ABSTRACT
Renal cell carcinoma (RCC) is an immunogenic tumor for which immunotherapeutic approaches could be associated with clinically relevant responses. It was recently shown, that induction of T-cell responses against multiple tumor-associated antigen (TAA) epitopes results in prolonged overall survival in RCC patients. In 2003–2005, we performed a phase I/II trial testing an mRNA-based vaccine formulation consisting of a mixture of in vitro transcribed RNA coding for six different TAAs (MUC1, CEA, Her2/neu, telomerase, survivin, MAGE-A1) in 30 metastatic RCC (mRCC) patients. In the first 14 patients, vaccinations were applied i.d. on days 0, 14, 28, and 42. In the consecutive 16 patients, an intensified protocol consisting of i.d. injections (daily on days 0–3, 7–10, 28, and 42) was used. After the respective induction periods, patients in both cohorts were vaccinated monthly until tumor progression. At survival update performed in July 2015, one of the 30 patients was still alive. One patient was lost to follow-up. Median survival of 24.5 mo (all patients) and 89 mo (favorable risk patients) exceeded predicted survival according to Memorial Sloan Kettering Cancer Center (MSKCC) risk score. Impressively, long-term survivors displayed immunological responses to the applied antigens while vice versa no patient without detectable immune response had survived more than 33 mo. The current survival update shows a clear correlation between survival and immunological responses to TAAs encoded by the naked mRNA vaccine. This is one of the first vaccination studies and the only RNA trial that reports on safety and efficacy after a follow-up of more than 10 y.
KEYWORDS: Cancer, dendritic cell, human, immunotherapy, phase I/II, renal cell carcinoma, RNA, survival, trial, vaccine
Abbreviations
- ADR
Adrenal
- BRA
Brain
- CD
Cluster of Differentiation
- CEA
Carcinoembryonic Antigen
- CT
Computed Tomography
- CTLA-4
Cytotoxic T-lymphocyte-associated Protein 4
- CTL
Cytotoxic T Lymphocyte
- DC
Dendritic Cell
- EGFP
Enhanced Green Fluorescent Protein
- ELISpot
Enzyme-Linked ImmunoSpot
- FDA
Food and Drug Administration
- GM-CSF
Granulocyte-macrophage Colony-stimulating Factor
- HEP
Hepatic
- Her2
Human Epidermal Growth Factor Receptor 2
- HLA
Human Leukocyte Antigen
- i.d.
Intradermally
- IFN
Interferon
- IL
Interleukin
- ITT
Intention-to-treat
- LYM
lymph nodes
- MAGE
Melanoma-associated Antigen
- mo, Month; MSKCC
Memorial Sloan Kettering Cancer Center
- mTOR
Mammalian Target Of Rapamycin
- MUC1
Mucin 1
- NK-cell
Natural Killer Cell
- No
Number
- OS
Overall Survival
- OSS
Osseos
- OTH
Others
- PBMC
Peripheral Blood Mononuclear Cell
- PBS
Phosphate Buffered Saline
- PD
Progressive Disease
- PD-1
Programmed Cell Death Protein 1
- PD-L1
Programmed Death-ligand 1
- PLE
Pleura
- PR
Partial Response
- PUL
Pulmonary
- RCC
Renal Cell Carcinoma
- RECIST
Response Evaluation Criteria In Solid Tumors
- RIG-I
Retinoic Acid-Inducible Gene-I
- RNA
Ribonucleic Acid
- SD
Stable Disease
- TAA
Tumor-Associated Antigen
- TLR
Toll Like Receptor
- VEGF
Vascular Endothelial Growth Factor
- yr
Year
Introduction
Anticancer immunotherapies have now been performed for a century. Although considerable progress has been made in understanding the biology of immune cells involved in induction of therapeutic immunity, most vaccination strategies so far have failed to demonstrate positive correlation between the induction of specific immune responses and clinical benefit in patients.
