Abstract
Background
The drawbacks of iliac crest autograft as graft material for spine fusion are well reported. Despite continued modifications to improve bone healing capacity, the efficacy of synthetic graft materials as stand-alone replacements remains uncertain. The rabbit posterolateral fusion model is an established environment for testing of fusion concepts. It offers the opportunity to obtain radiographic, biomechanical and histological data on novel fusion materials. The objective of this study was to compare the spine fusion capability of two synthetic bone graft products in an established rabbit posterolateral spine fusion (PLF) model: Signafuse® Bioactive Bone Graft Putty and Actifuse® ABX.
Methods
Bilateral intertransverse spine fusion was performed at the L5-L6 transverse processes (TPs) of New Zealand White rabbits using either Signafuse or Actifuse ABX as the bone graft material. Bone remodeling and spine fusion were assessed at 6 and 12 weeks using radiographic, biomechanical and histological endpoints.
Results
Fusion rate by manual palpation at 6 weeks was greater for Signafuse (33%) compared to Actifuse ABX (0%), and equivalent in both groups at 12 weeks (50%). Biomechanical fusion rate based on flexion-extension data was 80% in Signafuse group and 44% for Actifuse ABX. Histology revealed a normal healing response in both groups. MicroCT and histomorphometric data at 6 weeks showed greater new bone formation in the Signafuse group compared to Actifuse ABX (p <0.05), with no differences detected at 12 weeks. Histological fusion scores were greater in the Signafuse group at 6 and 12 weeks, indicated by higher degree structural remodeling and tendency towards complete bridging of the fusion bed compared to the Actifuse ABX group.
Conclusion
Confirmed by several metrics, Signafuse outperformed Actifuse ABX as a standalone synthetic bone graft in an established PLF model, demonstrating greater rates of bone remodeling and spine fusion. The combination of 45S5 bioactive glass and biphasic HA/βTCP granules of Signafuse appear to provide greater bone healing capability in comparison to the 0.8% silicate-substituted hydroxyapatite material of Actifuse ABX.
Background
Iliac crest autograft (ICBG) has been used for many years as a bone graft in spinal fusion procedures despite issues associated with donor site morbidity1-11. Synthetic alternatives to autograft are commercially available in a number of forms and have been shown to support bone healing used as stand-alone, extender, or enhancer type products. Calcium phosphate materials have been used clinically as the foundation of synthetic bone void fillers for several decades. Such products have been demonstrated to be osteoconductive, providing a scaffold for cell. Calcium phosphate grafts are similar in composition and crystalline structure to human bone and generally comprise some form of hydroxyapatite (HA), beta tricalcium phosphate (βTCP), or a blend of the two (HA/ βTCP), referred to as biphasic calcium phosphate (BCP). Depending on specific composition, these biomaterials differ in terms of resorption rate, osteoconductivity, and remodeling capability12.
In an attempt to enhance the bone healing capabilities of calcium phosphate materials, certain modifications have been incorporated into several products with claims that these changes bestow “bioactive” or “stimulative” properties. More recently, products have been introduced that contain synthetic granules with collagen or resorbable polymers to render the graft moldable to improve delivery and retention of the granular materials at the surgical site.
In this investigation, Signafuse® Bioactive Bone Graft Putty (BioStructures, LLC, New Port Beach, CA, USA), comprised of biphasic calcium phosphate granules (1-2 mm) and 45S5 bioactive glass (212-420 μm) suspended in a resorbable alkylene oxide polymer (AOP) matrix, was compared to Actifuse ABX (Baxter Healthcare, Deerfiled IL, USA), a phase-pure, silicate-substituted hydroxyapatite granules (1-2 mm) suspended in a resorbable alkylene oxide copolymer (AOC) matrix (Figure 1), using an established posterolateral spine fusion (PLF) rabbit model13-19. The aims of the study were to directly compare the fusion performance of two synthetic bone graft products as well as determine potential correlations between the “claimed” biological properties of each material and the actual bone healing and fusion capability observed in vivo.
Figure 1.
