of TH could contribute to the renewal of thymopoiesis and the replenishment of peripheral CD4+ T cell pool following chemotherapy in adults.
KEYWORDS: Adult, chemotherapy, recent thymic emigrants, single-joint T-cell receptor excision circles, thymic hyperplasia
Introduction
A variety of factors other than age such as stress and toxicity could result in an alteration in the structure and cellular composition of the thymus. However, the thymus tissue is plastic and has the capacity for renewal on recovery.1 Thymus atrophy has been observed following chemotherapy for malignancies, with a reduction in cortical lymphocytes, a shrinkage of the thymic lobules, and a decrease in the production of naïve T cells. After the cessation of therapy, the thymus regrows, and restores the output of naïve T cells.2 Excessive re-growth of the thymus may sometimes occur during recovery, and is termed rebound TH.2 TH may be related with a robust thymic regeneration, and is characterized by an increase in thymic size and density without changing its normal shape and histological appearance.3 This phenomenon is common in children and adolescents, and can occasionally be observed in young adults, but is rare in older patients,2,4-7 possible due to a decreased regenerative capacity of thymus with age. However, the small number of adults studied to date has provided only limited knowledge on the renewal capacity of adult thymus.
The assessment of thymic activity after chemotherapy is mainly based on studies of the regeneration of T lymphocytes with naïve phenotype (CD45RA+CD4+) and thymic imaging.2,8-10 The main limitation of these approaches is that they do not actually measure RTE but rather the more mature naïve T cells, which can proliferate in the peripheral blood or be converted from memory T cells.11 Moreover, thymic function is not always consistent with its mass. Therefore, more reliable approaches are needed for deep understanding of the dynamics of thymic activity. Over the past decade, sjTREC formed during T cell receptor rearrangement and CD31+ naïve CD4+ T cells were regarded as more reliable markers of thymic output, and have been widely used in the studies of haematopoietic stem cell transplantation (HSCT), human immuno-deficiency virus (HIV) infection and congenital immunodeficiency disorders, but less in chemotherapy.10-14
Full immune recovery after chemotherapy depends on thymic output of new RTE to replenish the peripheral naïve T cells pool. In the young, TH predicts a renewal of thymic activity, and contributes to the reconstitution of naïve T cells with a wide T cell receptor (TCR) repertoire.9 In contrast, homeostatic proliferation is thought to be the major contributor to immune reconstitution in adults, leading to a delayed recovery of naïve T cell populations after chemotherapy.15 No correlation has been found between thymic size and early recovery of peripheral naïve CD4+ counts after chemotherapy in adults.16 However, aged thymus still has the renewal capacity to rebuild a T cell repertoire.17,18 TH after chemotherapy observed in younger adults was found to be correlated with a faster and more complete recovery of naïve T cells.19 Patients' age-related thymic output and long-term consequences for their immune system remains to be fully understood.
The present study was designed to investigate the thymic regenerative potential in adults with lymphoma after chemotherapy, by assessing thymic structural change with serial CT scans and correlated these with measurements of thymic output by concurrent analysis of sjTREC and CD31+ RTE. Furthermore, the consequence of thymic renewal on peripheral CD4+ T cells recovery after chemotherapy was evaluated.
Results
Dynamics of thymic volume during and after chemotherapy
Time-dependent changes of thymic size were observed in 54 patients studied: thymic volume at baseline showed an inverse correlation with the age (Spearman test r = –0.707, p < 0.001); during treatment, the thymic soft tissue mass reduced in 16/54 (30%) and remained stable in 37/54 (69%) patients, yet increased in one patient; after the end of treatment, thymic enlargement was obvious in 24/54 (44%) patients, minimal change of thymus was detected in the remaining 30/54 (56%) cases.
TH was identified in 20/54 (37%) patients aged 18–53 y (median 33 y), whose clinical characteristics are shown in Table 1. The change in their thymic size was particularly remarkable (Fig. 1): prior to therapy, 17/20 (85%) of them had a TI of 0–2 with no or minimal soft tissue in the thymus; during therapy, all but one patient (95%) attained a TI of 0–2; yet, at the onset of TH, 11/20 (55%) of them achieved a TI of at least three with moderate to mass-like thymic soft tissue. Thus, the thymic size recovered from chemotherapy in these patients not merely returned to the pretreatment status but to a higher level.
Table 1.
