ABSTRACT
T cells engineered to express chimeric antigen receptors (CARs) targeted to CD19 are effective in treatment of B-lymphoid malignancies. However, CARs recognize all CD19 positive (pos) cells, and durable responses are linked to profound depletion of normal B cells. Here, we designed a strategy to specifically target patient B cells by utilizing the fact that T-cell receptors (TCRs), in contrast to CARs, are restricted by HLA. Two TCRs recognizing a peptide from CD20 (SLFLGILSV) in the context of foreign HLA-A*02:01 (CD20p/HLA-A2) were expressed as 2A-bicistronic constructs. T cells re-directed with the A23 and A94 TCR constructs efficiently recognized malignant HLA-A2pos B cells endogenously expressing CD20, including patient-derived follicular lymphoma and chronic lymphocytic leukemia (CLL) cells. In contrast, a wide range of HLA-A2posCD20neg cells representing different tissue origins, and HLA-A2negCD20pos cells, were not recognized. Cytotoxic T cells re-directed with CD20p/HLA-A2-specific TCRs or CD19 CARs responded with similar potencies to cells endogenously expressing comparable levels of CD20 and CD19. The CD20p/HLA-A2-specific TCRs recognized CD20p bound to HLA-A2 with high functional avidity. The results show that T cells expressing CD20p/HLA-A2-specific TCRs efficiently and specifically target B cells. When used in context of an HLA-haploidentical allogeneic stem cell transplantation where the donor is HLA-A2neg and the patient HLA-A2pos, these T cells would selectively kill patient-derived B cells and allow reconstitution of the B-cell compartment with HLA-A2neg donor cells. These results should pave the way for clinical testing of T cells genetically engineered to target malignant B cells without permanent depletion of normal B cells.
KEYWORDS: B-cell malignancies, CD20, gene therapy, haploidentical allogeneic stem cell transplantation, immunotherapy, T-cell receptor
Abbreviations
- ALL
Acute lymphoblastic leukemia
- Caco-2
Heterogeneous human epithelial colorectal adenocarcinoma cell
- CAR
Chimeric antigen receptor
- CD
Cluster of differentiation
- cDNA
complementary DNA
- CDR
Complementary-determining region
- CFSE
Carboxyfluorescein succimidyl ester
- CLL
Chronic lymphocytic leukemia
- CMV
Cytomegalovirus
- CTL
Cytotoxic T lymphocyte
- CTV
Cell trace violet
- DLCL2
Diffuse large cell lymphoma cell line
- DMF5
MART-1 TCR
- EBV-LCL
EBV-transformed lymphoblastoid cell
- EC50
Half maximal effective concentration
- FACS
Fluorescent-activated cell sorter
- FITC
Fluorescein isothiocyanate
- FL
Follicular lymphoma
- FM-81
Malignant melanoma cell
- FSCCL
Follicular small cleaved cell lymphoma cell line
- GVHD
Graft-versus-host disease
- HaCat
Cultured human keratinocyte cell
- Hc
Heteroclitic
- HCT-116
Human colon carcinoma cell
- HEK
Human embryonic kidney cells
- HeLa
Cervix adenocarcinoma cell
- HepG2
Human liver carcinoma cell
- HLA
Human leukocytes antigen
- HSC
Hematopoietic stem cell
- HSCT
Hematopoietic stem cell transplantation
- JVM-2
B-prolymphocytic leukemia cell line
- K562
Chronic myelogeneous leukemia cell
- MART-1
Melanoma antigen recognized by T cell 1
- MHC
Major histocompatibility complex
- mRNA
Messenger ribonucleic acid
- NCI-H522
Human lung adenocarcinoma
- PBMC
Peripheral blood mononuclear cells
- SCT
Single-chain trimer
- SupT1
T-/B-lymphoblastoid cell
- TCR
T-cell receptor
- TRAJ
T-cell receptor α joining gene
- TRAV
T-cell receptor α variable gene
- TRBJ
T-cell receptor β joining gene
- TRBV
T-cell receptor β variable gene
- WT
Wild type
Introduction
The B-cell surface proteins CD19 and CD20 are self-antigens with a highly cell-type specific expression. Anti-CD20 antibodies are now part of the standard treatment of B-cell lymphomas and have dramatically improved the prognosis of these diseases, although the treatment is not curative when used as single agent.1-3 By engrafting fusion proteins conferring the specificity of anti-CD19 antibodies combined with T-cell activation domains onto T cells (CARs), it is possible to obtain complete remission in patients with several types of B-cell malignancies who are resistant to standard treatment.4-10 However, the benefit appears to come at the cost of profound depletion of normal B cells.5,11 This on-target toxicity represents a clinical challenge over time, particularly in pediatric patients.
For patients with acute lymphoblastic leukemia (ALL) or malignant lymphoma who relapse after chemotherapy, allogeneic hematopoietic stem cell transplantation (HSCT) is a treatment option. Traditionally, patients have received grafts from HLA-matched donors to reduce the risk of graft-versus-host disease (GVHD).12 However, there is now evidence that HLA-haploidentical blood or marrow transplantation (haploHSCT) has an acceptable safety profile while being effective when combined with post-transplantation cyclophosphamide.13,14 In addition to increasing the number of patients that become eligible for HSCT, the use of haploidentical donors opens for a new type of immunotherapy where T cells are engineered to recognize patient-derived cells only. Unlike CARs, T-cell receptors (TCRs) are restricted to a specific HLA molecule. For example, T cells that recognize peptides from a B-cell protein presented on HLA-A2 could be used to kill leukemic B cells in an HLA-A2 positive (pos) patient while permitting the reconstitution of a healthy B-cell compartment from HLA-A2 negative (neg) donor HSC. In this situation, a long-lived population of memory T cells that recognize HLA-A2pos B cells would provide the desired therapeutic effect without causing depletion of healthy B cells.
Although the optimal TCR affinity for clinical use remains to be identified, high-affinity TCRs are regarded superior to achieve tumor regression compared to low- or intermediate-affinity TCRs.15-19 This represents a major challenge when targeting self-antigens, as the autologous T-cell repertoire is normally devoid of T cells recognizing self-peptides with high affinity due to negative selection in the thymus. As first demonstrated by Stauss and colleagues, tolerance to self can be overcome in a setting in which self-antigens are presented on allogeneic HLA and utilized to target cancer cells.20,21
Here, we performed a preclinical study to explore the efficacy and specificity of T cells genetically engineered to express two TCRs specific for a peptide from CD20 in the context of foreign HLA-A*02:01 (CD20p/HLA-A2). TCR-transduced T cells efficiently recognized and killed a range of malignant HLA-A2posCD20pos B cells, including primary leukemia and lymphoma cells. Importantly, extensive testing of potential off-target toxicities demonstrated exquisite specificity and dependence on cellular expression of the cognate ligand. We therefore propose that adoptive transfer of T cells expressing CD20p/HLA-A2-targeted TCRs combined with a haploHSCT from an HLA-A2neg donor might represent a new option to target B-cell malignancies while permitting reconstitution of the B-cell compartment by donor-derived cells.