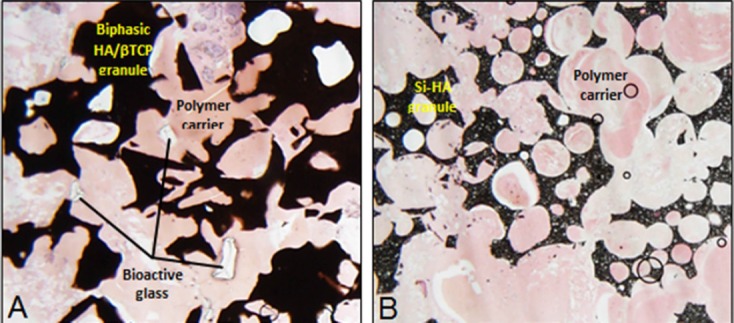
Time 0 histology showing the granulate and polymer carrier components of the Signafuse (A) and Actifuse ABX (B) graft materials.
Methods
Bilateral intertransverse spine fusion was performed at the L5-L6 transverse processes (TPs) of New Zealand White rabbits using either Signafuse or Actifuse ABX as the bone graft material. All surgeries were performed following IACUC approval (1208187). Animals were sacrificed at 6 weeks (n=6) and 12 weeks (n=10) for each treatment group. The experimental design is shown in Table I.
Table I.
Experimental Design
| Endpoint | Animals per Time Point (n) | |
|---|---|---|
| 6 weeks | 12 weeks | |
| Radiographic | 6 | 10 |
| Macroscopic Evaluation | 6 | 10 |
| MicroCT | 6 | 10 |
| Manual Palpation | 6 | 10 |
| Biomechanical Testing | - | 10 |
| Histo pathology | 3 | 5 |
| Histomorphometry | 3 | 5 |
| Histological Fusion | 3 | 5 |
Surgical Procedure
A dorsal midline skin incision, approximately 15 centimeters long, was made from L1 to the sacrum. Overlying fascia and muscle were incised over the L5-L6 TPs. The TPs were then decorticated with a high-speed burr. Approximately 2.5-3.0 cc per side of test article was placed in the paraspinal bed between the TPs. The fascia and skin were closed in the routine manner consistent with good surgical practice. Post-operative care of the animals was performed in accordance with good husbandry practices as understood in the art.
Necropsy/ Macroscopic Evaluation
Animals were euthanized using Euthasol solution (120 mg/kg IV). Necropsy was conducted on all study animals according to standard operating procedures under the supervision of the principal investigator (PI). The entire lumbar column was removed “en-bloc”. Soft tissues were immediately removed from the surgically treated spinal unit after the spine was dissected out of the body. The grafted site was examined for graft migration, infection, and soft tissue abnormalities. Spines from the 6 week animals were placed in 10% neutral buffered formalin. Spines from the 12 week animals were immediately biomechanically tested.
Radiographic Assessment
Ventral/dorsal radiographs were obtained with a Simon DR (Quantum) RAD-X High Frequency Radiographic Imaging System, (model: E7242X), and stored using Whitecap PACs system. Radiographic images were obtained immediately postoperatively and at 6 and 12 weeks post-surgery. Animals received sedation prior to radiography and images were assessed for osteolysis, fracture, and/or any other adverse radiographic events including infection and graft migration. Radiographic assessment of fusion was not performed as calcium phosphate-based materials, such as those contained in the test articles, look similar to bone preventing adequate radiographic evaluation13,20.
MicroCT Assessment
Microcomputed tomography (microCT) scans of the fusion defects were obtained at 6 and 12 weeks postoperatively. Bilateral morphometric analysis of sagittal scans was performed using a rectangular region of interest (ROI) of 250 mm2 placed across the fusion site inclusive of the TPs. Bone area was determined based on validated contrast parameters and reported as a percentage of the ROI.
Manual Palpation
Following spine removal at 12 weeks, three blinded independent observers graded the fusion mass as fused or not fused. Fused meant that there was no noticeable movement of the fusion mass in flexion and extension, while not fused meant movement of the fusion mass. Consensus among 2 of the 3 observers decided final results13-19.