Comparison of clinical, laboratory and imaging characteristics between patients with and without thymic hyperplasia (TH) after chemotherapy.
| characteristics | patients with TH (n = 20) | patients without TH (n = 18) | p |
|---|---|---|---|
| Median age (year) | 33(18−53) | 37(28−49) | 0.082 |
| Gender (%) | |||
| Male | 9/20 (45) | 11/18 (61) | 0.341 |
| Female | 11/20 (55) | 7/18 (39) | |
| Disease type (%) | |||
| DLBCL | 9/20 (45) | 11/18 (61) | 0.341 |
| HL | 5/20 (25) | 2/18 (11) | |
| Other types | 6/20 (30) | 5/18 (28) | |
| Disease stage (%) | |||
| I–II | 7/20 (35) | 7/18 (39) | 1.000 |
| III–IV | 13/20 (65) | 11/18 (61) | |
| CD4+T cells counts (× 109/L) | |||
| At baseline | 676 ± 382 | 572 ± 358 | 0.382 |
| At the nadir | 351 ± 215 | 329 ± 189 | 0.597 |
| Thymic index at baseline (%) | |||
| 0 | 2/20 (10) | 5/18 (28) | 0.410 |
| 1 | 6/20 (30) | 3/18 (17) | |
| 2 | 9/20 (45) | 9/18 (50) | |
| 3 | 2/20 (10) | 1/18 (5) | |
| 4 | 1/20 (5) | 0/18 (0) | |
| Thymic output at baseline | |||
| CD31+RTE (× 109/L) | 155 ± 127 | 136 ± 124 | 0.742 |
| sjTREC (copies/106PBMCs) | 9873 ± 9621 | 8526 ± 8625 | 0.793 |
Abbreviations: DLBCL, diffuse large B cell lymphoma; HL, Hodgkin lymphoma; RTE, recent thymic emigrants; sjTREC, single-joint T-cell receptor excision circles.
Figure 1.

Constituent ratios of thymic index (TI) in 20 patients with thymic hyperplasia (TH) following chemotherapy. The change in thymic size before, during and after treatment were assessed by examination of serial thoracic CT scans using TI on a 0–5 scale. At the onset of TH, 55% of patients achieved a TI above 2; in contrast, 85% of patients prior to therapy and 95% of patients during therapy had a TI of 0–2.
Dynamics of thymic output during and after chemotherapy
To determine the change of thymic output during and after chemotherapy, CD31+ RTE counts and sjTREC levels in peripheral blood were measured serially from baseline to 12 mo post-chemotherapy (Fig. 2). Both the numbers of CD31+ RTE and sjTREC levels started to reduce with the administration of cytotoxic drugs, and got down to the nadir at the end of treatment (p = 0.003, 0.015, respectively), then remained low during the first half year of follow-up; thereafter, CD31+ RTE counts and sjTREC levels rose significantly at 9 and 6 mo after the end of chemotherapy respectively (p < 0.001), and were not significantly different from the pretreatment levels (p > 0.05).
Figure 2.
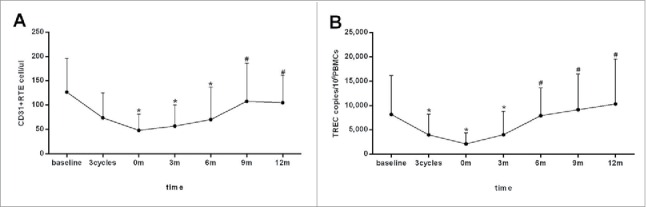
Dynamic change of thymic output during and after chemotherapy in 54 patients with B cell lymphoma. Thymic output was monitored by measuring CD31+ recent thymic emigrants (RTE) (A) counts and single-joint T-cell receptor excision circles (sjTREC) (B) levels in the peripheral blood prior (baseline), during (after three cycles of chemotherapy) and after (0, 3, 6, 9, 12 mo post-chemotherapy) treatment. Both of the CD31+ RTE counts and sjTREC levels decreased to the nadir at the end of treatment and recovered within one year of follow-up. Data are shown as mean ± SD. p values were assessed by general linear model analysis for repeated measure data. ★p < 0.05, versus baseline levels;#p < 0.001,vs. the nadir at the end of treatment.
The impact of thymic hyperplasia on thymic output
It should be noted that, the level of thymic output will vary according to a number of background factors, including age, gender, disease status and thymic activity at baseline. To assist the evaluation of the influence of TH on thymic output after chemotherapy, we compared quantitative changes of CD31+ RTE and sjTREC in peripheral blood prior, during and after chemotherapy in patients with (n = 20) and without (n=18) TH (Table 1). Patients in two groups were not significantly different in age, gender, diagnosis, disease stage, thymic volume and output function (CD31+ RTE and sjTREC) at baseline, and CD4+ cell numbers at baseline and the nadir (p > 0.05). By general linear models repeated measure analysis, impact of the presence of TH was found on the recovery of both CD31+ RTE counts and sjTREC levels between two groups (p = 0.035, 0.047, respectively).The numbers of CD31+ RTE and sjTREC levels in subjects with TH were higher than those in subjects without TH at each time point beyond 6 mo of follow-up (p < 0.05), indicating a fast recovery of thymic output in patients with TH (Fig. 3A and B ).