Dendritic cells (DC) represent potent antigen-presenting cells and may have the unique ability to induce, maintain, and regulate primary immune responses.1 These are not restricted to combat infection but also include anticancer immune responses. For example, an infiltration of tumors by DC was demonstrated to be of clinical significance in terms of favorable prognosis for cancers patients.2-4 Furthermore defects in DC were shown to enhance tumor progression and impair effectiveness of chemotherapy.5-8 Portrayal of their potential in inducing robust immune responses including anticancer immune surveillance gave rise to the development of DC-based immunotherapies. These are characterized by being mainly well tolerated and have been proven to induce tumor-specific immune responses.9-11 Multiple approaches were employed in previous studies including ex vivo loading of autologous DC with TAAs (peptide- or protein pulsing, transfection with TAA-coding mRNA, tumor lysates, etc.). Another technique is the induction of anticancer immune responses via in vivo loading of DC using TAA-derived peptides or TAA-coding nucleic acids. Because it is a safe approach, we chose to evaluate the impact of an experimental mRNA-based vaccine that is directly injected intradermally.12,13 The deployment of TAA-coding in vitro-transcribed mRNA allows the application of different antigens and enables induction of both polyclonal CD4+ and CD8+ restricted T cells recognizing multiple epitopes.14 In addition, in contrast to a peptide approach, mRNA vaccines injected directly into the dermis are not limited by HLA-types. They furthermore preclude escape due to antigen loss, allow frequent vaccinations thereby boosting immune responses and avoid the often laborious DC generation or culture in vitro. At the same time mRNA can be recognized by pattern recognition receptors and may be designed to be self-adjuvanting, a property peptide- and protein-based vaccines lack.15 The binding to specific ubiquitously expressed RNA sensors, leads to the activation of different cells in the dermis and thereby to a strong induction of innate immunity. As the uptake of mRNA is not cell specific, different cells in the dermis can contribute to the antigen production and support its cross-presentation by professional antigen-presenting cells, which seems to be very important for efficient induction of immune response.16,33
Although several immunotherapeutic trials employing vaccines in cancer patients are currently recruiting or have been completed, clinical benefit rarely met expectations.17 Many questions and problems are still unsolved including the repertory of the TAAs, the format of the antigen, the way of application, frequency of delivery, and the type of the adjuvant. Despite the fact that antitumor responses have been observed frequently in patients treated with immunotherapeutic approaches, immunological monitoring and correlation with clinical benefit remained challenging not only due to the fact that most patients enrolled in clinical trials were heavily pretreated and/or suffered from late stage cancer. Nevertheless with sipuleucel-T (Provenge®) in 2010, the first cellular vaccine for the treatment of cancer (prostate cancer) was approved by the FDA.18 Hereupon in 2012, Walter and colleagues published promising data for the first time demonstrating a positive correlation between the induction of immunological responses against multiple tumor-associated peptides and clinical efficacy. This peptide-based vaccine (IMA901) is based on T-cell induction by in vivo loading of DC residing at the injection site and its immunogenicity was found to correlate with disease control in patients suffering from advanced kidney cancer.19
RCC, including renal pelvis represents a relatively rare cancer and accounts for 3.8% of newly diagnosed cancer patients per year. About 64% of patients are diagnosed at the local stage. However, despite development of various new treatment options only 12% of patients diagnosed at metastasized stage survive 5 y (National Cancer Institute, http://seer.cancer.gov/statfacts/html/kidrp.html, July 2014).
Before December 2005 when with sorafenib the first targeted therapy was approved by the FDA for treatment of advanced RCC, treatment options were limited to a few catatonic drugs or recombinant IL-2 (Interleukin 2) and Interferon (IFN)-α. The currently marketed therapies for advanced RCC include the tyrosine kinase inhibitors sunitinib, sorafenib, pazopanib, and axitinib, the mTOR inhibitors temsirolimus and everolimus, cytokine therapy with IFNα or IL-2, and the anti-VEGF monoclonal antibody bevacizumab.20 All comprise limitations due mainly to toxicities and mechanisms of drug resistance indicating an urgent need for the development of tolerable treatment options.
Between August 2003 and November 2005, we had performed a phase I/II study to test feasibility and safety of an mRNA-based vaccine formulation in patients with stage IV RCC.21 We here provide an update on long-term survival and correlative analyses of immunological and clinical responses. It is of note that this study was performed in the pre-tyrosine kinase inhibitor era.