Biomechanical Testing & Fusion Analysis
Non-destructive biomechanical stiffness testing was performed following manual palpation of the 12 week animals. Testing consisted of flexion/extension, lateral bending, and torsion to a pre-determined, sub-failure load. The vertebral bodies cranial and caudal to the fused motion segment were mounted in a biaxial servohydraulic materials testing machine (858 Bionix II, MTS Corporation, Eden Prairie, MN) retrofitted with two spine gimbals and a passive XZ table. Custom-made rigid body markers were placed on each vertebral body and both gimbals to track segmental motion. Non-destructive flexibility tests were performed about each axis of rotation (i.e., flexion-extension, right-left lateral bending, and right-left axial rotation) by applying an isolated ±0.27 Nm moment about each of the primary axes. Each test initiated and concluded in the neutral position with zero load. Three loading and unloading cycles were performed with motion data collected on the third cycle. The displacement of each vertebrae was measured using an optoelectronic motion capture system, the output of which was synchronized with that of the MTS. During testing, the specimens were kept moist with saline solution spray. Stiffness was determined and compared to normal controls. Range of motion (ROM) data was compared between test groups and to historical internal laboratory data of normal unfused rabbit spines. Normal motion of the rabbit lumbar spine was determined by testing 10 normal (untreated/ unfused) rabbit lumbar spinal columns using the same testing methods as described above. Biomechanical determination of spine fusion was based on the flexionextension ROM data, which provides a direct correlation to the manual palpation evaluation16. Specimens showing a total ROM of less than 5 degrees were deemed to be fused. This threshold has been previously reported in the PLF rabbit model using ICBG, where Elruker and colleagues demonstrated that solid fusion, as initially determined by manual palpation, correlates to ROM of less than 5o in flexion-extension23.
Histologic Processing
Fusion sites were sectioned in the sagittal plane to obtain a total of 6 sections per animal (3 per side of the vertebral body). For each side, sections were created adjacent to the vertebral body, through the center of the fusion mass, and through the lateral aspect of the fusion mass, spaced approximately 3 mm apart. A total of six rabbits per test group were processed at 6 weeks and ten rabbits per test group at 12 weeks. At each time point, animals were evenly allocated for either decalcified paraffin embedded or non-decalcified plastic embedded processing. Routine hematoxylin and eosin (H&E) staining was applied to all slides.
Histopathology
Decalcified, paraffin-embedded sections were evaluated for histopathologic changes at 6 and 12 weeks using low and high magnification fields from each slide. Slides were viewed by the PI and a board certified veterinary pathologist. Areas of bone tissue, soft tissue (e.g. fibrous tissue, fibrocartilage, bone marrow), and graft were labelled on representative images from each group and time period. Areas of inflammation, osteoconduction (e.g. centripetal bone growth through open pores), osteointegration (bone-biomaterial contact), and resorption were also identified. The host biological response was scored and semiquantitativly assessed based on ISO 10993-620.
Histomorphometry
Non-decalcified plastic embedded sections were subject to bilateral histomorphometry analysis at 6 and 12 weeks. A rectangular ROI of 85.8 mm2 placed across the middle of the fusion bed between but not inclusive of the TPs was analyzed for each slide. Bone area was determined based on validated color pixel parameters and reported as a percentage of the ROI.
Histologic Fusion Analysis
Non-decalcified plastic embedded histology sections for each test group were semiquantitatively assessed for fusion at 6 and 12 weeks by 3 blinded observers according to the scale scoring shown in Table II19. Scores for each slide from the 3 observers were averaged to obtain a fusion score for each animal. The final fusion scores were determined as the mean of the animal fusion scores for each test group and time point.
Table II.
