ABSTRACT
A novel approach to immunotherapy is the activation of toll-like receptor 8 (TLR8). Motolimod, a selective TLR8 agonist can act in concert with approved immunotherapies to sensitize T cells and augment natural killer (NK) cell function. Despite treatment with chemotherapeutic agents and advance disease, cancer patients remain sensitive to motolimod.
Introduction
An emerging paradigm in the treatment of cancer is to harness the individual's immune system to actively participate in the eradication of tumor cells. When successful, the development of an adaptive immune response to tumor-expressed antigens results in long-term tumor cell surveillance and translates into a durable clinical response. One promising pathway to evoke an innate immune response is through TLR8 activation. Activation of TLR8 in endosomal compartments of monocytes and myeloid dendritic cells (mDC) stimulates the release of distinct inflammatory mediators, including Th1-polarizing cytokines.1-3 The pathway increases expression of costimulatory molecules on antigen presenting cells (APC), facilitating more effective presentation of tumor-expressed antigens to responsive T cells (Fig. 1). TLR8 agonists also enhance NK cell function, leading to an augmented antibody-dependent cell cytotoxicity (ADCC) and the production of IFNγ.4
Figure 1.
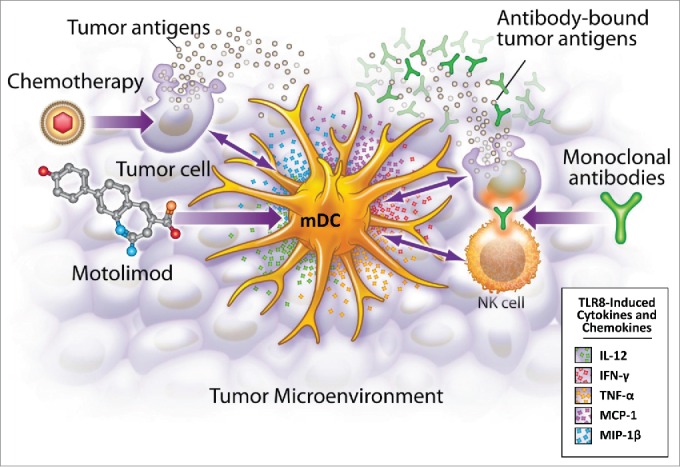
Motolimod is a selective TLR8 agonist that activates myeloid dendritic cells (mDC), resulting in the production of mediators that recruit and activate other inflammatory cells in the tumor microenvironment. Additionally, motolimod increases NK mediated antibody-dependent cell-mediated cytotoxicity (ADCC), and augments the presentation of tumor-derived antigens to the adaptive immune system.
Motolimod (VTX2337) is a potent and selective TLR8 agonist in clinical development as an immunotherapy for multiple cancer types. This therapeutic approach relies on robust activation of the immune system, yet there is the perception that cancer patients have weak immune systems due to repeat cycles of immunosuppressive chemotherapy,5 advanced age and/or deregulated immune function related to the malignancy. For example, tumors can exert negative effects on the immune system through the release of either soluble mediators6,7 or expression of immunomodulatory cell surface antigens.8,9 To address these concerns, a series of investigations, including clinical studies, have fully characterized motolimod activity in advanced-stage cancer patients.
TLR8 activation in advanced cancer patients
In this study,10 we show that motolimod activation of peripheral blood monocytes (PBMCs) from healthy volunteers induces a specific set of cytokines and chemokines. Consistent with the hypothesis that TLR8 activation facilitates the development of tumor-directed adaptive immune responses, motolimod induced Th1 polarizing cytokines, IL-12p70, TNF-α and IFNγ, in addition to an array of other cytokines and chemokines. To translate motolimod in vitro activity into a meaningful measure of immune activation, cynomolgus monkeys were administered escalating dose levels of motolimod. Generally, plasma analytes with the greatest dynamic response to increasing doses of motolimod were a subset of analytes induced to high levels in TLR8 activated human blood. While not all mediators induced in motolimod activated PBMC appear in plasma, this was expected. The production, consumption, and clearance of cytokines/chemokines in vivo is a highly dynamic process, leading to large changes in plasma levels over time. However, the collective results from nonclinical studies provided a framework to assess qualitative and quantitative features of the motolimod pharmacodynamic response in humans.