Results
T cells re-directed with CD20p/HLA-A2-reactive TCRs are functional and sequence modifications increase expression
TCR sequences derived from cytotoxic T cell (CTL) clones restricted by allogeneic HLA-A2 and specific for a peptide derived from CD20 (SLFLGILSV, CD20p) 22 were cloned and expressed to investigate their potential for future clinical applications. Two CD20p/HLA-A2-reactive TCRs, A23 and A94, were expressed in PBMC as wild type (wt) and modified (mod) versions to investigate if expression levels could be improved. Modifications included (i) replacement of the constant (C-) domains of the human TCRs by the murine counterparts (murinization) 23,24 and ii) insertion of a second cysteine bridge in the constant part.25-27 All constructs were cloned as 2A bicistronic vectors to ensure efficient production of equimolar amounts of the TCRα and β chains.28
Results obtained by measuring binding of peptide-HLA (pHLA) multimers showed that there was a substantial increase in the fraction of CD8pos T cells expressing A23mod and A94mod relative to wt constructs (Fig. 1A). Expression measured by labeling with HLA multimers and by anti-mouse TCR-β chain antibodies was highly similar for cells engineered with the modified TCR constructs containing mouse constant parts (Fig. S1). As expected, the functionality of A23mod and A94mod was considerably higher than for wt constructs, as determined by measurements of the ability of cells to degranulate upon encounter with HLA-A2pos SupT1 cells induced to present the CD20p by mRNA transfection or peptide loading, or transduced to express a single-chain trimer (SCT) of HLA-A2 fused to the CD20p (Fig. 1B and C). The degree of improvement obtained with the modified constructs was, however, consistently higher for A94 than for A23. Degranulation responses induced in T cells engineered to express A94mod or A23mod were strong and similar upon activation by target cells expressing CD20p/HLA-A2, whereas very low responses were seen following stimulation with target cells presenting the irrelevant MART-1-derived peptide EEAGIGILTV (MART-1pwt/HLA-A2) or the heteroclitic peptide-analog with improved binding to HLA-A2 (ELAGIGILTV—MART-1phc/HLA-A2), respectively).29 For comparison, we included the high-affinity TCR DMF5 as a control, since this particular TCR has sufficient affinity for its cognate peptide/HLA-A2 complex to cause tumor regression in patients with malignant melanoma with minimal off-target toxicity.30,31 T cells expressing DMF5 responded weakly to target cells expressing the CD20p, whereas strong responses were seen to target cells presenting MART-1pwt or MART-1phc. Collectively, the data show that the CD20p/HLA-A2-reactive TCRs are functional when introduced into T cells, and confirm earlier observations showing that the expression and functionality of cloned TCRs is enhanced by murinization and introduction of a cysteine bridge.
Figure 1.
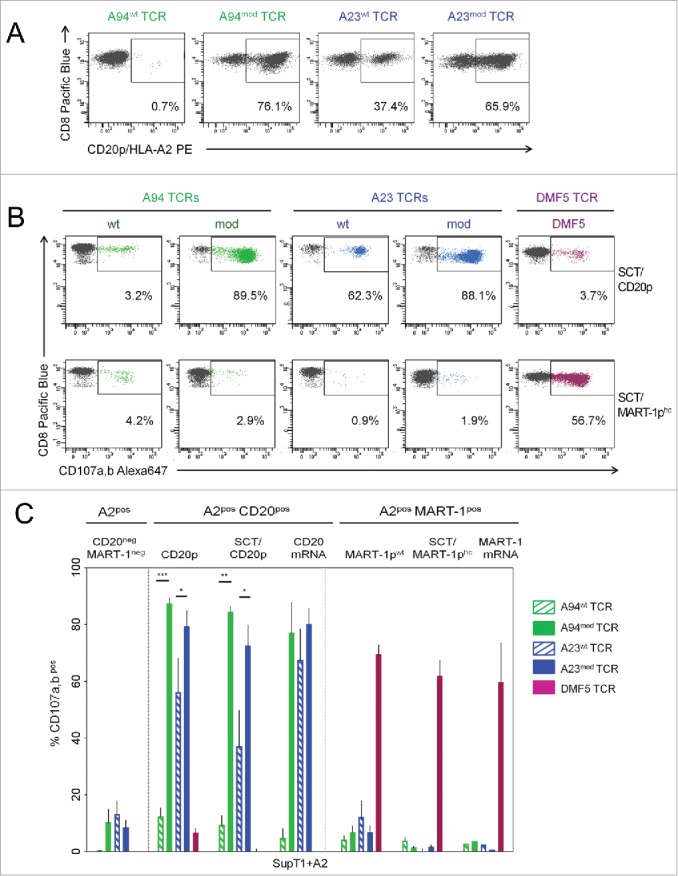
Enhanced expression and functional avidity of CTLs retrovirally transduced to express modified relative to wt CD20p/HLA-A2-specific TCRs. (A) Surface expression of CD20p/HLA-A2-reactive TCRs. Flow cytometric analysis of PE-labeled CD20p/HLA-A2 multimers was used to measure the frequency of CTLs expressing the retrovirally transduced wild type (wt) or gene-modified (mod) CD20p/HLA-A2-reactive TCRs (A23wt, A23mod, A94wt, A94mod TCRs). Dot plots are gated on CD8pos peripheral blood T cells and show flow cytometric analysis of frequencies of CD20p/HLA-A2 multimerpos cells. Results shown are representative of five experiments and two donors. (B) The TCR-transduced CTLs shown in (A) were tested for CD107a,b mobilization upon stimulation with SupT1 cells engineered to express single chain trimer HLA-A2 (SCT) molecules complexed with the CD20p or the heteroclitic MART-1p (SCT/CD20p and SCT/MART-1phc, respectively). T cells transduced with the MART-1-specific TCR DMF5 were used as a control. The experiment shown is representative of three performed. (C) Bar graph showing frequencies (y-axis) of CD107a,bpos events among CD8pos T cells re-directed with A94wt TCR (striped green bars), A94mod TCR (green bars), A23wt TCR (striped blue bars), A23mod TCR (blue bars) or DMF5 TCR (purple bars) upon stimulation with: SupT1 cells retrovirally transduced to express HLA-A2 (+A2) only (CD20neg, MART-1neg), or SupT1+A2 cells that were either loaded with indicated peptide (CD20p, MART-1pwt) or electroporated with indicated mRNA, or SupT1 cells transduced to express indicated SCT. Values for CTLs alone were subtracted. Error bars indicate SEM and each bar represents the mean of two–four independent experiments.
T cells re-directed with CD20p/HLA-A2-reactive TCRs are specific for HLA-A2 and CD20p and recognize antigen-expressing target cells with similar potencies as T cells re-directed with a CD19 CAR
Next, we assessed the reactivity of expanded T cells expressing A94mod and A23mod to a panel of cell lines positive or negative for the target antigen. To ensure equal conditions for T cells re-directed with A94mod, A23mod or the control TCR DMF5, respectively, the three populations were color-coded before they were combined and tested for reactivity to various target cells. T cells with receptors for CD20p/HLA-A2 or MART-1/HLA-A2, respectively, responded with strong degranulation only to target cells expressing the cognate ligand (Fig. 2A). Furthermore, T cells expressing CD20p/HLA-A2 TCRs responded to HLA-A2pos target cells endogenously expressing CD20, including EBV-transformed lymphoblastoid cell lines (EBV-LCL), the B-prolymphocytic leukemia cell line JVM-2, the follicular small cleaved cell lymphoma cell line FSCCL and diffuse large cell lymphoma cell line DLCL2 (Fig. 2B). In contrast, there was negligible reactivity to a panel of HLA-A2posCD20neg cell lines derived from liver carcinoma (HepG2), colon carcinoma (Caco-2, HCT-116) lung adenocarcinoma (NCI-H522), keratinocytes (HaCat), malignant melanoma (FM81), cervix adenocarcinoma (HeLa), chronic myelogeneous leukemia (K562), the T-/B-lymphoblastoid cell line SupT1 and human embryonic kidney cells (HEK). However, responses were elicited when these target cells were loaded with CD20p. Collectively, the data show that the A94 and A23 TCRs confer a high degree of CD20p/HLA-A2-specificity and lack of cross-reactivity to a wide range of cell types.