Figure 3.
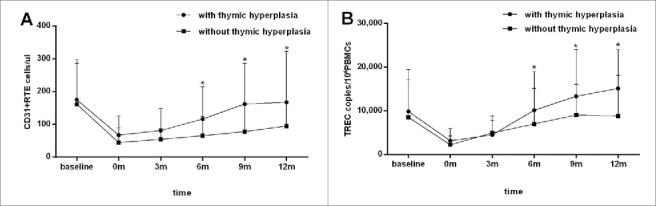
Evaluation the influence of thymic hyperplasia (TH) on the recovery of thymic output after chemotherapy. CD31+ recent thymic emigrants (RTE) (A) counts and single-joint T-cell receptor excision circles (sjTREC) (B) levels in the peripheral blood were measured serially before (baseline) and after (0, 3, 6, 9, 12 mo post-chemotherapy) treatment in 20 patients with TH (•) and 18 patients without TH (▪) following chemotherapy. The presence of TH was found to influence the recovery of both CD31+ RTE counts and sjTREC levels after chemotherapy (p = 0.035, 0.047), and higher CD31+ RTE counts and sjTREC levels were found in patients with TH in the second half year of follow-up. Data are shown as mean ± SD. p values were assessed by general linear model analysis for repeated measure data and Mann–Whitney U test.★p < 0.05, compared with the control at the same time point.
The impact of thymic hyperplasia on CD4+ T cells repopulation
Next, we assessed whether the presence of TH could promote the reconstitution of naïve CD4+T cells after chemotherapy. Since the replenishment of both naïve CD4+ T cells and natural regulatory CD4+T cells (nTregs) in the peripheral blood following lymphopenia relies on thymic de novo production,20,21 we performed a serial analysis of peripheral naïve CD4+ T cells and nTregs counts from baseline to 12 mo post-chemotherapy in patients with (n = 20) and without TH (n = 18) (Table 1). By repeated-measure analyses adjusted for the presence of thymic rebound using general linear models, it was found that, subjects with TH could reconstitute the peripheral naïve CD4+ T cell pool significantly faster than subjects without TH during the 12 mo follow-up (p = 0.042). The numbers of peripheral naïve CD4+ T cells in both groups got down to the nadir at the end of chemotherapy (p = 0.005, 0.019, respectively), and rose significantly at 6 and 9 mo after chemotherapy discontinuation in cases with and without TH, respectively (p = 0.028, 0.036) (Fig. 4A). Similarly, the repopulation of nTreg was found to be faster in subjects with TH (p = 0.038), in whom the numbers of nTreg rose significantly at 6 mo after the completion of treatment (p = 0.032) before declining to the nadir at the end of treatment (p = 0.007). Yet, no significant increase in nTreg counts was observed in those without TH within one year of follow-up (p > 0.05) (Fig. 4B).
Figure 4.

Evaluation the influence of thymic hyperplasia (TH) on the recovery of CD4+ T cell subsets after chemotherapy. Numbers of naive CD4+T cells (A) and natural CD4+ regulatory T cells (nTreg) (B) in peripheral blood were measured serially before (baseline) and after (0, 3, 6, 9, 12 mo post-chemotherapy) treatment in 20 patients with TH (•) and 18 patients without TH (▪) following chemotherapy. The presence of TH was found to influence the repopulation of naïve CD4+ T cells and nTregs after chemotherapy (p = 0.042, 0.038), and earlier recovery of naïve CD4+ T cells and nTregs was found in subjects with TH after chemotherapy. Data are shown as mean ± SD. p values were assessed by general linear model analysis for repeated measure data.★p < 0.05, compared with the nadir at the end of therapy in the same group.
Discussion
During the involutional process, the densely cortical and medullary tissues of the thymus reduce and the thymopoietic productivity decline. In adults over 40 y, the thymus is usually mostly fatty in composition, but remains active and generates new T cells at a lower rate.22,23 Since thymic activity is dependent upon age, the renewal ability of the thymus maybe also influenced by host age. Information on the thymic renewal capacity following chemotherapy has been obtained from different age groups. Thymic atrophy was reported to be observed in over 90% of young patients (2−35, mean 17 y) receiving chemotherapy for malignancies; withdrawal of chemotherapy resulted in variable enlargement of the thymus; reactive TH or rebound, defined as a diffuse enlargement of thymus (re-growth 50% or greater than baseline volume) on CT scans was observed in 25% of them, suggesting a high regenerative capacity of the thymus in children, adolescents and young adults.2 In a group of older adults (26−63, mean 46 y), however, this thymic renewal capacity is limited. Only a low frequency (22%) of thymic atrophy followed by a low incidence (11%) and minimal degree of hyperplasia was observed after high-dose chemotherapy and autologous stem cell transplantation.6 In this study, the thymus appeared to atrophy during chemotherapy in 30% of adults aged 18−59 y (median 35 y), and regrow on recovery in nearly half of them. Moreover, TH was found to be a relatively common phenomenon occurring in 37% of those adults, of whom the eldest was 53 y old. No TH was found in those above 60 y. It is concluded that adult thymus retains the capacity of regeneration, especially in young adults. In agreement with our findings, Sfikakis et al.19 demonstrated an enlargement of the previously atrophic thymus in 63% of younger adults (18−49 y, median 30 y), but in none of the elderly.