Results
In the years 2003 to 2005, we had performed a phase I/II vaccination trial including patients suffering from advanced RCC. Patient characteristics at entry are displayed in . 30 patients were enrolled, 14 in cohort A and 16 in cohort B. Mean age of all patients was 63.5 y and ranged from 36 to 79 y. For the prediction of median survival patients were categorized in risk groups according to the MSKCC risk score, which was established in the pre-tyrosine kinase inhibitor era.22 11 patients of our study population were favorable-risk and 19 intermediate-risk patients. All 30 patients had undergone nephrectomy and some had received cytokine therapy, radiation or resection of metastases as displayed in . All patients suffered from stage IV RCC at enrollment with metastases at numerous anatomic sites. In this immunotherapeutic trial in vitro transcribed naked mRNA, which was generated using plasmids coding for the mentioned TAAs was applied directly into the dermis. The vaccination schedule is depicted in our previous publication.21 Stabilization of metastasized disease for 3 mo or more was achieved in 50% of patients and 27% of the patients had survived more than 5 y. Importantly, immunological work-up revealed induction of CD4+ as well as CD8+ immune responses against multiple epitopes applied through the vaccine as shown by IFNγ Enzyme-Linked Immuno Spot (ELISpot) and 51-chromium release assays. With the exception of an allergic reaction to GM-CSF in one patient, the safety profile of the vaccine was favorable.
Survival data exceed predicted survival
In July 2015, we performed a survival update. One of the 30 patients was still alive. One patient was lost to follow-up. While MSKCC risk score predicts a median survival of 20 mo for patients with favorable risk and 10 mo for patients grouped intermediate risk, survival monitoring in our patients revealed a median survival of 89 mo (mean 73.6 mo) for favorable-risk patients and a median time to death of 13 mo (mean 25.4 mo) for intermediate-risk patients. Taken together, for the intent-to-treat (ITT) population, we observed a median survival of 24.5 mo (Fig. 1A). Of note, we performed these survival analyses from the time point of study entry, not from date of documented stage IV disease.
Figure 1.
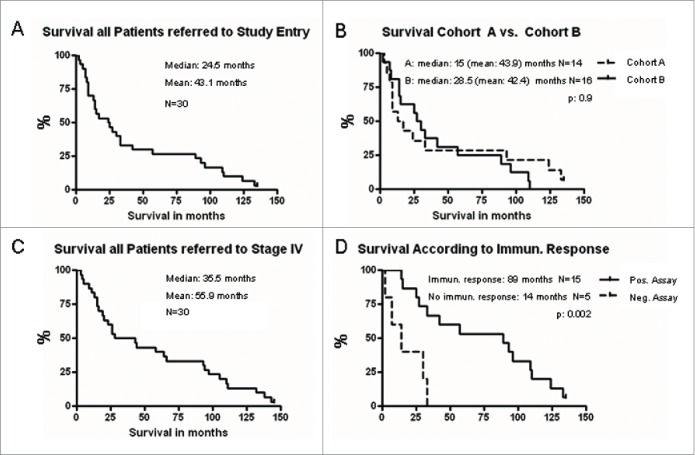
Overall survival is depicted in Kaplan–Meier Plots (GraphPad Prism). The current update revealed median (mean) survival of 24.5 (43.1) mo for all patients included in the study based on timepoint of study entry (A). Overall survival of patients treated in cohort A versus cohort B. p = 0.9 (logrank test), Kaplan–Meier Plots (GraphPad Prism) (B). Overall survival based on the date of documented stage IV disease. Median (mean) survival reached 35.5 (55.9) mo as depicted in Kaplan–Meier Plots (GraphPad Prism) (C). Overall survival of patients with detectable immunological responses to the vaccinated TAA was significantly prolonged compared to patients with negative immunological assays (89 mo vs. 14 mo, p = 0.002, logrank test) as displayed. Of note, immunological assays could only be performed in 20 out of 30 patients (D).
In order to evaluate the impact of post vaccination therapies with targeted drugs on the observed prolonged survival, we analyzed post-study treatment in our patients. As shown in , half of the patients were treated with two or more systemic therapies including one or sequentially different multi tyrosine kinase receptor inhibitors (sunitinib, pazopanib, axitinib, sorafenib). Several patients received mTOR inhibitors and two patients received bevacizumab. In addition, patients were treated with chemotherapy, radiation therapy, IFN-α, and other treatment options.
The current follow-up furthermore revealed long-term survival of 7 y and more after study entry in 27% of the study population treated with this mRNA vaccine. Detailed patient characteristics of five patients surviving astonishing 9 y after study entry are shown in . Patients were diagnosed stage IV RCC between 2002 and 2005. All of them had undergone prior nephrectomy and showed metastases at multiple anatomic regions. Two patients had received no prior treatment except for nephrectomy. The other three had received INF-α, IL-2 or had undergone resection of metastases. Patients were categorized intermediate (patients 5A, 6A, 3B) and favorable risk (patients 8A, 16B) according to the Heng risk model. Study and post-study treatments of surviving patients are summarized in . Three of the five patients received several molecular targeted therapies as displayed.
