ABSTRACT
Promising cancer immunotherapeutics depend on mobilization of cytotoxic T cells across tumor vascular barriers through mechanisms that are poorly understood. Recently, we discovered that the CXCR3 chemokine receptor uniquely functions as the master-regulator of cytotoxic CD8+ T cell extravasation and tumor control despite the multiplicity of chemokines available in the tumor landscape.
KEYWORDS: Adoptive T cell transfer, cancer immunotherapy, chemokine receptor, CXCR3, T cell trafficking, tumor vessels
Abbreviations
- CCR2
CC chemokine receptor 2
- CCR5
CC chemokine receptor 5
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- CXCR3
CXC chemokine receptor 3
- DC
dendritic cell
- DPPIV
dipeptidylpeptidase IV
- PD-1
programmed death-1
- PD-L1
programmed death-1 ligand
- RGS1
regulator of G protein signaling 1
Cancer immunotherapy is enjoying an unprecedented renaissance after decades of unrealized potential. This transformation is fueled by recognition that cytotoxic CD8+ T cells are necessary for the efficacy of conventional cancer treatments (i.e., chemotherapy and radiation),1 together with promising clinical results for therapeutics that relieve tumor-mediated immune suppression.2 It is now feasible to ramp up the T cell arm of tumor immunity in patients through vaccination, blockade of immune checkpoints (i.e., cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1)/PD-1 ligand (PD-L1)), or adoptive transfer of billions of patient-derived T cells.2,3 However, observations that only a subset of cancer patients experience long-term benefits suggest that additional ill-defined factors limit the efficacy of T cell-based therapies.2,3
One open area of investigation centers on the mechanisms that govern the delivery of blood-borne T cells across tumor vascular checkpoints, a physical barrier that must be breached for T cells to deliver lethal hits to tumor targets. Prior correlative studies raised the specter that redundant chemokine receptor/chemokine pairs might participate in T cell extravasation in patient tumors, such as the CXC chemokine receptor CXCR3 (cognate ligands CXCL9/10/11), CC chemokine receptor CCR5 (CCL3/4/5), and CCR2 (CCL2).3-5 However, the precise function of these individual chemokine receptors during T cell trafficking at the vascular interface has remained obscure since they could alternatively impact intratumoral accumulation by regulating T cell retention, survival, and proliferation in situ.4,5 Further clouding this issue are reports that cytotoxic T cells can bypass prototypical chemokine requirements for adhesion within vessel walls during extravasation in non-cancerous tissues.5,6
To address the question of chemokine receptor requirements for tumor immunity, our studies focused on CXCR3, CCR5, and CCR2 because of their correlation with T cell accumulation and cancer patient survival.5 We found that both human and murine cytotoxic CD8+ T cells are equipped with all three functional chemokine receptors.5 Similarly, human and murine melanoma contain stromally derived chemokines for each of these receptors (CXCL9/10, CCL5, and CCL2) as measured by standard proteomic profiling.5 We further established using an antibody-conjugated bead-based detection assay that these chemokines are displayed on murine tumor vessel walls, a prerequisite for interactions with circulating T cells5 (Fig. 1). It remains to be determined whether the spatial heterogeneity observed for chemokine distribution in this assay reflects “hotspots” of chemokine availability that create high-throughput hubs for T cell trafficking in tumor vessels.
Figure 1.