In consistent with the changes in thymic size, time-dependent changes of thymic output could be observed during and after chemotherapy. Thymic output monitored by determining the numbers of CD31+ RTE and indirectly by measuring the levels of sjTREC in peripheral blood decreased to the nadir at the end of chemotherapy, but recovered to baseline levels within one year after completion of chemotherapy. It can be speculated that, cytotoxic drugs and glucocorticoid hormones used in chemotherapy could effectively extinguish thymic output, followed by a restoration of thymic output after the removal of drug toxicity and the elimination of stress imposed by the tumor with successful therapy. Furthermore, it is confirmed that, TH observed in young adults post-chemotherapy could influence the regeneration rate of both CD31+ RTE and sjTREC in the peripheral blood. The faster recovery of thymic output observed in subjects with TH following chemotherapy suggested that thymic structural re-growth served as a basis for the renewed thymopoiesis. Thymic enlargement concurrent with the restoration of thymic output further supported the hypothesis that TH after chemotherapy predicts a robust renewal of thymopoiesis. Multiple mechanisms including the numeric and functional recovery of cortical thymic epithelial cells that determine the overall lymphopoietic capacity of the thymus might play important roles in the thymic regeneration and contribute to the process of TH following chemotherapy.24 In support of this point, HSCT recipients with TH was reported to display a robust renewal of thymopoiesis during recovery from lymphopenia, as evidenced by increases in sjTREC bearing cells with broad TCR diversity in the peripheral blood.10
The potential role of the adult thymus in T cell immune recovery following chemotherapy-induced lymphopenia remains the subject of debate. CD4+T cell repopulation subsequent to lymphopenia relies on two pathways: production of T cells de novo by the thymus or expansion of the mature peripheral T cells.20 The thymic pathway is commonly compromised in adults, and CD4+ T cells rely more upon expansion to restore peripheral T cell pool, which could reduce the TCR diversities and increase the risk for infections and cancer.9,10,15 In the study of Vedel et al., no association was found between thymic size and early recovery of peripheral naïve CD4+ counts after chemotherapy, suggesting a limited role of adult thymus in CD4+ T cells repopulation.16 However, other two studies revealed that thymic output was still an important pathway for CD4+ T cell reconstitution in adults, and TH correlated significantly with a faster and more complete recovery of naïve CD4+ T cells after chemotherapy.19,25 As reported, TH after chemotherapy, observed in this cohort of adults we studied, could promote the repopulation of naïve CD4+ T cells within one year of follow-up, although no impact of TH has been found on the numeric recovery of total CD4+ T cells in our previous study.26 Hence, it is concluded that, TH after chemotherapy in adults, as in children and adolescents, predicts a renewal of thymic activity, and contributes to the reconstitution of peripheral T cell populations by generating naïve T cells de novo. Besides, thymic enlargement occurring in adults under HSCT and anti-viral therapy for HIV, was reported to be correlated with higher TREC frequency and greater numbers of naive and total CD4+ T cells as well, consistent with the idea that the adult thymus remains active late in life and retains the capacity to orchestrate normal T lymphopoiesis.10,27
Furthermore, this data explored the role of adult thymus in maintaining peripheral Tregs pool after chemotherapy. Tregs is a heterogeneous population that consists of thymic derived natural (n)Treg and peripheral converted induced (i)Treg cells.21 It has been shown that, most recovering Tregs after chemotherapy were peripherally derived, possibly due to the excessive proliferation of iTregs under immune activation and the impaired thymic output of nTregs.28 This study revealed that, the numbers of peripheral nTregs declined significantly with cytoreductive therapy; during recovery, the repopulation of nTregs was faster in subjects with TH, indicating an increased thymic production of nTregs correlated with thymic renewal. This data provided the first evidence of an important role of renewed thymopoiesis in the Tregs repopulation following chemotherapy. Likewise, the replenishment of nTregs subsequent to lymphopenia induced by HSCT, HIV infection or autoimmune disease, was found to rely on de novo thymic production.29-31
Admittedly, there are some limitations to this study. sjTRECs are not replicated during mitosis and thus, become diluted with each successive iteration of cell division. Therefore, levels of sjTRECs in rapidly dividing cells can underestimate the ability of thymic regeneration.32 On the other hand, besides the sole thymic T-cell neogenesis, CD31+ naïve CD4+ T cells can proliferate and maintain their CD31 expression in peripheral blood. Thus, the CD31+ RTE data potentially underestimate thymic derivation as well.33 Thymic activity evaluation by sjTRECs in naïve CD4+ T cells could be more sensitive and informative.