Several authors published survival data of patients suffering from stage IV RCC treated in the pre- and post-targeted therapy era.23,24Kroeger et al. demonstrated prolonged survival data in 2370 non-clear cell carcinoma patients who were treated between 2003 and 2012 with first-line targeted therapy.25 In another analysis, Heng and colleagues compared various risk stratification models in patients treated with VEGF-targeted therapy (sunitinib, sorafenib, and bevacizumab) introducing the Heng model which identified a Karnofsky performance status of less than 80%, a situation of less than 1 y from diagnosis to treatment, anemia, hypercalcemia, neutrophilia, and thrombocytosis as relevant risk factors. These factors allocate patients to favorable (no factors, median OS 43.2 mo), intermediate (one or 2 factors, median OS 22.5 mo), and poor risk (more than three factors, median OS 7.8 mo).26 In order to rank achieved survival, we applied this model to our patients showing that a majority would be characterized as intermediate-risk patients prognosticating an OS of 22.5 mo (19/30 patients), seven were categorized favorable risk, and four were at poor risk.
The majority of our patients was diagnosed stage IV RCC in the pre-tyrosine kinase era with 23 being diagnosed in 2004 or earlier. However, our ITT patients reached a median survival of 24.5 mo (Fig. 1A) thereby meeting survival data of patients treated in the post targeted therapy era as noted by Pal, Heng, Kroeger, and colleagues (median survival according to risk factors for the whole cohorts 16, 18.8, and 20.9 mo, respectively).23-25 Comparison of the two cohorts in our study revealed median survival of 15 mo of patients in cohort A (mean 43.9) and 28.5 mo (mean 42.4) in cohort B (Fig. 1B). As noted above, our survival data were based on the date of study entry. Due to lack of beneficial therapies before targeting drugs were available for RCC treatment, many of our patients were diagnosed stage IV disease and had remained without specific treatment long before the date of study entry. In order to correlate our survival data with published data, we performed survival analysis from date of documented stage IV disease. This evaluation disclosed an astonishing median survival of 35.5 (mean 55.9) mo (Fig. 1C).
Survival correlates with immunological response
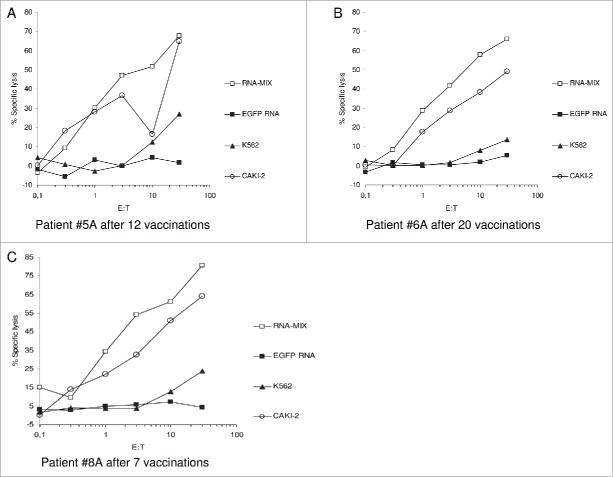
As Walter et al. had shown clear correlation between the extent of immune response to a multi-peptide vaccine and OS for RCC patients,19 we analyzed the effect of the induced immune responses in our patients on the survival benefit. Due to progress of disease and thus insufficient blood samples merely 20 out of 30 treated patients were eligible for evaluation of immune responses. Vaccine-induced CD4+ and CD8+ T cell responses specific for the utilized TAAs were analyzed by applying IFNγ ELISpot and 51-chromium release assays. 15 of 20 analyzed patients showed immune responses toward TAAs included in the vaccine in one or more assays revealing an immune response rate of 75%. In five analyzed patients, no immune responses could be detected. Examples of the lytic activity of vaccine-induced T cells are displayed in Fig. 2 for three of the long-term survivors. Immunological data are extensively displayed in our previous publication.21
Figure 2.