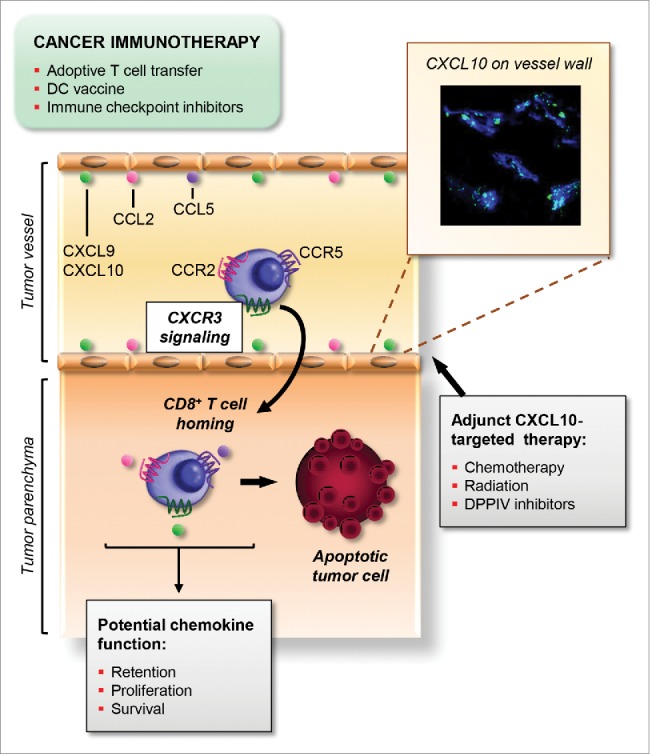
Schematic outline of the non-redundant role of CXCR3 for trafficking of cytotoxic T cells across tumor vessels. The success of T cell-based cancer immunotherapies including adoptive T cell transfer, dendritic cell (DC) vaccines, and immune checkpoint inhibitors, hinges on efficient trafficking of blood-borne cytotoxic CD8+ T cells at tumor vascular checkpoints. Functional chemokine receptors including CXCR3, CCR2, and CCR5, are present on circulating tumor-reactive T cells. Moreover, cognate chemokine ligands (CXCL9/10, CCL2, and CCL5) decorate vessel walls (e.g., inset, CXCL10-detection beads [green] within CD31+murine melanoma vessels [blue]). Despite this rich chemokine landscape, CXCR3 has emerged as the master-regulator of cytotoxic CD8+ T cell trafficking in the intravascular compartment. CXCR3 engagement by CXCL9/10 triggers firm adhesion to vascular endothelium and subsequent migration into the underlying tumor parenchyma. By contrast, CCR2 and CCR5 are dispensable for T cell extravasation although they, together with CXCR3, could potentially regulate T cell retention, proliferation, or survival in the intratumoral compartment. Altogether, this CXCR3-dependent process allows CD8+T cells to initiate apoptotic pathways leading to tumor cell destruction. This unique role for CXCR3 suggests that the efficacy of cancer immunotherapy could be enhanced by adjuvant treatments (i.e., chemotherapy, radiation, and dipeptidylpeptidase IV [DPPIV] inhibitors) that boost CXCL10 availability at vascular checkpoints.
We used live-imaging microscopy and short-term homing assays to interrogate the chemokine receptor requirements for T cell trafficking in tumors. Visualization of adoptively transferred cytotoxic CD8+ T cells in real-time revealed that signaling through a single chemokine receptor, CXCR3, is indispensable for stable adhesion at the vessel wall in murine melanoma.5 Surprisingly, loss of CXCR3 or its ligands CXCL9/10 blocks T cell trafficking to the same extent as global inhibition of Gαi protein signaling by pertussis toxin, while CCR5 and CCR2 are non-essential for homing at tumor sites5 (Fig. 1). The requirement for CXCR3 is not unique to adoptively transferred cells as it was also shared by the endogenous T cell pool in murine melanoma. We further showed that CXCR3 dominance is maintained for trafficking of adoptively transferred human CD8+ effector T cells in human melanoma xenografts.5 Notably, we discovered that expression of Cxcr3 by cytotoxic T cells is absolutely required for tumor control and survival in a preclinical murine model of adoptive T cell transfer therapy, whereas mice treated with Cxcr3-deficient T cells fared no better than untreated cohorts.5
These studies reveal a unique role for CXCR3 and its ligands in cancer immunotherapy that could not be predicted from genomic or proteomic profiling of the tumor microenvironment. While our data do not exclude a role for CCR5, CCR2, or CXCR3 for accrual of T cells in the tumor parenchyma (Fig. 1), these activities are insufficient for therapeutic efficacy in the absence of CXCR3-guided trafficking in the intravascular compartment.5 The molecular basis for our paradoxical observations that intravascular CCR2 supports intratumoral extravasation of monocytes, but not T cells, must still be explored.5 Answers to this conundrum may lie in cell-specific usage of post-translationally modified CCL2 within tumors which favors recruitment of immune-suppressive myeloid cells,3 but could also suggest differential cell-intrinsic regulation of Gαi protein signaling. Provocatively, differential expression of negative regulators of Gαi protein signaling (e.g., Rgs1) can be found in T cells compared with macrophages which may dictate chemokine receptor sensitivity.5