Histological Fusion Scoring Parameters
| Fusion Status | Score |
|---|---|
| Union of TPs by mature bone; complete bridge | 10 |
| Union of TPs by immature bone and cartilage; com¬plete bridge | 9 |
| Union of TPs by cartilage with little fibrocartilage | 8 |
| Partial union with more bone (>75%) than cartilage and fibrocartilage | 7 |
| Partial union with more (56%-75%) than other tissues (i.e., cartilage, fibrocartilage and fibrous tissue) | 6 |
| Partial bridge; equal amounts of bone (4596-55%) and other tissues | 5 |
| Minimal bridge with less bone (259644%) than other tissues | 4 |
| Minimal bone (<25%) with predominantly other tissues | 3 |
| Little new bone with predominantly fibrous tissue | 2 |
| Fibrous tissue only between TP; full (across the defect) | 1 |
Statistical Analysis
Statistical analysis was performed on the biomechanical flexibility data as well as the normalized microCT and histomorphometric area percentages. First, a normality test was performed on each data set from each time point. If the data was normal, a student’s t-test was conducted (α=0.05). If the data was not normal, a nonparametric Mann-Whitney test was used (α=0.05). All statistical analysis was performed using Minitab software (version 15.1.1.0: Minitab Inc., State College, PA, USA). All morphometry data was analyzed to a 95% confidence level (p<0.05) with a 2-tailed Student’s t-test, assuming unequal variance using Microsoft Excel.
Results
Necropsy/ Macroscopic Evaluation
Necropsy of the animals was unremarkable regardless of test group. Macroscopic analysis of the implant sites demonstrated healthy tissue with no apparent adverse effects such as inflammation, tissue necrosis, or devascularized tissue surrounding the defect sites.
Radiography
Radiographic observations at 6 and 12 weeks indicated a normal healing response over time in both test groups, with no evidence of fractures, osteolysis, or other adverse reactions.
MicroCT Assessment
The 6 week morphometric data showed significantly greater bone area in the Signafuse group compared to Actifuse ABX (19.7% vs. 12.3%; p <0.05), while the 12 week data showed no statistical differences between groups (Table III). Although the area-based morphometric bone data was similar at 12 weeks, distinctions in the alignment and structural development of new bone across the fusion bed were observed between groups. The Signafuse group generally demonstrated a more developed fusion structure compared to Actifuse ABX, characterized by a higher tendency of mature bone spanning the fusion bed. The Actifuse ABX group generally demonstrated a lesser degree of mature bone formation and structural development across the fusion defect (Figure 2).
Table III.
MicroCT Morphometric Results
| Test Group | Normalized Bone Area (%) | |
|---|---|---|
| 6 weeks | 12 weeks | |
| Signafuse® | 19.7 ±6.2 | 23.2 ±7.7 |
| Actifuse ABX | 12.3 ±3.4 | 21.4 ±7.6 |
| p-value | 0.002 | 0.478 |
Figure 2.
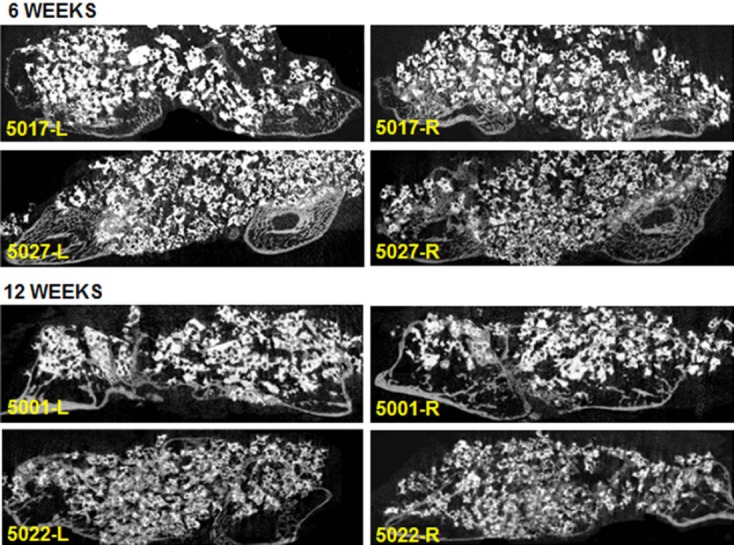
Representative bilateral microCT sagittal images of the fusion defects at 6 and 12 weeks for Signafuse (5017, 5001) and Actifuse ABX (5027, 5022).