Exemplary Immunological Assays in long-term Survivors. Cytotoxic T cells (CTL) were generated out of the patients (HLA-A2-) PBMC (obtained after onset of vaccination as mentioned) by stimulation with autologous DC transfected with an RNA-Mix (containing RNA coding for the vaccinated TAAs MAGE-A1, MUC1, CEA, and survivin). After re-stimulations CTL were used as effector cells in 51-chromium release assays. These vaccine-induced CTL efficiently lysed autologous DC electroporated with the RNA-Mix as well as the tumor cell line Caki-2 (RCC, HLA-A2-) while DC electroporated with irrelevant EGFP-RNA were not lysed. K562 was used to exclude NK-cell mediated toxicity.
Thus, the induction of immune responses in patients treated with the mRNA vaccine correlated with a clinical benefit. When referred to study entry, median survival in immune responders in our trial reached 89 mo compared to merely 14 mo in patients without immune response (non-responders) and this difference in survival reached significance (p: 0.002, Fig. 1D).
Discussion
Here, we report on long-term follow-up of survival data and correlated immune responses for patients with mRCC who were treated with an mRNA-based vaccine. The primary end points defined as feasibility and safety of this vaccine consisting of an mRNA mix coding multiple TAAs and adjuvant GM-CSF were analyzed and previously published.21
With a follow-up of 10 y, we show that the median survival of all treated patients reached 24.5 mo (mean: 43.1) when referred to study entry and astonishing 35.5 mo (mean 55.9) when referred to date of recorded stage IV disease. Despite restriction of inclusion criteria concerning performance status, organ function and exclusion of patients with detected active brain metastases, classification of our patients according to the Heng model revealed a heterogeneous cohort. Encouragingly median survival of 35.5 mo in our patients exceeded predicted survival even when the Heng model was applied, which was established and evaluated for patients treated in the post tyrosine kinase inhibitors era. Moreover, of five long-term survivors only two were at favorable risk and three at intermediate risk predicting median survival of 43.3 and 22.5 mo, respectively. Follow-up analyses revealed a survival of over 9 y in these five patients. Thus, these survival data are not due to a selection of patients according to risk factors or baseline characteristics.
In order to elucidate other possible reasons for the prolonged survival, we correlated survival between the two cohorts. Although median survival was higher in cohort B treated with an intensified induction period (28.5 in cohort B vs. 15 mo in cohort A) this difference was not significant and may in part be due to the fact that cohort B was treated later than patients in cohort A, when the first targeted therapies were available for patients after study medication. However, since merely 50% of our patients proceeded to the treatment with one or more of the novel agents, sustained survival in our patients is presumably not exclusively associated with the efficacy of these therapies. Whether post-study regimens evoked prolonged survival can surely not be distinctively verified in this non-randomized setting and immediate comparisons to other studies are not feasible. However, as displayed above and by Harrison, Herrmann, Wahlgren, Shek, and colleagues,27-30 survival documented here exceeded survival published for patients with comparable baseline characteristics even when patients were diagnosed mRCC after approval of TKI and mTOR inhibitors. Median overall survival in our patients reached 24.5 mo after randomization and is comparable with recently published survival data of mRCC patients treated with the programmed death 1 (PD-1) checkpoint inhibitor antibody nivolumab. In contrast to our patients, Motzer and colleagues report on patients who had received at least one antiangiogenic regimen before randomization and over 50% received systemic subsequent treatment.31 With >55% of patients in the nivolumab group still being alive at the last follow-up (30 mo after study inclusion) long-term survival can be expected. In our trial, we observed not only a median survival of nearly 3 y but a high percentage of long-term survivors which may possibly be attributed to applied immunotherapy. 27% of our patients survived 7 y, 17% lived for more than 9 y, and the 10 y survival rate in this cohort is 10%.
As a next step, we compared the individual immunological data with the survival in our treated patients. Unfortunately, immunological assays could not be performed in 33% of all patients mainly due to lack of sufficient blood samples. However, vaccine-specific immune responses could be detected in 15 of 20 patients. In these immunologically responding patients, survival was significantly prolonged with 89 mo compared to 14 mo in the patients without confirmed immune responses (p = 0.002). Induced adaptive immunity comprised CD4+ and CD8+ T cell responses against several antigens encoded by the mRNA vaccine. Neither baseline characteristics nor prognostic factors varied significantly between responding and non-responding patients. While median age slightly differed (66 y in non-responding versus 64 y in responding patients) three non-responding patients were at intermediate risk and two even at favorable risk according to MSKCC. The reason for missing ability to induce antigen-specific immune responses could not be illuminated, however no biomarker analysis or characterization of immune cell composition were performed. Although this trial was primarily performed to assess feasibility and safety, in line with data obtained after peptide vaccination, prolonged survival after treatment with our mRNA vaccine also showed a clear correlation with in vitro detectable immunological responses to the applied TAAs. Hence, this proof of principle study suggests that long-term clinical responses can be achieved by induction of CD4+ and CD8+ T cells after an mRNA-based immunotherapy. Importantly, this is one of the first vaccination studies and the only RNA trial that reports on safety and efficacy after a follow up of more than 10 y. In this vaccination trial, we used a direct intra dermal application of free unmodified mRNA allowing vaccination independently of the patients HLA-type. However, recent data had shown that protamine-complexed (two component) mRNA seems to be more effective and increases antigen expression and immune stimulation.15 These self-adjuvanting mRNA-based vaccines, applied without any additional adjuvant, induce an efficient and protective immune response in mice, consisting of antigen-specific CD4+ T cells, CD8+ T cells, and B cells.