Our findings raise the possibility that adjuvant targeting of the CXCR3/CXCR3 ligand axis could be a viable option to improve cancer immunotherapy (Fig. 1). It is unlikely that CXCR3 expression will need to be augmented in a therapeutic setting given its uniformly high expression by cytotoxic CD8+ T cells.5 A benefit may instead come from boosting CXCR3 ligands in the tumor locale to enhance T cell extravasation, thereby overcoming the variability in the chemokine landscape observed among patients.3-5 Promising strategies already in clinical use have recently been shown to enhance CXCL10 production in murine tumors including chemotherapeutics and radiation that trigger type I-interferons.7,8 Reports of post-translational inactivation of CXCL10 within tumors by dipeptidylpeptidase IV (DPPIV) suggest that both chemokine function as well as availability are important considerations during therapeutic targeting.9 However, manipulating the chemokine microenvironment is not without risk. CXCR3 expression by tumor cells is implicated in metastasis10 while CXCR3 is also expressed by immunosuppressive regulatory T cells and macrophages that accelerate tumor outgrowth.4,5 Thus, a major challenge moving forward will be to exploit the antitumorigenic function of CXCR3 in cytotoxic T cells without invoking pro-tumorigenic activities of CXCR3 ligands that unintentionally promote spread from the tumor nidus.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the NIH (CA79765 and AI082039 to S.S. Evans; T32 CA085183 and 1F30 CA177210 to M.E. Mikucki; CA069212 to A.D. Luster; CA184433 to J. Frelinger; CA127475 to T. Gajewski; CA158318–01A1 and the RPCI-UPCI Ovarian Cancer SPORE P50CA159981–01A1 to K. Odunsi); the Joanna M. Nicolay Foundation and the University at Buffalo Mark Diamond Research Fund to M.E. Mikucki; the Jennifer Linscott Tietgen Family Foundation to S.S. Evans and J.J. Skitzki; and the Roswell Park/Wilmot Collaborative Grant to S.S. Evans and J. Frelinger.
References
- 1.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/ 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 2.Lizee G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med 2013; 64:71-90; PMID:23092383; http://dx.doi.org/ 10.1146/annurev-med-112311-083918 [DOI] [PubMed] [Google Scholar]
- 3.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015; 348(6230):74-80; PMID:25838376; http://dx.doi.org/ 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 4.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014; 32:659-702; PMID:24655300; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120145 [DOI] [PubMed] [Google Scholar]
- 5.Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, Ku AW, Frelinger JG, Odunsi K, Gajewski TF et al.. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 2015; 6:7458; PMID:26109379; http://dx.doi.org/ 10.1038/ncomms8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walch JM, Zeng Q, Li Q, Oberbarnscheidt MH, Hoffman RA, Williams AL, Rothstein DM, Shlomchik WD, Kim JV, Camirand G et al.. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest 2013; 123(6):2663-71; PMID:23676459; http://dx.doi.org/ 10.1172/JCI66722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C et al.. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014; 20(11):1301-9; PMID:25344738; http://dx.doi.org/ 10.1038/nm.3708 [DOI] [PubMed] [Google Scholar]
- 8.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 2015; 33:445-74; PMID:25622193; http://dx.doi.org/ 10.1146/annurev-immunol-032414-112043 [DOI] [PubMed] [Google Scholar]
- 9.Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, Albert ML. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat Immunol 2015; 16(8):850-8; PMID:26075911; http://dx.doi.org/ 10.1038/ni.3201 [DOI] [PubMed] [Google Scholar]
- 10.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res 2011; 71(4):1214-8; PMID:21212413; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3277 [DOI] [PubMed] [Google Scholar]