Manual Palpation
At 6 weeks, the fusion rate by manual palpation was 33% (2/6 animals) for Signafuse and 0% (0/6 animals) for Actifuse ABX. At 12 weeks, the fusion rate was 50% (5/10 animals) in both test groups, and thus no differences were detected between groups.
Biomechanical Testing & Fusion Analysis
At 12 weeks, both groups had statistically significant less ROM in all motion planes compared to the normal controls (p <0.001), and no differences were detected between test groups in any motion plain (Table IV). However, the mean flexion-extension ROM for the Signafuse group (4.19o) met the fusion criteria (< 5o) while Actifuse ABX did not (5.35o). Analysis of individual specimens based on this flexion-extension fusion criteria revealed a biomechanical fusion rate of 80% (8/10 animals) in the Signafuse group and 44% (4/9 animals) for Actifuse ABX (Table IV).
Table IV.
Biomechanical Range of Motion Results
| Test Group | Flex-Ext (°) | Lat Bend (°) | Axial Rot (°) |
|---|---|---|---|
| Normal Unfused* | 14.57 | 13.43 | 2.92 |
| Signafuse | 4.19 | 2.04 | 0.93 |
| Actifuse ABX | 5.35 | 3.42 | 0.99 |
Obtained from historical internal laboratory data of normal unfused rabbits.
Histopathology
Histopathology analysis of decalcified paraffin sections generally revealed minimal inflammation and a normal healing response regardless of implant type or time point, largely characterized by very low numbers of macrophages and multinucleated giant cells (which in some cases could be osteoclasts) with some scattered, often rare lymphocytes and plasma cells. All fusion sections demonstrated moderate neovascularization, fibroconnective tissue and new bone formation, which was more mature in the 12 weeks animals compared to the 6 weeks animals. New bone formation was not specifically scored but at both 6 and 12 weeks points it appeared that Signafuse had more abundant new bone formation and remodeling as compared to Actifuse ABX. Semiquantitative histology analysis at 6 weeks indicated a similar biological response in both groups (Table VI). The scoring at 12 weeks indicated an increased response in both groups, due to an increase in macrophages and giant cells likely associated bone and/or tissue remodeling. There were more giant cells associated with new bone and/or implant material suggestive of osteoclast remodeling. In lieu of this, there were, as expected, an increased number of macrophages to clean up associated cellular debris. Neovascularization and fibrosis were similar to that at 6 weeks in both groups. The increased response of the Signafuse treated defects compared to Actifuse ABX at 12 weeks correlates with the observation of more abundant new bone formation and remodeling in the Signafuse sections.
Table VI.
Histopathology Analysis
| Test Group | Semiquantitative Analysis (ISO 10993-6) | |
|---|---|---|
| 6 weeks | 12 weeks | |
| Signafuse® | 25.60 | 68.27 |
| Actifuse ABX | 24.00 | 52.40 |
Table V.
Biomechanical Fusion Analysis
| Treatment Group | Biomechanical Fusion Rate (< 5o ROM, Flexion-Extension) |
|
|---|---|---|
| Signafuse® | 8/10 rabbits | 80% |
| Actifuse ABX | 4/9 rabbits* | 44% |
One of the fusion masses broke before biomechanical testing.
Histomorphometry
Histomorphometry analysis of the nondecalcified sections revealed significantly greater bone area in the Signafuse group at 6 weeks compared to Actifuse ABX (29.4% vs. 24.4%; p <0.05), while the 12 week data showed no statistical differences between groups (Table VII).
Table VII.