Further trials with an increased number of patients and extensive immunological monitoring are essential to further prove the predictive power of vaccine-induced immune responses. In addition, incorporation of protamine-complexed RNA and more potent adjuvants such as TLR or RIG-I ligands as well as combinations with targeted therapies (like sunitinib) and monoclonal antibodies blocking CTLA-4 or PD-1/PD-L1 interaction will be included in the next immunotherapy protocol in order to improve the efficacy of the mRNA-vaccine approach.32
Material and methods
Material and Methods are extensively described in our previous publication.21
Study design
In this phase I/II trial, a formulation of naked mRNA coding for the TAAs survivin, MUC1, Her-2/neu, CEA, telomerase, and MAGE-A1 was administered intradermally. The mRNA in the formulation was transcribed from DNA (collaboration with Curevac GmbH, Tuebingen, Germany). GM-CSF (granulocyte macrophage colony-stimulating factor) applied subcutaneously served as an adjuvant. Patients suffering from advanced RCC with prior therapy or untreated patients could be included. Inclusion criteria comprised histological confirmation of RCC, bi-dimensionally measurable lesions (thus all patients are stage IV), an interval of at least 6 weeks to last therapy, a Karnofsky score of >70%, and absence of severe hepatic or renal impairment. Patients with history of further malignancies, pregnancy, severe heart disease, and brain metastases were not eligible. Physical examination, computed tomography (CT) scans and laboratory testing was performed prior to the first vaccination in every patient. The clinical protocol complied with the regulations of the Declaration of Helsinki and had been approved by the local institutional ethics committee at Eberhard-Karls-University Tuebingen, Germany. Furthermore, written informed consent was gained from each patient before study inclusion. Evaluation of safety and feasibility were the primary end points. Secondary endpoints comprised analysis of immunological responses as well as possible antitumor activity. The injection schedule is displayed in our previous publication. The first 14 patients (cohort A) were vaccinated with 20µg of coding mRNA per antigen dissolved in 150µL phosphate buffered saline on days 0, 14, 28, and 42. In the following 16 patients (cohort B), the formulation consisted of 50µg of RNA per antigen dissolved in 150µL PBS and an intensified schedule was applied with injections on days 0–3, 7–10, 28, and 42. In both cohorts, the RNA-solution was injected intradermally at two different sites (lower abdomen or upper thigh), which could be varied during the subsequent treatment. Furthermore, on the day following RNA application GM-CSF (100µg/m2 in cohort A and 250µg abs. in cohort B) was administered subcutaneously at one of the RNA injection sites.
Clinical response was monitored by restaging via CT scan according to RECIST (Response Evaluation Criteria in Solid Tumors) around week 7. In the course of treatment, CT scans were repeated every 6 to 8 weeks. Vaccinations were performed monthly in case of clinical response until progression.
Evaluation of immune response
In order to evaluate vaccine-induced immune responses, patients' peripheral blood was drawn before the first injection and at various time points during study treatment. Immune assays including INFγ ELISpot assays and cytotoxic T-lymphocyte assays were performed using cryopreserved PBMCs from the different time points simultaneously after the end of treatment as described previously.21 CD4+ and CD8+ immune responses specific for the applied antigens were considered significant when a stimulation index of ≥2 was detected for two or more TAA-derived peptides in the CD4+ and CD8+ ELISpot assays at at least one time point after initiation of study treatment as compared to the number of spots before vaccination. Those data were reported in our previous manuscript.21 Furthermore, antigen-specific lytic activity of vaccine-induced T cells was evaluated after in vitro restimulation in 51-chromium release assays.