Figure 2.
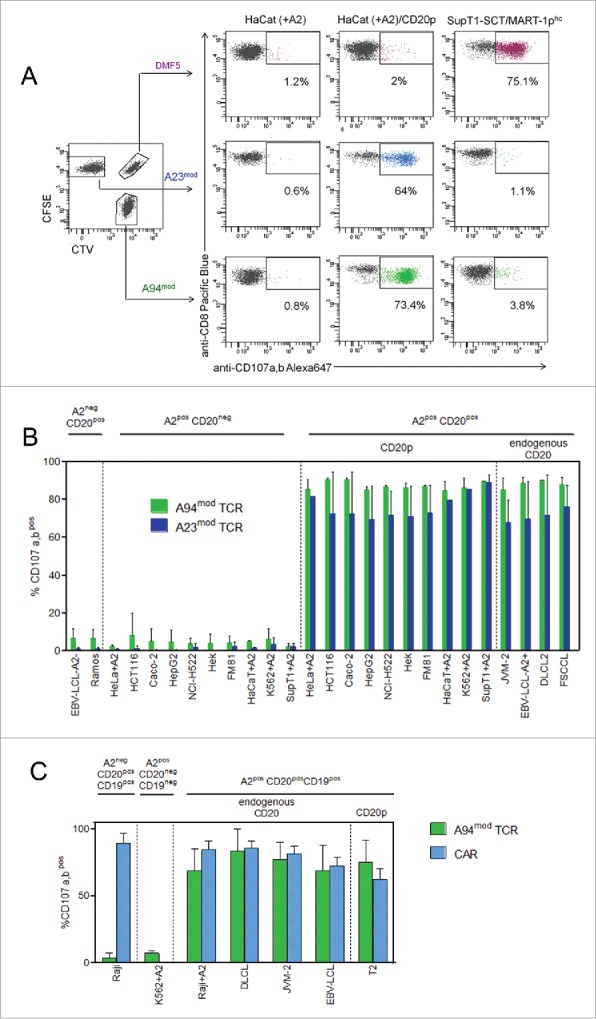
CTLs re-directed with CD20p/HLA-A2-reactive TCR display exquisite antigen specificity and mediate similar degranulation responses to antigen-positive target cells as CD19 CAR-transduced T cells. (A) PBMC from one donor were retrovirally transduced with three different TCRs and expanded. Each TCR-transduced population was subsequently color-coded to allow identification by flow cytometry; A94mod CTLs with CTV, A23mod CTLs with CFSE and DMF5 CTLs with CTV/CFSE, and combined into one sample, as shown in the left dot plot, gated on CD8pos T cells. Degranulation responses (mobilization of CD107a,b) were measured in the CTLs following incubation with indicated target cells; HaCaT cells transfected with HLA-A2 mRNA (+A2), either loaded or not with CD20 peptide, and SupT1 cells induced to express SCT/MART-1phc. (B) Summary of degranulation responses measured in T cells expressing A94mod or A23mod that were treated and analyzed as described in A following incubation with a panel of target cells (left to right); two HLA-A2negCD20pos B cell lines, 10 different HLA-A2posCD20neg cell lines of various tissue origins in the absence or presence of externally loaded peptide (CD20p), and four HLA-A2pos B-cell lines endogenously expressing CD20. HLA-A2 (A2pos) was either naturally expressed or induced (+A2), as indicated. Bars represent mean frequencies of CD107a,bpos events among stimulated CD8pos CTLs following subtraction of values for CTLs alone. Error bars indicate SD of duplicate samples from n = 3 experiments for all conditions, except for CD20p (n = 4) and CD20 mRNA (n = 2). The p values were calculated using Student’s t-test (one star p < 0.05; two stars p < 0.01; three stars p < 0.001). (C) Degranulation of expanded PBMCs retrovirally transduced with A94mod or a CD19 CAR was measured following co-incubation with indicated target cell lines: Raji (HLA-A2negCD20posCD19pos); K562 transduced with HLA-A2 (HLA-A2posCD20negCD19neg); or HLA-A2pos cell lines endogenously expressing CD19 and CD20 (Raji+A2 (transduced with HLA-A2), DLCL, JVM-2, HLA-A2pos EBV-LCL), or expressing CD19 and loaded with CD20p (T2 cells), as indicated. Bars represent mean frequencies of CD107a,bpos events among CD8pos CTLs following subtraction of values for CTLs alone and normalized according to the transduction efficiency. Error bars indicate SD of triplicate samples from n = 3 experiments for JVM-2, Raji and T2; n = 2 experiments for DLCL, Raji+A2 and EBV-LCL; n = 1 for K562+A2.
Next, we compared the potency of T cells re-directed with A94mod with T cells engineered to express a CD19 CAR construct (41BB-Zeta)32 that has been extensively used in vivo and demonstrated to be clinically effective.8,32 Notably, strong and highly similar degranulation responses were seen in CD8pos T cells transduced with either receptor when stimulated by a panel of HLA-A2pos target cells endogenously expressing both CD19 and CD20 at high and similar levels (DLCL, JVM-2, EBV-LCL, and Raji transduced with HLA-A2) (Fig. 2C and Fig. S2). The re-directed T cells also responded similarly to CD19pos T2 cells loaded with CD20p. Negligible responses were seen to K562 cells induced to express HLA-A2, but lacking the target antigens, while only CD19 CAR T cells recognized wt Raji cells, as expected, expressing both target antigens but lacking HLA-A2. The data show that T cells re-directed with the described CD20p/HLA-A2-specific TCR or the CD19 specific CAR recognized target cells expressing the respective cognate antigens with comparable potencies.
T cells re-directed with A23mod- or A94mod-TCRs show high peptide sensitivity
TCRs recognizing their cognate peptide-HLA target on tumor cells with high affinity are regarded superior to achieve clinical responses in adoptive T-cell therapy.16,17,31 We therefore determined the avidity with which T cells expressing A23mod and A94mod recognized the CD20p presented on HLA-A2 using two different assays; measurements of degranulation responses by flow cytometry (Fig. 3A), and a standard Chromium-51 release cytotoxicity assay (Fig. 3B). The predicted affinity of the CD20p for HLA-A2 is 11nM using the computer algorithm Net MHC 3.4, making it a strong binder. The sensitivity of A94mod and A23mod was similar and found to be in the pM range in both assays. For reference, we determined the peptide avidity of T cells expressing the control TCR DMF5.30,31 The sensitivity of DMF5 for the improved MART-1phc was manyfold (range 52–312) higher than for the wt MART-1p (MART-1pwt), which represents the clinically relevant TCR-peptide interaction. These data suggest that CTLs re-directed with the A94mod and A23mod TCRs recognize their cognate target with similar sensitivities, which is higher than the sensitivity of the DMF5 TCR for its in vivo target.
Figure 3.