Histomorphometry Analysis
| Test Group | Normalized Bone Area (%) | |
|---|---|---|
| 6 weeks | 12 weeks | |
| Signafuse® | 29.4 ±6.0 | 22.9 ±6.8 |
| Actifuse ABX | 24.4 ±6.4 | 20.9 ±7.7 |
| p-value | 0.019 | 0.288 |
Histological Fusion Analysis
Although the area-based bone histomorphometry data was similar at 12 weeks, distinctions in the structural development of new bone across the fusion bed were observed between groups, as demonstrated by the histological fusion scores (Table VIII). The scoring suggests more advanced remodeling of the fusion defects in the Signafuse group at both 6 and 12 weeks, characterized by a greater presence of mature bone and tendency toward complete bridging of the transverse processes, which was less apparent in the Actifuse ABX group (Figure 3). The bioactive glass component of Signafuse was appreciably resorbed at 6 weeks and completely replaced by host bone at 12 weeks. The ceramic component in both groups showed new bone formation in direct apposition to and bridging between granules. The biphasic granules of Signafuse demonstrated a loss of distinction at the new bone interface indicating a normal resorption process, which was less apparent for the silicatesubstituted HA granules of Actifuse ABX (Figure 4). The greater structural development of the fusion masses observed over time in the Signafuse group also supports the microCT assessment and biomechanical fusion data.
Table VIII.
Histological Fusion Scores
| Test Group | Fusion Score | |
|---|---|---|
| 6 weeks | 12 weeks | |
| Signafuse® | 5.46 ±0.79 | 6.76 ±0.67 |
| Actifuse ABX | 3.83 ±0.62 | 4.13 ±0.65 |
| p-value | 0.156 | 0.047 |
Figure 3.
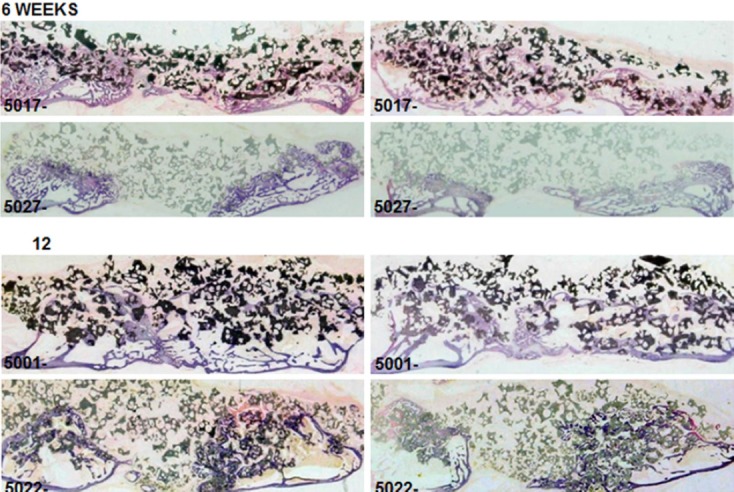
Representative bilateral non-decalcified histology of the fusion defects at 6 and 12 weeks for Signafuse (5017, 5001) and Actifuse ABX (5027, 5022).
Figure 4.
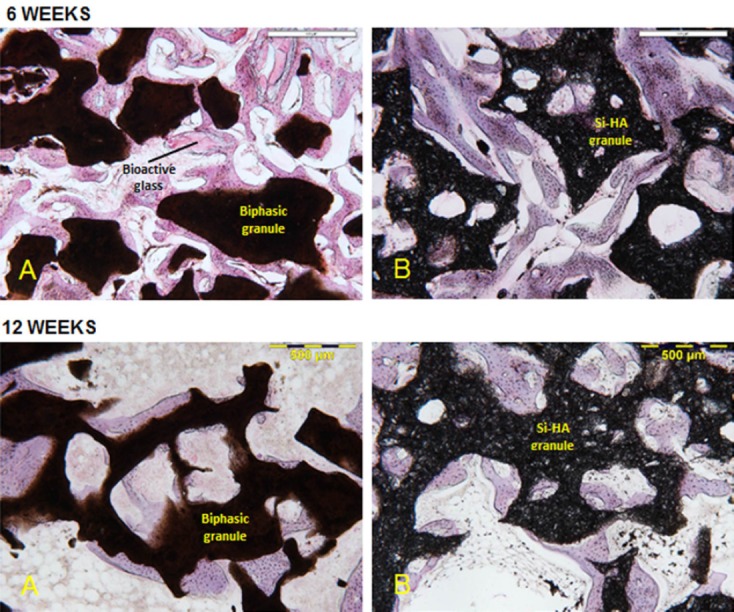
Representative hi-magnification non-decalcified histology at 6 and 12 weeks showing the remodeling progression of the graft materials for Signafuse (A) and Actifuse ABX (B).