In conclusion, this study confirmed adult thymus retains the capacity of regeneration after chemotherapy, especially in young adults, manifesting as an increase in thymic volume and output function. TH was related to a faster recovery of CD4+ naïve T cells and nTreg after chemotherapy, indicating that the thymic output pathway may play a role in the reconstitution of CD4+ T cells in young adults. Adult thymus appears to be pivotal for reconstitution of T lymphoid immunity following chemotherapy, and could be the target of therapeutic improvements.
Materials and methods
Patients
Eighty-four lymphoma patients with mature B cell lymphoma admitted at the Department of Hematology, the First Affiliated Hospital of Nanjing Medical University between January 2013 and January 2015 were studied. All of them accepted chemotherapy at lymphoma diagnosis. Patients with diffuse large B cell lymphoma (DLBCL) were treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-DA-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) regimen. For patients with Hodgkin lymphoma (HL), ABVD (adriamycin, bleomycin, vinblastin and dacarbazine) regimen was administered. Patients with follicular lymphoma (FL) or marginal zone lymphoma (MZL) accepted R-CHOP regimen mentioned above or R-COP (rituximab, cyclophosphamide, vincristine and prednisone) regimen. Eight patients who had no response to therapy or had disease progression within one year after the end of treatment were excluded. Sixteen patients who had response to chemotherapy and underwent HSCT as first-line consolidation were excluded. In six patients, it was not possible to determine the size of the thymus because of the lymphoma growth. The remaining 54 patients (32 DLBCL, 12 HL, 6 FL, 4 MZL), aged 18−59 y (median 35 y), were included. CT examinations were performed prior (baseline), during (after three cycles of chemotherapy) and after (0, 3, 6, 9, 12 mo post-chemotherapy) treatment. Simultaneously, blood samples were collected for flow cytometric analyses and the obtainment of peripheral blood mononuclear cells (PBMC) by density gradient centrifugation. This single-center retrospective study was approved by hospital ethical committee and admitted by the patients.
Imaging of thymus
Serial analysis of thymus structural changes was performed by reviewing CT images. The thymic size was scored using thymic index (TI) on a scale from 0 to 5 as described elsewhere:19 0, no soft tissue; 1, minimal soft tissue, barely recognizable; 2, minimal soft tissue, more obvious; 3, moderate soft tissue; 4, moderate soft tissue of greater extent, almost mass-like; and 5, mass-like appearance, suggesting hyperplasia or thymoma. Thymic enlargement, or subsequent regression, was defined as a change of at least 1 on the 0–5 scale. Enlargement of the thymus over baseline in the absent of any clinical, laboratory or radiological sign of disease progression was interpreted as TH.
Detection of CD31+RTE by flow cytometry
Repeated analyses of peripheral blood lymphocyte subsets were performed in parallel with the CT examination. Peripheral blood samples were collected and stained immediately using the whole blood lysis technique. Phenotypic analyses were made with combinations of FITC, PE, APC and PerCP-conjugated mAbs (Becton Dickinson, USA) against CD3, CD4+, CD45RA, CCR7, CD31, CD25, CD127 and isotype control, using FACS Calibur (Becton Dickinson, USA) as described.34,35 The number of CD4+ cells expressing naive phenotype (CD3+CD4+CD45RA+CCR7+), CD31+ naïve CD4+T cells (CD3+CD4+CD45RA+CD31+) and CD4+regulatory T cells (Tregs) with naïve phenotype (CD4+CD25+CD127lowCD45RA+) was determined. The fluorescence of 40,000 cells was measured. Whole blood lymphocyte counts were performed with an automated analyzer, and the absolute numbers of cell subsets were determined by multiplying the total lymphocyte count by the fraction of lymphocytes bearing the specific phenotypic markers.