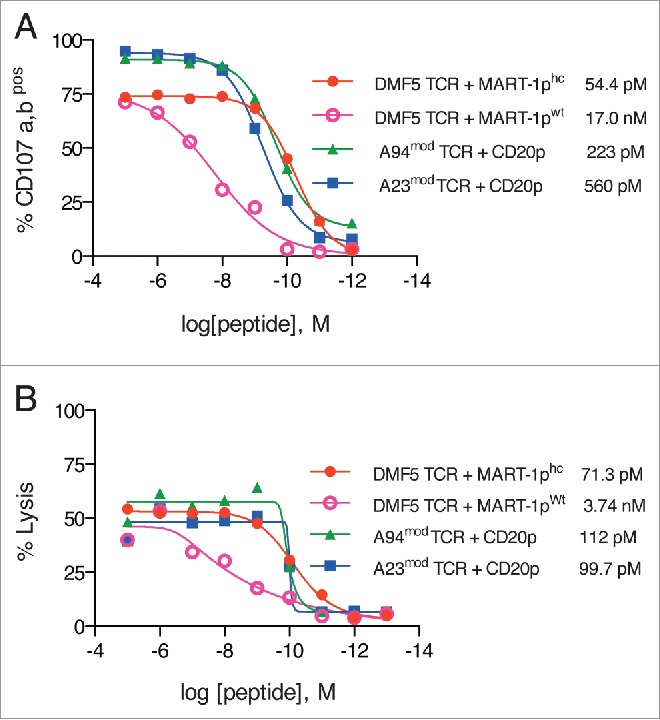
Functional avidity of T cells re-directed with CD20p/HLA-A2-reactive TCRs. PBMCs were retrovirally transduced with either A94mod or A23mod or DMF5 TCR and expanded. Re-directed T cells were co-incubated with target cells loaded with indicated concentrations of relevant peptide (CD20p, MART-1pwt or MART-1phc), ranging from 1012 M to 10−5 M, and functional avidities were measured by two different assays. (A) Flow cytometric measurements of degranulation: T cells and HLA-A2-transduced peptide-loaded SupT1 cells were co-incubated for 5 h at an effector: target (E:T) ratio of 1:2. The functional avidity of each TCR for the cognate peptide was measured as frequencies of CD107a,bpos cells out of CD8pos T cells. Data shown represent mean of duplicates and are representative of two experiments performed. (B) Chromium-51 release cytotoxicity assay: TCR-engineered T cells were stimulated with peptide-loaded T2 cells (CD20p, MART-1pwt or MART-1phc) for 4 h at a ratio of 5:1 (E:T). The cytotoxic activity of the effectors was measured by detecting the amount of 51Cr released in the supernatant, which was then used to calculate the percentage of lysed target cells loaded with different concentrations of peptide. Data represent mean of three parallels and are representative of two experiments performed. Numbers shown in (A) and (B) represent EC50, which was calculated to compare the avidities of the three different TCRs: DMF5 TCR avidity for MART-1phc (•red line); DMF5 avidity for MART-1pwt (○ purple line), A94mod TCR avidity for the CD20p (▵ green line) and A23mod TCR avidity for the CD20p (▭ blue line). Similar transduction efficiencies were seen for all three TCRs in (A) (>90%) and in (B) (approximately 50%).
T cells re-directed with CD20p/HLA-A2-specific TCRs target patient-derived follicular lymphoma and chronic lymphocytic leukemia cells
We next tested the ability of CTLs re-directed with A94mod or A23mod to degranulate in response to a panel of patient-derived follicular lymphoma (FL) cells and CLL cells. TCR-transduced peripheral blood T cells (transduction efficiencies ranging from 50–80%) were co-cultured with the lymphoma or leukemia cells. As shown in Fig. 4A and B, TCR-engineered CTLs mobilized CD107a,b when stimulated with HLA-A2pos but not HLA-A2neg primary FL cells. Similar responses were measured against primary CLL cells, confirming the ability of the TCRs to target endogenous CD20 in HLA-A2pos primary cancer cells.
Figure 4.

CTLs re-directed with CD20p/HLA-A2-reactive TCRs target primary lymphoma and leukemia cells. PBMC were transduced with either A94mod or A23mod TCRs, expanded and color-coded as described in Fig. 2A. (A) CTLs were co-cultured with primary follicular lymphoma (FL) cells from 2 HLA-A2pos patients and one HLA-A2neg patient and degranulation responses were measured as described in Fig. 2A. (B) Bar graphs show frequencies of CD107a,bpos events among CD8pos T cells upon co-culture with patient-derived FL cells (left) or chronic lymphocytic leukemia (CLL) cells (right), corrected for TCR transduction efficiencies of 50–80%. Results from two separate experiments are shown (FL and CLL, respectively). Bars represent mean values of duplicate (CLL) or triplicate (FL) samples of one experiment (n = 1) with values for CTLs alone subtracted, and error bars represent SD. (C) Flow cytometric analysis strategy in cytotoxicity assay. Upper plot: A gate is set to include beads and tumor cells, which can be separated from CTV-labeled T cells (green dots). Middle plots: Gates identify SSChi FITC-labeled counting beads (red dots) used for quantification of target cells (black dots), including lysed and disintegrated target cells. Lower plots: Only tumor cells are shown. Gates identify the fraction of live tumor cells, which are negative for the apoptosis marker activated caspase-3 (FITC) and the dead cell marker live/dead aqua. (D) Percentage of CTL-induced lysis of the DLCL2 and FSCCL lymphoma cell lines after 4 h incubation with either A94mod or A23mod TCR transduced T cells at different effector cell/target cell ratios, as indicated. Samples were analyzed as shown in 4(C) and the % specific CTL-induced target cell lysis was calculated according to the formula in Materials and Methods. HLA-A2pos CD4pos T cells were used as a CD20neg control and were not lysed unless loaded with the CD20 peptide. Data represent mean±SD of triplicates of one experiment, representative of a total of five performed including DLCL2 and FSCCL cells.
Degranulation is a highly correlated, but indirect, method of assessing cytotoxicity. This assay is preferred over direct assays for cytotoxicity when targeting patient-derived leukemia and lymphoma cells that poorly survive even short-term in vitro incubation. However, to ascertain that TCR re-directed T cells were able to kill cancer cells, we developed a bead-calibrated flow cytometry-based killing assay that allowed sensitive and accurate quantification of cell death in lymphoma cell lines (Fig. 4C). The difference between the number of viable target cells at start and end of culture was less than 5% when cultured alone. Using this assay, we demonstrated that T cells re-directed with either the A94mod or A23mod TCRs efficiently lysed the HLA-A2pos lymphoma cell lines DLCL or FSCCL, whereas HLA-A2posCD4pos T cells, used as a CD20neg control target, were spared unless loaded with the CD20p.(Fig. 4D)
Discussion
Here, we performed a preclinical study to characterize the utility of T cells re-directed with CD20p/HLA-A2-specific TCRs for use in adoptive immunotherapy of B-cell malignancies. We cloned and expressed two candidate TCRs and optimized the constructs with mouse constant parts and an added cysteine bridge. Immune responses to murine TCR sequences used in patients in previous studies did not limit the persistence of re-directed T cells or reduce clinical efficacy.31,33 As expected, the modified TCRs A94mod and A23mod were expressed at higher levels than wt constructs when introduced by retroviral transduction into peripheral blood T cells. The TCRs efficiently recognized B cells expressing the cognate antigen, including patient-derived lymphoma and leukemia cells. In contrast, cells derived from a variety of tissues lacking the target peptide/HLA-A2 complex were spared, showing a high degree of specificity. Moreover, CD20 has a well-characterized and highly cell-type restricted expression,34,35 and the toxicity of anti-CD20 antibodies is limited to on-target effects on healthy B cells.36 Collectively, the data suggest that the CD20p/HLA-A2-specific TCRs are good candidates for a first-in-man safety trial in which patients suffering from treatment-refractory B-cell malignancies are treated with TCR re-directed T cells.