DISCUSSION
Synthetic bone graft materials continue to be developed as alternatives to ICBG, with the ultimate goal of optimizing implant resorption and bone substitution such that solid bony fusion can be reliably achieved12. This study evaluated the fusion performance of two synthetic bone graft products for which the base materials have been reported to influence the biologic healing response in bony defects, namely Signafuse and Actifuse ABX. Confirmed by multiple metrics, Signafuse outperformed Actifuse ABX as a standalone synthetic bone graft in an established PLF rabbit model, demonstrating greater rates of bone remodeling and spine fusion. The distinctions in performance between the grafts were observed in all endpoints including radiographic, biomechanical and histological evaluations.
The 50% fusion rate by manual palpation observed in both test groups at 12 weeks is typical of this animal model. The majority of reported PLF rabbit studies have used ICBG as the standard of care control, as it best mirrors in situ fusions in humans. Published metaanalyses of these studies have revealed manual palpation fusion rates of 56.8% and 58.3%14-15. Manual palpation has historically been considered an accurate indicator of solid fusion in this animal model because it allows direct functional assessment, as would be performed during surgical exploration in the clinical setting. However, manual palpation is a subjective evaluation dependent on perceived relative rigidity of the fusion segments, and therefore comprehensive evaluation of additional endpoints is often required to judge actual fusion status. In this study, biomechanical ROM of the fused segments in flexion-extension was used as an objective determinant of solid fusion, based on previously reported ROM data for ICBG in the rabbit PLF model. Elruker and colleagues, using almost identical method as employed in this study, demonstrated that solid fusion, as initially determined by manual palpation, correlates to total ROM in flexion-extension of less than five degrees (< 5o) in ICBG treated fusions16. Due to the established efficacy of ICBG in the rabbit PLF model, this value was used as the determinant of biomechanical fusion in the current study. This measurement is also relevant in the clinical setting, where less than 5° ROM, as observed in lateral flexion-extension radiographs, has been used as a criterion for successful fusion21.
Of significant note is that the 80% (8/10 animals) fusion rate in the Signafuse group is higher than the 63% (5/8 animals) fusion rate reported in the referenced ICBG study18. Although this data represents fusion at 5 weeks, it has been reported that ICBG fusion rates do not significantly increase past this time point, and therefore a casual comparison of Signafuse fusion rates to ICBG may be warranted.
The greater bone area across the fusion bed measured in the Signafuse group at 6 weeks was likely due to the presence of the bioactive glass inter-dispersed among the BCP granules. Aside from the potential biologic effects previously reported22-25, the rapid dissolution and apatite layer formation may have produced a viable osteoconductive substrate for cellular attachment earlier in healing process. The biphasic HA/βTCP granules demonstrated intimate surface bonding with host bone and indicated an active remodeling process based on changes in granular appearance and loss of distinction at the new bone interface. The silicate-substituted hydroxyapatite (Si-HA) material of Actifuse ABX did demonstrate sufficient bone bonding capability, however new bone formation was largely associated with the decorticated transverse processes, with limited formation across the defect. In addition, a more distinct surface demarcation was evident at the host bone interface, indicating low solubility and a limited resorption profile.