Correlative analyses
For presentation of correlative analyses, we used the Kaplan–Meier method and logrank test (GraphPad Prism 4.03). A p value of <0.05 was considered significant. Update of survival data was based on information given by patients, their physician, or family members.
Supplementary Material
Disclosure of potential conflicts of interest
I. Hoerr: Employment and Leadership Position at Curevac GmbH, Role Held: Managing Director, Stock Ownership: Curevac GmbH. I. Hoerr has a patent null issued. H.-G. Rammensee is stock shareholder at Curevac. S. Pascolo was founder and CSO for Curevac but left the company in 2006 and has no longer any link to the company.
Acknowledgments
We thank Sylvia Stephan, Renate Dreher, and Corinna Walker for excellent technical assistance.
Funding
This project was supported by the European Social Fond in Baden-Wuerttemberg.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18:767-811; PMID:10837075; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.767 [DOI] [PubMed] [Google Scholar]
- 2.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, Aridome K, Hokita S, Aikou T. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett 2000; 159:103-8; PMID:10974412; http://dx.doi.org/ 10.1016/S0304-3835(00)00542-5 [DOI] [PubMed] [Google Scholar]
- 3.Ishigami S, Natsugoe S, Matsumoto M, Okumura H, Sakita H, Nakashima S, Takao S, Aikou T. Clinical implications of intratumoral dendritic cell infiltration in esophageal squamous cell carcinoma. Oncol Rep 2003; 10:1237-40; PMID:12883687; http://dx.doi.org/ 10.3892/or.10.5.1237 [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer 2003; 104:92-7; PMID:12532424; http://dx.doi.org/18836473 10.1002/ijc.10915 [DOI] [PubMed] [Google Scholar]
- 5.Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene 2008; 27:5920-31; PMID:18836473; http://dx.doi.org/ 10.1038/onc.2008.270 [DOI] [PubMed] [Google Scholar]
- 6.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic Cell Death in Cancer Therapy. Ann Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/23243626 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 7.Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and CD8(+) T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology 2012; 1:1451-3; PMID:23243626; http://dx.doi.org/ 10.4161/onci.21356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S et al.. Impaired IFN-α Production by Plasmacytoid Dendritic Cells Favors Regulatory T-cell Expansion That May Contribute to Breast Cancer Progression. Cancer Res 2012; 72:5188-97; PMID:22836755; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3468 [DOI] [PubMed] [Google Scholar]
- 9.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 2004; 199:251-63; PMID:15233739; http://dx.doi.org/ 10.1111/j.0105-2896.2004.00139.x [DOI] [PubMed] [Google Scholar]
- 10.Heiser A, Dahm P, Yancey DR, Maurice MA, Boczkowski D, Nair SK, Gilboa E, Vieweg J. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol 2000; 164:5508-14; PMID:10799919; http://dx.doi.org/ 10.4049/jimmunol.164.10.5508 [DOI] [PubMed] [Google Scholar]
- 11.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 2003; 63:2127-33; PMID:12727829 [PubMed] [Google Scholar]
- 12.Carralot JP, Probst J, Hoerr I, Scheel B, Teufel R, Jung G, Rammensee HG, Pascolo S. Polarization of immunity induced by direct injection of naked sequence-stabilized mRNA vaccines. Cell Mol Life Sci 2004; 61:2418-24; PMID:15378210; http://dx.doi.org/ 10.1007/s00018-004-4255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol 2000; 30:1-7; PMID:10602021; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 14.Heine A, Holderried TAW, Brossart P. Immunotherapy in renal cell carcinoma. Immunotherapy 2009; 1:97-107; PMID:20635977; http://dx.doi.org/ 10.2217/1750743X.1.1.97 [DOI] [PubMed] [Google Scholar]
- 15.Kallen KJ, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M. A novel, disruptive vaccination technology: self-adjuvantedRNActive((R)) vaccines. Hum VaccinImmunother 2013; 9:2263-76; PMID:23921513; http://dx.doi.org/ 10.4161/hv.25181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz C, Fotin-Mleczek M, Roth G, Becker C, Dam TC, Verdurmen WPR, Brock R, Probst J, Schlake T. Protein expression from exogenous mRNA Uptake by receptor-mediated endocytosis and trafficking via the lysosomal pathway. RNA Biol 2011; 8:627-36; PMID:21654214; http://dx.doi.org/ 10.4161/rna.8.4.15394 [DOI] [PubMed] [Google Scholar]