Detection of sjTREC by RQ-PCR
Serial quantification of sjTREC in DNA of PBMCs was done by TaqMan real-time quantitative PCR assay using a StepOnePlus (Applied Biosystems, USA) according to the method of Tang et al.36 DNA was extracted from PBMCs using the QIAamp® Blood DNA Mini Kit (QIAGEN, German) according to instructions, and the DNA concentration was determined by spectrophotometry (Eppendorf, German) prior to further analysis. The sequences of the forward and reverse primers and probe for the sjTREC were 5′ AACAGCCTTTGGGACACTATCG 3′, 5′ GCTGAACTTATTGCA ACTCGTGAG 3′, and 5′ 6-FAM-CCACATCCCTTTCAACCATGCTGACACCTC-TAMRA 3′. As an internal control, the expression of the RAG2 gene was simultaneously analyzed using the following forward and reverse primers and probe: forward primer, 5′ GCAACATGGGAAATGGAACTG 3′; reverse primer, 5′ GGTGT CAAATTCATCATCACCATC 3′, and the probe 5′ 6-FAM-CCCCTGGATCTTCTG TTGATGTTTGACTGTTTGTGA-TAMRA 3′. Each PCR mixture contained around 10,000 copies of genomic DNA, 0.8 μL TREC or RAG2 probe, 0.4 μL each sjTREC or RAG2 primer, 0.4 μL ROX Reference Dye (50 ×) and 10 μL Premix Ex Taq (2 ×) (TaKaRa, China). The thermal cycling conditions were 2s at 95°C, and 50 cycles of 1s at 94°C and 20s at 60°C. A standard curve based on a plasmid preparation containing the sjTREC target sequence was plotted, and sjTREC values for samples were calculated using the StepOne software (Applied Biosystems, USA). Samples were analyzed in triplicates, and the median was calculated. The percentage of the copies of the sjTREC gene to that of the RAG2 gene was defined as the sjTREC level.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) and categorical ones as number of cases (percentage). K–S test was used for normality test. Pearson or Spearman correlation analysis was applied for correlation analysis of two factors. Differences in numerical data were compared by the independent t test or Mann–Whitney U test. Differences in categorical data were compared using the chi-square test or Fisher exact test. The changes of each individual's thymic output and lymphoctyte subsets at different time points were assessed by general linear models repeated-measure analysis using the statistical tests within subject contrasts. The impact of TH on the recovery of thymic output and lymphocyte subsets within individual patients was evaluated by general linear models repeated-measure analysis using between-subject contrasts adjusted for the presence of TH. Data analysis was performed using SPSS21 statistical software. A value of p < 0.05 was considered to be significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by National Natural Science Foundation of China (81170485, 81170488, 81370657, 81470328), Key Projects of Health Department of Jiangsu Province (K201108), Jiangsu Province's Medical Elite Program (RC2011169), National Public Health Grand Research Foundation (201202017), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (JX10231801), Program for Development of Innovative Research Teams in the First Affiliated Hospital of Nanjing Medical University, Project of National Key Clinical Specialty. National Science & Technology Pillar Program (2014BAI09B12), and Project funded by Jiangsu Provincial Special Program of Medical Science (BL2014086).
References
- 1.Pearse G. Histopathology of the thymus. Toxicol Pathol 2006; 34:515-47; PMID:17067942; http://dx.doi.org/ 10.1080/01926230600978458 [DOI] [PubMed] [Google Scholar]
- 2.Choyke PL, Zeman RK, Gootenberg JE, Greenberg JN, Hoffer F, Frank JA. Thymic atrophy and regrowth in response to chemotherapy. AJR Am J Roentgenol 1987; 149:269-72; PMID:3496749; http://dx.doi.org/ 10.2214/ajr.149.2.269 [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Hill CA, Cangir A, Sullivan MP. Thymic rebound after treatment of childhood tumors. AJR Am J Roentgenol 1980; 135:151-6; PMID:6771982; http://dx.doi.org/ 10.2214/ajr.135.1.151 [DOI] [PubMed] [Google Scholar]
- 4.Zhen Z, Sun X, Xia Y, Ling J, Cai Y, Wang J, Guan Z. Clinical analysis of thymic regrowth following chemotherapy in children and adolescents with malignant lymphoma. Jpn J Clin Oncol 2010; 40:1128-34; PMID:20693549; http://dx.doi.org/ 10.1093/jjco/hyq149 [DOI] [PubMed] [Google Scholar]