Adoptive transfer of T cells engineered to express CARs recognizing CD19 has been very successful in the treatment of B-cell malignancies.4-10 The high affinity of the CAR permits efficient binding to the target, while the ability of serial killing is retained.37 CAR-transduced T cells can persist as long-lived memory T cells, and this seems to be critical to obtain stable remission, although the follow-up time for treated patients is still limited.5 A clinical application for the CD20p/HLA-A2 specific TCRs could be the adoptive transfer of autologous, engineered T cells to patients with B-cell malignancies, which might provide additional—or synergistic—therapeutic effects relative to CAR-therapy. An interesting alternative might, however, be the use of genetically modified allogeneic T cells. To this end, there is evidence that donor-derived T cells modified with CD19 CARs can induce regression of B-cell malignancies in patients who experience a relapse following alloHSCT. In one study, lymphodepletion was omitted to limit the risk of GVHD upon infusion of gene-modified donor-derived T cells,6 whereas in another study all 18 patients were T cell depleted.5 It is worth noting that transfer of re-directed T cells did not cause GVHD in any of these studies. However, as for autologous CD19 CAR-modified T cells, a long-lasting therapeutic effect of engineered donor-derived T cells seemed to be correlated with a concomitant B-cell aplasia, and recurring normal B cells predicted relapse.5
CD8pos T cells engineered to express either CD20p/HLA-A2-specific TCRs or CD19 CARs responded with comparable potencies to target cells endogenously expressing similar levels of CD20 and CD19. However, in a therapeutic setting the HLA-dependency of a therapeutic TCR might provide an opportunity for outgrowth of normal B cells while the malignancy is controlled. For instance, T cells attacking recurring malignant B cells in an HLA-A2pos patient would allow unperturbed formation of healthy B cells derived from an HLA-A2neg stem cell donor. The adoptive transfer of donor-derived T cells re-directed with TCRs targeting the CD20p/HLA-A2 complex combined with an alloHSCT mismatched on HLA-A2 could represent a therapeutic setting in agreement with current protocols for transplantation. To this end, the success of HLA-haploidentical transplantation (haploHSCT) not only provides the opportunity for expeditious identification of donors for almost all patients, it also opens the possibility for combining stem cell transplants with various adoptive T-cell therapies, such as T cells genetically engineered to express therapeutic TCRs.38 Successful haploHSCT has been performed following in vitro T-cell depletion of the graft prior to transplantation,39,40 or following reduction of T-cell numbers in vivo by post-transplantation treatment with cyclophosphamide day 3–4.14,41,13 This protocol has acceptable toxicity and non-transplant related mortality.41 An alternative approach to target malignant B cells while sparing a proportion of healthy cells might be to re-direct T cells with a CAR that recognizes only lambda or kappa positive B cells.42
Self-reactive T cells can be difficult to identify in the autologous T cell repertoire.43 Moreover, allo-reactive TCRs can readily be obtained that react to self-antigens with superior affinity relative to those identified in an autologous setting.44,45 Strong binding of re-directed T cells to the intended target is important for clinical efficacy,11,46 but on-target and off-target binding to cells other than the intended can potentially cause hazardous toxicities,31,47-51 and reviewed in15. T-cell allo-recognition has long been believed to be more cross-reactive or polyspecific than conventional recognition. The concept that allo-reactivity has low peptide-specificity has likely arisen from earlier studies performed in artificial settings that favor promiscuity. For instance, negative selection in mice expressing only one pMHC molecule can lead to an abnormal degree of cross-reactivity across MHC classes.52-54 In contrast, the normal human TCR repertoire has undergone negative selection against a vast variety of peptides presented on up to 12 HLA molecules. It is now clear that peptide recognition on foreign MHC can have very high specificity.22,43,44,55-60 Furthermore, it is interesting to note that while studies on allo-reactivity recently have identified a number of highly specific TCRs, there is increasing evidence that conventional recognition is more polyspecific than previously anticipated.55,61,62 In contrast to the allo-restricted TCRs used in the current study, TCRs with a sequence that has been mutated to improve the affinity have not been negatively selected against any HLA molecule. It is therefore maybe not surprising that serious off-target toxicities from the therapeutic use of T cells re-directed with such affinity-enhanced TCRs have been reported in patients.49
A recent study showed that although a single TCR can react with a large number of peptides restricted by a particular self-HLA class I molecule, these peptides have highly related and conserved amino acids in positions critical for contact with the TCR.62 Cross-reactivity is therefore predictable to a certain degree.62,63 However, rather than attempting to get a complete picture of which peptides in a vast repertoire that a TCR intended for therapeutic use can recognize, it might be preferable to determine clinically relevant cross-reactivity. Testing in vitro reactivity against panels of human cell types originating from a wide range of tissues might therefore be more relevant for safety assessments, although comparisons suggest that reactivity against cell panels might overestimate the in vivo cross-reactivity, rather than underestimate it.64 Thus, third-party donor-derived T cells reactive to CMV showed cross-reactivity in vitro, but did not induce GVHD upon transplantation to patients,64 indicating that low levels of in vitro cross-reactivity does not necessarily predict clinical toxicity.
In conclusion, we provide data showing that the off-target reactivity of the CD20p/HLA-A2-specific TCRs was very low to the predicted >58.000 different peptide ligands presented on HLA-A2 on each cell type 65-67 in the selected target cell panel, as well as to the large number of irrelevant HLA-alleles on these cells. Collectively, the data presented here pave the way for the adoptive transfer of T cells re-directed with CD20p/HLA-A2-specific TCRs to enter the clinic. When applying this approach in the haploHSCT setting, from an HLA-A2neg donor to an HLA-A2pos recipient, transduced donor-derived T cells would target malignant B cells without causing permanent depletion of normal B cells.
Materials and methods
Cell lines and culture conditions
This study was approved by the Regional Ethics Committee and the Institutional Review Board and performed in accordance with the declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy Blood Bank donors. CD4pos T cells from healthy donors were isolated using a Dynabeads CD4 positive isolation kit (Life Technologies, Oslo, Norway) and stored in liquid nitrogen. After informed, written consent, patients with lymphoma donated an affected lymph node and patients with CLL donated peripheral blood. The human T-cell lymphoblastic lymphoma cell line SupT1 (ECACC, 95013123) was a kind gift from M. Pule (UCL Cancer Institute, University College London, UK). The SupT1 cells were maintained in RPMI 1640 (Gibco, Invitrogen, Paisley, UK) supplemented with 10% FCS (Gibco) and 1% penicillin/streptomycin (Sigma-Aldrich, St Louis, MO, USA). SupT1 cells were retrovirally transduced to express HLA-A2 or single chain trimer HLA-A2 (SCT) molecules in complex with the indicated peptide. Expression of HLA-A2 or SCT was measured by staining with anti-HLA-A2 antibody. Sup-T1 cells were used as target cells in assays measuring T-cell responses. For source of other cell lines and culture conditions, see Supplemental Methods.