- 17.Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G et al.. Trial Watch Dendritic cell-based interventions for cancer therapy. Oncoimmunology 2013 Oct 1; 2(10); PMID:24286020; http://dx.doi.org/ 10.4161/onci.25771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al.. IMPACT Study Investigators. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/ 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 19.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al.. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254-61; PMID:22842478; http://dx.doi.org/ 10.1038/nm.2883 [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, Mulders P, Kataja V; ESMO Guidelines Working Group. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23:65-71; PMID:21436185; http://dx.doi.org/ 10.1093/annonc/mdr034 [DOI] [PubMed] [Google Scholar]
- 21.Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W, Horger MS, Maksimovic O, Stenzl A, Hoerr I et al.. Intradermal Vaccinations With RNA Coding for TAA Generate CD8(+) and CD4(+) Immune Responses and Induce Clinical Benefit in Vaccinated Patients. MolTher 2011; 19:990-9; PMID:21189474; http://dx.doi.org/ 10.1038/mt.2010.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J ClinOncol 1999; 17:2530-40; PMID:10561319 [DOI] [PubMed] [Google Scholar]
- 23.Heng DYC, Xie WL, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S et al.. Prognostic Factors for Overall Survival in Patients With Metastatic Renal Cell Carcinoma Treated With Vascular Endothelial Growth Factor-Targeted Agents: Results From a Large, Multicenter Study. J ClinOncol 2009; 27:5794-9; PMID:19826129; http://dx.doi.org/ 10.1200/JCO.2008.21.4809 [DOI] [PubMed] [Google Scholar]
- 24.Pal SK, Nelson RA, Vogelzang N. Disease-Specific Survival in De Novo Metastatic Renal Cell Carcinoma in the Cytokine and Targeted Therapy Era. PLoS One 2013; 8 (5):e63341; PMID:23658823; http://dx.doi.org/ 10.1371/journal.pone.0063341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroeger N, Xie WL, Lee JL, Bjarnason GA, Knox JJ, MacKenzie MJ, Wood L, Srinivas S, Vaishamayan UN, Rha SY et al.. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013; 119:2999-3006; PMID:23696129; http://dx.doi.org/ 10.1002/cncr.28151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heng DYC, Xie WL, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH et al.. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013; 14:141-8; PMID:23312463; http://dx.doi.org/ 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison MR, Hirsch BR, George DJ, Walker MS, Chen C, Korytowsky B, Stepanski E, Abernethy AP. Real-world outcomes in metastatic renal cell carcinoma: insights from a Joint Community-Academic Registry. J OncolPract 2014; 10(2):e63-72; PMID:24281152; http://dx.doi.org/ 10.1200/JOP.2013.001180 [DOI] [PubMed] [Google Scholar]
- 28.Herrmann E, Marschner N, Grimm MO, Ohlmann CH, Hutzschenreuter U, Overkamp F, Groschek M, Blumenstengel K, Pühse G, Steiner T. Sequential therapies with sorafenib and sunitinib in advanced or metastatic renal cell carcinoma. World J Urol 2011; 29(3):361-6; PMID:21461939; http://dx.doi.org/ 10.1007/s00345-011-0673-4 [DOI] [PubMed] [Google Scholar]
- 29.Wahlgren T, Harmenberg U, Sandström P, Lundstam S, Kowalski J, Jakobsson M, Sandin R, Ljungberg B. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000-2008). Br J Cancer 2013; 108(7):1541-9; PMID:23531701; http://dx.doi.org/ 10.1038/bjc.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shek D, Tomlinson B, Brown M, Brunson A, Pan CX, Lara PN Jr. Epidemiologic trends in renal cell carcinoma in the cytokine and post-cytokine eras: a registry analysis of 28,252 patients. ClinGenitourin Cancer 2012; 10(2):93-98; PMID:22382008; http://dx.doi.org/ 10.1016/j.clgc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER et al.. Nivolumabvs.Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; Nov 5; 373(19):1803-13; PMID:26406148; http://dx.doi.org/24800168 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf D, Heine A, Brossart P. Implementing combinatorial immunotherapeutic regimens against cancer: The concept of immunological conditioning. Oncoimmunology 2014; 3:e27588; PMID:24800168; http://dx.doi.org/ 10.4161/onci.27588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Probst J, Weide B, Scheel B, Pichler BJ, Hoerr I, Rammensee HG, Pascolo S.Gene Ther. 2007 Aug; 14(15):1175-80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.