- 5.Yarom N, Zissin R, Apter S, Hertz M, Rahimi-Levene N, Gayer G. Rebound thymic enlargement on CT in adults. Int J Clin Pract 2007; 61:562-8; PMID:17263694; http://dx.doi.org/ 10.1111/j.1742-1241.2006.00950.x [DOI] [PubMed] [Google Scholar]
- 6.Hara M, McAdams HP, Vredenburgh JJ, Herndon JE, Patz EF Jr. Thymic hyperplasia after high-dose chemotherapy and autologous stem cell transplantation. AJR Am J Roentgenol 1999; 173:1341-4; PMID:10541115; http://dx.doi.org/ 10.2214/ajr.173.5.10541115 [DOI] [PubMed] [Google Scholar]
- 7.Sehbai AS, Tallaksen RJ, Bennett J, Abraham J. Thymic hyperplasia after adjuvant chemotherapy in breast cancer. J Thorac Imaging 2006; 21:43-6; PMID:16538156; http://dx.doi.org/ 10.1097/01.rti.0000185143.17436.f9 [DOI] [PubMed] [Google Scholar]
- 8.Ferrando-Martínez S, Ruiz-Mateos E, Hernández A, Gutiérrez E, Rodríguez-Méndez Mdel M, Ordoñez A, Leal M. Age-related deregulation of naïve T cell homeostasis in elderly humans. Age (Dordr) 2011; 33:197-207; PMID:20700658; http://dx.doi.org/7800006 10.1007/s11357-010-9170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al.. AGE, THYMOPOIESIS, AND CD4. N Engl J Med 1995; 332:143-9; PMID:7800006; http://dx.doi.org/ 10.1056/NEJM199501193320303 [DOI] [PubMed] [Google Scholar]
- 10.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, et al.. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 2005; 115:930-9; PMID:15776111; http://dx.doi.org/ 10.1172/JCI200522492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naïve CD4+ T-cell subsets. Blood 2009; 113:769-74; PMID:18583570; http://dx.doi.org/ 10.1182/blood-2008-02-139154 [DOI] [PubMed] [Google Scholar]
- 12.Sandgaard KS, Lewis J, Adams S, Klein N, Callard R. Antiretroviral therapy increases thymic output in children with HIV. AIDS 2014; 28:209-14; PMID:24072195; http://dx.doi.org/ 10.1097/QAD.0000000000000063 [DOI] [PubMed] [Google Scholar]
- 13.Guazzi V, Aiuti F, Mezzaroma I, Mazzetta F, Andolfi G, Mortellaro A, Pierdominici M, Fantini R, Marziali M, Aiuti A. Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles. Clin Exp Immunol 2002; 129:346-53; PMID:12165093; http://dx.doi.org/ 10.1046/j.1365-2249.2002.01893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resino S, Seoane E, Perez A, Ruiz-Mateos E, Leal M, Munoz-Fernandez MA. Different profiles of immune reconstitution in children and adults with HIV-infection after highly active antiretroviral therapy. BMC Infect Dis 2006; 6:112; PMID:16839416; http://dx.doi.org/ 10.1186/1471-2334-6-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakim FT, Cepeda R, Kaimei S, Mackall CL, McAtee N, Zujewski J, Cowan K, Gress RE. Constraints on CD4 recovery postchemotherapy in adults: thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood 1997; 90:3789-98; PMID:9345067 [PubMed] [Google Scholar]
- 16.Vedel SJ, Tholstrup D, Kolte L, Gaardbo J, Ryder LP, Ersbøll A, Albrecht-Beste E, Jurlander J, Nielsen JO, Nielsen SD. Limited impact of the thymus on immunological recovery during and after chemotherapy in patients with diffuse large B-cell lymphoma. Scand J Immunol 2009; 69:547-54; PMID:19439016; http://dx.doi.org/ 10.1111/j.1365-3083.2009.02252.x [DOI] [PubMed] [Google Scholar]
- 17.Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000; 355:1875-81; PMID:10866444; http://dx.doi.org/ 10.1016/S0140-6736(00)02293-5 [DOI] [PubMed] [Google Scholar]
- 18.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol 2009; 30:366-73; PMID:19540807; http://dx.doi.org/ 10.1016/j.it.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur J Clin Invest 2005; 35:380-7; PMID:15948899; http://dx.doi.org/ 10.1111/j.1365-2362.2005.01499.x [DOI] [PubMed] [Google Scholar]
- 20.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007; 19:318-30; PMID:18023361; http://dx.doi.org/ 10.1016/j.smim.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Povoleri GA, Scottà C, Nova-Lamperti EA, John S, Lombardi G, Afzali B. Thymic versus induced regulatory T cells who regulates the regulators. Front Immunol 2013; 4:169; PMID:23818888; http://dx.doi.org/ 10.3389/fimmu.2013.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis IR, Glazer GM, Bookstein FL, Gross BH. The thymus: reexamination of age-related changes in size and shape. AJR Am J Roentgenol 1985; 145:249-54; PMID:3875220; http://dx.doi.org/ 10.2214/ajr.145.2.249 [DOI] [PubMed] [Google Scholar]