DNA constructs
The cDNA encoding CD20p/HLA-A2 specific TCR α and TCR β chains were cloned from our originally established cytotoxic T-cell lines and clones.43 The TCR cloning procedure was performed as described.68 The A23 TCR sequence is: TRBV19- CATAPGLSYEQYF (CDR3)-TRBJ2-7 TRAV30- CGTQGGSEKLVF (CDR3) – TRAJ-57, and the A94 TCR sequence is as follows: TRBV12-4-CASSPGLTYEQYF (CDR3)-TRBJ2-7 TRAV5-CAEWGQGGKLIF (CDR3)-TRAJ-23. The MART-1/HLA-A2-specific TCR DMF5 was a kind gift from R. Morgan (National Institute of Health, Bethesda, MD, USA). The CD19 CAR used was originally described in reference32 and cloned into a retroviral SFG vector by M. Pule and colleagues (MP12783. SFGmR.RQR8-2A-aCD19fmc63-CD8STK-41BBZ), and was a kind gift from M. Pule (University College London, UK).
HLA-A2 and CD20 cDNA were previously described.22 Single-chain trimer constructs (SCT) were cloned as described.69-71 The retroviral vector pMP71 was modified to a GatewayTM-compatible version and used to express all retroviral constructs, whereas a GatewayTM compatible pCIpA102 vector (SSL 2002) was used to generate in vitro transcribed mRNA for electroporation.68
Retroviral supernatant production and retroviral TCR gene transfer
Production of viral particles and retroviral transduction was performed as described,68 with modifications indicated in Supplemental Methods.
Antibodies and dyes, peptides and HLA-multimers
Cells were labeled for FACS analysis with the following antibodies, in-house conjugated to Pacific Blue, AlexaFluor 647 or Atto488: anti-HLA-A2 (BB7.2; AbD Serotec, Oxford, UK), -CD8+ (RPA-T8), -CD107a (H4A3) and -CD107b (H4B4) (all from BDB, San Jose, CA, USA). Anti-mouse TCR-β PE (H57-597) was from Becton Dickinson Biosciences (BDB; CA, USA). The cell proliferation dyes Cell Trace Violet (CTV, Life Technologies, Thermo Fisher Scientific Inc. Waltham, MA, USA) and Carboxyfluorescein succinimidyl ester (CFSE, Life Technologies) were used to trace T-cell populations. Caspase-3 substrate VAD-FITC (Abcam, Cambridge, UK) was used as an apoptosis marker and LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life technologies) as a dead cell marker.
The CD20188-196 peptide (SLFLGILSV, CD20p), MART-126–35 wt peptide (EAAGIGILTV, MART-1pwt), and the heteroclitic MART-126–35 peptide (ELAGIGILTV, MART-1phc) were synthesized by ProImmune Ltd. (Oxford, UK) or GenScript Inc. (NJ, USA). The pMHC multimers of HLA-A*02:01 included CD20p (SLFLGILSV) pentamer-PE (Proimmune Ltd), MART-1phc (ELAGIGILTV) dextramer-PE (Immudex, Denmark) and MART-1phc (ELAGIGILTV) tetramers synthesized by UV-mediated ligand exchange as described.72
Measuring degranulation and cytotoxicity by flow cytometry
Degranulation was measured as surface mobilization of CD107a,b in an FACS-based assay as described,56 with some modifications indicated in Supplemental Methods. For measurements of cytotoxic activity, TCR-transduced T cells were labeled with CTV to allow subsequent exclusion by flow cytometry. FITC-conjugated latex particles in solution, referred to as counting beads, (a kind gift from F. Lund-Johansen, Oslo University Hospital Rikshospitalet, Norway) were quantified with True count beads (BDB) by flow cytometry. TCR-transduced T cells and tumor cells were counted separately by flow cytometry using counting beads before co-culture at effector:target (E:T) ratios of 0:1 (3 × 104 tumor cells only), 2:1, 5:1 and 10:1 in 200 µL/well X-vivo 20 medium in U-bottomed polypropylene 96-well plates at 37°C for 4 h. The caspase 3 inhibitor DEVD-FMK-FITC (Abcam) was added to all wells at the start of culture for detection of apoptotic cells, as recommended by the manufacturer. At harvest, cells were washed and stained with LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life technologies) in V-bottomed polypropylene 96-well plates. Finally, cells were washed and a fixed number of counting beads was added to each well before flow cytometric analysis to allow for quantification of live cells left in the sample. Percentage of cytotoxic T lymphocyte (CTL)-induced target cell lysis was calculated according to the following formula: 100-((number of live target cells in CTL co-cultures/number of live target cells cultured alone) × 100). Cells were analyzed on a FACS LSRII (BDB) and data was analyzed with DIVA software (BDB) or FlowJo software (Tree Star, Inc.).
Chromium-51 release cytotoxicity assay
T2 target cells were labeled for 1 h with 10 μCi of 51Cr per 1 × 106 target cells at 37°C. Target cells were washed twice to remove excess 51Cr. The target cells were then loaded with relevant or irrelevant peptides at a concentration of 10 uM to 1 pM for 2 h. The labeled, peptide-loaded target cells were washed thrice to remove any free peptides and added to 96-well round-bottom plates in triplicates (5 × 103 labeled target cells). T cells re-directed with either CD20 TCRs (A94 and A23) or the control TCR DMF5 were added to the plates at E:T cell ratios of 5:1. Effectors and targets were incubated in RPMI with 10% FCS for 4 h at 37°C. The amount of 51Cr released, corresponding to target cell death, was measured by a gamma scintillation counter, and the percentage lysed target cells was calculated as follows: [(experimental lysis - spontaneous lysis)/(maximal lysis - spontaneous lysis)] × 100. To determine maximal lysis, 51Cr-labeled target cells were treated with 5 % Triton X for 4 h.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Jehad Charo (previously Max-Delbrück Center for Molecular Medicine, Berlin, Germany, presently DTA Oncology, Roche Innovation Center, Basel, Switzerland) for providing expert advice and guidance on retroviral transduction procedures, to Nomdo Christiaan Westerdaal for expert cell sorting and to Fridtjof Lund-Johansen and Terhi Kärpänen for helpful input to the manuscript.
Funding
This work was financed by the Research Council of Norway, the K.G. Jebsen Foundation, Oslo University Hospital Radiumhospitalet Gene therapy program, the University of Oslo and the Regional Health Authorities South-Eastern Norway/Helse Sør-Øst.