In the initial clinical study of motolimod in late-stage cancer patients doses of 2.0, 2.8 and 3.9 mg/m2 induced dose-related increases in plasma levels of multiple cytokines and chemokines. Most of these responsive mediators had been identified as biomarkers of motolimod activity in human PBMCs and motolimod-dosed cynomolgus monkeys. In a subsequent study, a 2.5 mg/m2 motolimod dose was given to healthy volunteers to characterize both the pharmacokinetic and pharmacodynamic response to motolimod. The 2.5 mg/m2 dose was considered safe, yet pharmacologically active. In these subjects, motolimod induced significant changes in the same array of analytes that were elevated in the plasma of advanced-stage cancer patients, with few exceptions. Overall, the magnitude of the increase in mediator levels in late-stage cancer patients administered 2.0 and 2.8 mg/m2 were comparable to healthy volunteers who received a dose of 2.5 mg/m2. For cancer patients who received the 3.9 mg/m2 dose of motolimod, the mediator response was considerably more robust than for healthy volunteers dosed at 2.5 mg/m2, indicating the response in cancer patients did not plateau.
The pharmacokinetic profile for healthy volunteers given a 2.5 mg/m2 motolimod dose was highly comparable to that observed in cancer patients at doses of 2.0–2.8 mg/m2. Therefore, changes in metabolism and subsequent clearance of motolimod due to concomitant medications or the poor health of cancer patients were not a complication when comparing the level of immune activation to that of healthy volunteers.
Our study concludes that late-stage cancer patients are highly sensitive to TLR8 activation by motolimod. As predicted by nonclinical studies and confirmed by comparison to the response in healthy volunteers, tumor-mediated immune suppression, increased age, and prior treatment history with cytotoxic agents, do not moderate the TLR8 response. This demonstration of robust immune activation in cancer patients has led to the initiation of additional clinical studies designed to determine if motolimod can augment the effectiveness of some standard of care oncology treatments. TLR8 activation is expected to enhance tumor-directed immune responses to “antigenic” tumor cell death mediated by anthracyclines, which are commonly used in the treatment of ovarian cancer. TLR8 activation of NK cells can augment the ADCC activity of approved mAb therapies such as cetuximab, which is used in the treatment of squamous cell carcinoma of the head and neck (SCCHN). Thus, motolimod is being assessed in randomized, placebo controlled Phase 2 clinical studies of ovarian cancer and SCCHN to determine if it can increase the effectiveness of these standard treatments.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11(5):373-84; PMID:20404851; http://dx.doi.org/ 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- 2.Ghosh TK, Mickelson DJ, Fink J, Solberg JC, IngleWeld JR, Hook D, Gupta SK, Gibson S, Alkan SS. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol 2006; 243(1):48-57; PMID:17250816; http://dx.doi.org/ 10.1016/j.cellimm.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005; 174:1259-68; PMID:15661881; http://dx.doi.org/ 10.4049/jimmunol.174.3.1259 [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M, Suni M, Maino VC, Henderson KE, Howbert JJ et al.. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res 2012; 18:499-509; PMID:22128302; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1625 [DOI] [PubMed] [Google Scholar]
- 5.Harris J, Sengar D, Stewart T, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer 1976; 37(2 Suppl):1058-69; PMID:766953; http://dx.doi.org/ 10.1002/1097-0142(197602)37:2+%3c1058::AID-CNCR2820370813%3e3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- 6.Nakamura I, Shibata M, Gonda K, Yazawa T, Shimura T, Anazawa T, Suzuki S, Sakurai K, Koyama Y, Ohto H et al.. Serum levels of vascular endothelial growth factor are increased and correlate with malnutrition, immunosuppression involving MDSCs and systemic inflammation in patients with cancer of the digestive system. Oncology letters 2013; 5(5):1682-6; PMID:23761834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO. Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol 2004; 165(6):1853-63; PMID:15579430; http://dx.doi.org/ 10.1016/S0002-9440(10)63238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014; 20(6):607-15; PMID:24793239; http://dx.doi.org/ 10.1038/nm.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8):793-800; PMID:12091876 [DOI] [PubMed] [Google Scholar]
- 10.Dietsch GN, Randall TD, Gottardo R, Northfelt DW, Ramanathan RK, Cohen PA, Manjarrez KL, Newkirk M, Bryan JK, Hershberg RM. Late Stage Cancer Patients Remain Highly Responsive to Immune Activation by the Selective TLR8 Agonist Motolimod (VTX 2337). Clin Cancer Res 2015; 21(24):5445-52; PMID: 26152744; http:/dx.doi.org/ 10.1158/1078-0432.CCR-15-0578. [DOI] [PubMed] [Google Scholar]