- 23.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity 1999; 10:569-75; PMID:10367902; http://dx.doi.org/ 10.1016/S1074-7613(00)80056-4 [DOI] [PubMed] [Google Scholar]
- 24.Boehm T, Swann JB. Thymus involution and regeneration: two sides of the same coin? Nat Rev Immunol 2013; 13:831-8; PMID:24052146; http://dx.doi.org/ 10.1038/nri3534 [DOI] [PubMed] [Google Scholar]
- 25.Aquino VM, Douek DC, Berryman B, Johnson M, Jain VK, Collins RH. Evaluation of thymic output by measurement of T-cell-receptor gene rearrangement excisional circles (TREC) in patients who have received fludarabine. Leuk Lymphoma 2003; 44:343-8; PMID:12688355; http://dx.doi.org/ 10.1080/1042819021000029696 [DOI] [PubMed] [Google Scholar]
- 26.Sun DP, Ding CY, Wang L, Liang JH, Fan L, Wu YJ, Tian T, Li TN, Xu W, Li JY. Thymic hyperplasia following chemotherapy in adults with lymphoma: F-fluorodeoxyglucose positron emission tomography/computed tomography findings and correlation with T cell repopulation. Leuk Lymphoma 2015; 56:2344-9; PMID:25407653; http://dx.doi.org/ 10.3109/10428194.2014.986480 [DOI] [PubMed] [Google Scholar]
- 27.Rubio A, Martínez-Moya M, Leal M, Franco JM, Ruiz-Mateos E, Merchante E, Sánchez-Quijano A, Lissen E. Changes in thymus volume in adult HIV-infected patients under HAART: correlation with the T-cell repopulation. Clin Exp Immunol 2002; 130:121-6; PMID:12296862; http://dx.doi.org/ 10.1046/j.1365-2249.2002.01950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, Briel J, Luznik L, Smith BD, Levitsky H, et al.. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood 2011; 117:608-17; PMID:20935254; http://dx.doi.org/ 10.1182/blood-2010-04-277939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Zhao H, Hao Y, Song C, Han J, Zhang J, Gao G, Han N, Yang D, Li Y, et al.. Excessive conversion and impaired thymic output contribute to disturbed regulatory T-cell homeostasis in AIDS patients with low CD4 cell counts. AIDS 2013; 27:1059-69; PMID:23299175; http://dx.doi.org/ 10.1097/QAD.0b013e32835e2b99 [DOI] [PubMed] [Google Scholar]
- 30.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naïve Tregs. Clin Exp Immunol 2009; 155:44-52; PMID:19016807; http://dx.doi.org/ 10.1111/j.1365-2249.2008.03803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, Fritz B, Suri-Payer E, Wildemann B. Interferon β-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naïve regulatory T cells. Arch Neurol 2008; 65:1434-9; PMID:19001161; http://dx.doi.org/ 10.1001/archneur.65.11.1434 [DOI] [PubMed] [Google Scholar]
- 32.Ferrando-Martínez S, Franco JM, Ruiz-Mateos E, Hernández A, Ordoñez A, Gutierrez E, Leal M. A reliable and simplified sj β-TREC ratio quantification method for human thymic output measurement. J Immunol Methods 2010; 352:111-7; PMID:19919841; http://dx.doi.org/19008454 10.1016/j.jim.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 33.Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, Sousa AE. IL-7 sustains CD31 expression in human naïve CD4+ T cells and preferrentially expands the CD31+ subset in a PI3K-dependent manner. Blood 2009; 113:2999-3007; PMID:19008454; http://dx.doi.org/ 10.1182/blood-2008-07-166223 [DOI] [PubMed] [Google Scholar]
- 34.Lario M, Muñoz L, Ubeda M, Borrero MJ, Martínez J, Monserrat J, Díaz D, Alvarez-Mon M, Albillos A. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol 2013; 59:723-30; PMID:23742913; http://dx.doi.org/ 10.1016/j.jhep.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 35.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural Naïve CD4+CD25+ CD127low Regulatory T Cell (Treg) Development andFunction Are Disturbed in Multiple Sclerosis Patients: Recovery of Memory Treg Homeostasis during Disease Progression. J Immunol 2008; 180:6411-20; PMID:18424765; http://dx.doi.org/ 10.4049/jimmunol.180.9.6411 [DOI] [PubMed] [Google Scholar]
- 36.Tang TT, Zhu ZF, Wang J, Zhang WC, Tu X, Xiao H, Du XL, Xia JH, Dong NG, Su W, et al.. Impaired thymic export and apoptosis contribute to regulatory T-cell defects in patients with chronic heart failure. PLoS One 2011; 6:e24272; PMID:21935395; http://dx.doi.org/ 10.1371/journal.pone.0024272 [DOI] [PMC free article] [PubMed] [Google Scholar]