References
- 1.Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia 2014; 28:1388–95; PMID:24577532; http://dx.doi.org/ 10.1038/leu.2014.91 [DOI] [PubMed] [Google Scholar]
- 2.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. Am J Hematol 2014; 89:429–36; PMID:24687887; http://dx.doi.org/ 10.1002/ajh.23674 [DOI] [PubMed] [Google Scholar]
- 3.Griffin MM, Morley N. Rituximab in the treatment of non-Hodgkin's lymphoma–a critical evaluation of randomized controlled trials. Expert Opin Biol Ther 2013; 13:803–11; PMID:23560506; http://dx.doi.org/ 10.1517/14712598.2013.786698 [DOI] [PubMed] [Google Scholar]
- 4.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM et al.. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33:540–9; PMID:25154820; http://dx.doi.org/ 10.1200/JCO.2014.56.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF et al.. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507–17; PMID:25317870; http://dx.doi.org/ 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM et al.. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013; 122:4129–39; PMID:24055823; http://dx.doi.org/ 10.1182/blood-2013-08-519413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM et al.. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119:2709–20; PMID:22160384; http://dx.doi.org/ 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF et al.. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509–18; PMID:23527958; http://dx.doi.org/ 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al.. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38; PMID:23515080; http://dx.doi.org/ 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O et al.. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118:4817–28; PMID:21849486; http://dx.doi.org/ 10.1182/blood-2011-04-348540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 2013; 10:267–76; PMID:23546520; http://dx.doi.org/ 10.1038/nrclinonc.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb HJ. Graft-vs.-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112:4371–83; PMID:19029455; http://dx.doi.org/ 10.1182/blood-2008-03-077974 [DOI] [PubMed] [Google Scholar]
- 13.Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 2012; 39:683–93; PMID:23206845; http://dx.doi.org/ 10.1053/j.seminoncol.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolanos-Meade J, Rosner GL, Kanakry CG, Perica K, Symons HJ, Brodsky RA et al.. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 2015; 125:3024–31; PMID:25814532; http://dx.doi.org/ 10.1182/blood-2015-01-623991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpanen T, Olweus J. T-cell receptor gene therapy - ready to go viral? Mol Oncol 2015; 9:2019–42; PMID:26548533; http://dx.doi.org/ 10.1016/j.molonc.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeh HJ 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol 1999; 162:989–94; PMID:9916724 [PubMed] [Google Scholar]
- 17.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A 1996; 93:4102–7; PMID:8633023; http://dx.doi.org/ 10.1073/pnas.93.9.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006; 6:383–93; PMID:16622476; http://dx.doi.org/ 10.1038/nri1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorritsma A, Gomez-Eerland R, Dokter M, van de Kasteele W, Zoet YM, Doxiadis II, Rufer N, Romero P, Morgan RA, Schumacher TN et al.. Selecting highly affine and well-expressed TCRs for gene therapy of melanoma. Blood 2007; 110:3564–72; PMID:17660381; http://dx.doi.org/ 10.1182/blood-2007-02-075010 [DOI] [PubMed] [Google Scholar]
- 20.Sadovnikova E, Jopling LA, Soo KS, Stauss HJ. Generation of human tumor-reactive cytotoxic T cells against peptides presented by non-self HLA class I molecules. Eur J Immunol 1998; 28:193–200; PMID:9485199; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199801)28:01%3c193::AID-IMMU193%3e3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 21.Savage P, Gao L, Vento K, Cowburn P, Man S, Steven N, Ogg G, McMichael A, Epenetos A, Goulmy E et al.. Use of B cell-bound HLA-A2 class I monomers to generate high-avidity, allo-restricted CTLs against the leukemia-associated protein Wilms tumor antigen. Blood 2004; 103:4613–5; PMID:14988155; http://dx.doi.org/ 10.1182/blood-2003-11-3903 [DOI] [PubMed] [Google Scholar]
- 22.Abrahamsen IW, Kjellevoll S, Greve-Isdahl M, Mensali N, Walchli S, Kumari S, Loland BF, Egeland T, Kolstad A, Olweus J. T cells raised against allogeneic HLA-A2/CD20 kill primary follicular lymphoma and acute lymphoblastic leukemia cells. Int J Cancer 2012; 130:1821–32; PMID:21630262; http://dx.doi.org/ 10.1002/ijc.26209 [DOI] [PubMed] [Google Scholar]
- 23.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res 2006; 66:8878–86; PMID:16951205; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommermeyer D, Neudorfer J, Weinhold M, Leisegang M, Engels B, Noessner E, Heemskerk MH, Charo J, Schendel DJ, Blankenstein T et al.. Designer T cells by T cell receptor replacement. Eur J Immunol 2006; 36:3052–9; PMID:17051621; http://dx.doi.org/ 10.1002/eji.200636539 [DOI] [PubMed] [Google Scholar]
- 25.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 2007; 109:2331–8; PMID:17082316; http://dx.doi.org/ 10.1182/blood-2006-05-023069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Cohen CJ, Peng PD, Zhao Y, Cassard L, Yu Z, Zheng Z, Jones S, Restifo NP, Rosenberg SA et al.. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther 2008; 15:1411–23; PMID:18496571; http://dx.doi.org/ 10.1038/gt.2008.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res 2007; 67:3898–903; PMID:17440104; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 2004; 22:589–94; PMID:15064769; http://dx.doi.org/ 10.1038/nbt957 [DOI] [PubMed] [Google Scholar]
- 29.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol 1998; 160:1750–8; PMID:9469433 [PubMed] [Google Scholar]
- 30.Johnson LA, Heemskerk B, Powell DJ Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol 2006; 177:6548–59; PMID:17056587; http://dx.doi.org/ 10.4049/jimmunol.177.9.6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR et al.. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009; 114:535–46; PMID:19451549; http://dx.doi.org/ 10.1182/blood-2009-03-211714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G et al.. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009; 17:1453–64; PMID:19384291; http://dx.doi.org/ 10.1038/mt.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res 2010; 16:5852–61; PMID:21138872; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol 1980; 125:1678–85; PMID:6157744 [PubMed] [Google Scholar]
- 35.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A 1981; 78:3848–52; PMID:6973760; http://dx.doi.org/ 10.1073/pnas.78.6.3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solal-Celigny P. Safety of rituximab maintenance therapy in follicular lymphomas. Leuk Res 2006; 30 Suppl 1:S16–21; PMID:16750674; http://dx.doi.org/ 10.1016/S0145-2126(06)80004-4 [DOI] [PubMed] [Google Scholar]
- 37.Davenport AJ, Jenkins MR, Cross RS, Yong CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK, Neeson PJ. CAR-T Cells Inflict Sequential Killing of Multiple Tumor Target Cells. Cancer Immunol Res 2015; 3:483–94; PMID:25711536; http://dx.doi.org/ 10.1158/2326-6066.CIR-15-0048 [DOI] [PubMed] [Google Scholar]
- 38.De Witte MA, Kierkels GJ, Straetemans T, Britten CM, Kuball J. Orchestrating an immune response against cancer with engineered immune cells expressing alphabetaTCRs, CARs, and innate immune receptors: an immunological and regulatory challenge. Cancer Immunol Immunother 2015; 64(7):893–902; PMID:25990073; http://dx.doi.org/ 10.1007/s00262-015-1710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, Pende D, Falco M, Handgretinger R, Moretta F et al.. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood 2014; 124:822–6; PMID:24869942; http://dx.doi.org/ 10.1182/blood-2014-03-563817 [DOI] [PubMed] [Google Scholar]
- 40.Reisner Y, Aversa F, Martelli MF. Haploidentical hematopoietic stem cell transplantation: state of art. Bone Marrow Transplant 2015; 50 Suppl 2:S1–5; PMID:26039199; http://dx.doi.org/ 10.1038/bmt.2015.86 [DOI] [PubMed] [Google Scholar]
- 41.Patriarca F, Luznik L, Medeot M, Zecca M, Bacigalupo A, Di Bartolomeo P, Arcese W, Corradini P, Ciceri F, Vago L et al.. Experts' considerations on HLA-haploidentical stem cell transplantation. Eur J Haematol 2014; 93:187–97; PMID:24660868 [DOI] [PubMed] [Google Scholar]
- 42.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, Wu J, Heslop HE, Rooney CM, Brenner MK et al.. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006; 108:3890–7; PMID:16926291; http://dx.doi.org/ 10.1182/blood-2006-04-017061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahamsen IW, Stronen E, Walchli S, Johansen JN, Kjellevoll S, Kumari S, Komada M, Gaudernack G, Tjonnfjord G, Toebes M et al.. Targeting B cell leukemia with highly specific allogeneic T cells with a public recognition motif. Leukemia 2010; 24:1901–9; PMID:20844564; http://dx.doi.org/ 10.1038/leu.2010.186 [DOI] [PubMed] [Google Scholar]
- 44.Wilde S, Sommermeyer D, Frankenberger B, Schiemann M, Milosevic S, Spranger S, Pohla H, Uckert W, Busch DH, Schendel DJ. Dendritic cells pulsed with RNA encoding allogeneic MHC and antigen induce T cells with superior antitumor activity and higher TCR functional avidity. Blood 2009; 114:2131–9; PMID:19587379; http://dx.doi.org/ 10.1182/blood-2009-03-209387 [DOI] [PubMed] [Google Scholar]
- 45.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, Schendel DJ. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest 2010; 120:3869–77; PMID:20978348; http://dx.doi.org/ 10.1172/JCI43437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL et al.. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917–24; PMID:21282551; http://dx.doi.org/ 10.1200/JCO.2010.32.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010; 18:843–51; PMID:20179677; http://dx.doi.org/ 10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G et al.. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Translational Med 2013; 5:197ra03; PMID:23926201; http://dx.doi.org/ 10.1126/scitranslmed.3006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ et al.. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122:863–71; PMID:23770775; http://dx.doi.org/ 10.1182/blood-2013-03-490565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM et al.. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013; 36:133–51; PMID:23377668; http://dx.doi.org/ 10.1097/CJI.0b013e3182829903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM et al.. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011; 19:620–6; PMID:21157437; http://dx.doi.org/ 10.1038/mt.2010.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell 2005; 122:247–60; PMID:16051149; http://dx.doi.org/ 10.1016/j.cell.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 53.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell 1996; 84:521–9; PMID:8598039; http://dx.doi.org/ 10.1016/S0092-8674(00)81028-4 [DOI] [PubMed] [Google Scholar]
- 54.Logunova NN, Viret C, Pobezinsky LA, Miller SA, Kazansky DB, Sundberg JP, Chervonsky AV. Restricted MHC-peptide repertoire predisposes to autoimmunity. J Exp Med 2005; 202:73–84; PMID:15998789; http://dx.doi.org/ 10.1084/jem.20050198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, Allen PM. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol 2007; 8:388–97; PMID:17322886; http://dx.doi.org/ 10.1038/ni1446 [DOI] [PubMed] [Google Scholar]
- 56.Stronen E, Abrahamsen IW, Gaudernack G, Walchli S, Munthe E, Buus S, Johansen FE, Lund-Johansen F, Olweus J. Dendritic cells engineered to express defined allo-HLA peptide complexes induce antigen-specific cytotoxic T cells efficiently killing tumour cells. Scand J Immunol 2009; 69:319–28; PMID:19284496; http://dx.doi.org/ 10.1111/j.1365-3083.2008.02223.x [DOI] [PubMed] [Google Scholar]
- 57.Amir AL, van der Steen DM, Hagedoorn RS, Kester MG, van Bergen CA, Drijfhout JW, de Ru AH, Falkenburg JH, van Veelen PA, Heemskerk MH. Allo-HLA-reactive T cells inducing graft-vs.-host disease are single peptide specific. Blood 2011; 118:6733–42; PMID:21972290; http://dx.doi.org/ 10.1182/blood-2011-05-354787 [DOI] [PubMed] [Google Scholar]
- 58.Housset D, Malissen B. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity Trends Immunol 2003; 24:429–37; PMID:12909456; http://dx.doi.org/ 10.1016/S1471-4906(03)00180-7 [DOI] [PubMed] [Google Scholar]
- 59.Udaka K, Tsomides TJ, Eisen HN. A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein. Cell 1992; 69:989–98; PMID:1606619; http://dx.doi.org/ 10.1016/0092-8674(92)90617-L [DOI] [PubMed] [Google Scholar]
- 60.Kronig H, Hofer K, Conrad H, Guilaume P, Muller J, Schiemann M, Lennerz V, Cosma A, Peschel C, Busch DH et al.. Allorestricted T lymphocytes with a high avidity T-cell receptor towards NY-ESO-1 have potent anti-tumor activity. Int J Cancer 2009; 125:649–55; PMID:19444908; http://dx.doi.org/ 10.1002/ijc.24414 [DOI] [PubMed] [Google Scholar]
- 61.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nat Rev Immunol 2007; 7:942–53; PMID:18007679; http://dx.doi.org/ 10.1038/nri2200 [DOI] [PubMed] [Google Scholar]
- 62.Birnbaum ME, Mendoza JL, Sethi DK, Dong S, Glanville J, Dobbins J, Ozkan E, Davis MM, Wucherpfennig KW, Garcia KC. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 2014; 157:1073–87; PMID:24855945; http://dx.doi.org/ 10.1016/j.cell.2014.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Archbold JK, Macdonald WA, Miles JJ, Brennan RM, Kjer-Nielsen L, McCluskey J, Burrows SR, Rossjohn J. Alloreactivity between disparate cognate and allogeneic pMHC-I complexes is the result of highly focused, peptide-dependent structural mimicry. J Biol Chem 2006; 281:34324–32; PMID:16963442; http://dx.doi.org/ 10.1074/jbc.M606755200 [DOI] [PubMed] [Google Scholar]
- 64.Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, Brenner MK, Barrett AJ, Heslop HE. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 2010; 116:4700–2; PMID:20709906; http://dx.doi.org/ 10.1182/blood-2010-06-289991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumari S, Walchli S, Fallang LE, Yang W, Lund-Johansen F, Schumacher TN, Olweus J. Alloreactive cytotoxic T cells provide means to decipher the immunopeptidome and reveal a plethora of tumor-associated self-epitopes. Proc Natl Acad Sci U S A 2014; 111:403–8; PMID:24344295; http://dx.doi.org/ 10.1073/pnas.1306549111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nielsen M, Lundegaard C, Blicher T, Lamberth K, Harndahl M, Justesen S, Roder G, Peters B, Sette A, Lund O et al.. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One 2007; 2:e796; PMID:17726526; http://dx.doi.org/ 10.1371/journal.pone.0000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Verteuil D, Granados DP, Thibault P, Perreault C. Origin and plasticity of MHC I-associated self peptides. Autoimmun Rev 2012; 11:627–35; PMID:22100331; http://dx.doi.org/ 10.1016/j.autrev.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 68.Walchli S, Loset GA, Kumari S, Johansen JN, Yang W, Sandlie I, Olweus J. A practical approach to T-cell receptor cloning and expression. PLoS One 2011; 6:e27930; PMID:22132171; http://dx.doi.org/ 10.1371/journal.pone.0027930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Truscott SM, Lybarger L, Martinko JM, Mitaksov VE, Kranz DM, Connolly JM, Fremont DH, Hansen TH. Disulfide bond engineering to trap peptides in the MHC class I binding groove. J Immunol 2007; 178:6280–9; PMID:17475856; http://dx.doi.org/ 10.4049/jimmunol.178.10.6280 [DOI] [PubMed] [Google Scholar]
- 70.Mitaksov V, Truscott SM, Lybarger L, Connolly JM, Hansen TH, Fremont DH. Structural engineering of pMHC reagents for T cell vaccines and diagnostics. Chem Biol 2007; 14:909–22; PMID:17719490; http://dx.doi.org/ 10.1016/j.chembiol.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walseng E, Walchli S, Fallang LE, Yang W, Vefferstad A, Areffard A, Olweus J. Soluble T-cell receptors produced in human cells for targeted delivery. PLoS One 2015; 10:e0119559; PMID:25875651; http://dx.doi.org/ 10.1371/journal.pone.0119559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodenko B, Toebes M, Hadrup SR, van Esch WJ, Molenaar AM, Schumacher TN, Ovaa H. Generation of peptide-MHC class I complexes through UV-mediated ligand exchange. Nat Protoc 2006; 1:1120–32; PMID:17406393; http://dx.doi.org/ 10.1038/nprot.2006.121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