Although the morphometric data at 12 weeks was similar in both groups, appreciable differences in the maturity and structure of new bone formation was observed between groups in the histological fusion scoring. As remodeling of the fusion mass progresses, new bone will condense and align directionally according to anatomical constraints and physiological stress, which can result in minimal change or a reduction in morphometric bone area in successfully fused specimens. The progression in bone remodeling toward a structurally mature fusion mass at 12 weeks, evidenced by mature bone and developed marrow spaces bridging across the defect, may be attributed to the continued dissolution and complete remodeling of bioactive glass, in addition to the gradual resporption properties of the BCP granules, which appeared to be compatible with the host remodeling process. The Si-HA granules of Actifuse ABX did support a progression in new bone formation and remodeling over time, but the fusion structures were relatively underdeveloped, evidenced by a lesser degree of mature bone and bridging between TPs and apparent minimal resporption of the Si-HA granules.
In conclusion, this study has demonstrated the efficacy of Signafuse as a viable standalone replacement for ICBG in spinal fusion procedures based on performance outcomes in an established rabbit spine fusion model. Fusion defects treated with Signafuse demonstrated greater levels of bone remodeling and higher fusion rates compared to Actifuse ABX, as confirmed across several endpoints. The combination of biphasic calcium phosphate and bioactive glass appears to elicit a greater bone healing response than the 0.8% silicate-substituted hydroxyapatite. The prevalence of structurally mature fusion development in the 12 week Signafuse animals, observed both biomechanically and histologically, is uncommon for a synthetic bone graft and compares favorably to historical ICBG data in this animal model.
References
- 1.Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin.Orthop.Relat Res. 1996:300–9. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J.Am.Acad.Orthop.Surg. 2001;9:210–8. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Hu R, Hearn T, Yang J. Bone graft harvest site as a determinant of iliac crest strength. Clin.Orthop. Relat Res. 1995:252–6. [PubMed] [Google Scholar]
- 4.Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin.Orthop.Relat Res. 1994:208–13. [PubMed] [Google Scholar]
- 5.Kahn B. Superior gluteal artery laceration, a complication of iliac bone graft surgery. Clin.Orthop.Relat Res. 1979:204–7. [PubMed] [Google Scholar]
- 6.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–31. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Sasso RC, Williams JI, Dimasi N, et al. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J.Bone Joint Surg.Am. 1998;80:631–5. doi: 10.2106/00004623-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28(2):134–9. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 9.St John TA, Vaccaro AR, Sah AP, et al. Physical and monetary costs associated with autogenous bone graft harvesting. American Journal of Orthopedics (Chatham, Nj) 2003;32(1):18–23. [PubMed] [Google Scholar]
- 10.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J.Bone Joint Surg.Br. 1989;71:677–80. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 11.Xu R, Ebraheim NA, Yeasting RA, et al. Anatomic considerations for posterior iliac bone harvesting. Spine. 1996;(21):1017–20. doi: 10.1097/00007632-199605010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003;14:195–200. doi: 10.1023/a:1022842404495. [DOI] [PubMed] [Google Scholar]
- 13.Boden SD, Schimandle JH, Hutton HC. An Experimental Intertransverse Process Spinal Fusion Model: Radiographic, Histologic & Biomechanical Healing Characteristics. Spine. 1995;20:412–20. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Riordan AM, Rangarajan R, Balts JW, Hsu WK, Anderson PA. Reliability of the rabbit postero-lateral spinal fusion model: a meta-analysis. J Orthop Res. 2013;31:1261–1269. doi: 10.1002/jor.22359. [DOI] [PubMed] [Google Scholar]
- 15.Ghodasra JH, Daley EL, Hsu EL, Hsu WK. Factors influencing arthrodesis rates in a rabbit posterolateral spine model with iliac crest autograft. Eur Spine J. 2014;23:426–434. doi: 10.1007/s00586-013-3074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erulker JS, Grauer JN, Patel TC, Panjabi MM. Flexibility analysis of posterolateral fusions in New Zealand white rabbit model. Spine (Phila Pa 1976) 2001;26:1125–1130. doi: 10.1097/00007632-200105150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler DL, Jenis LG, Kovach ME, Marini J. Turner AS. Efficacy of silicated calcium phosphate graft in posterolateral lumbar fusion in sheep. Spine J. 2007;7:308–317. doi: 10.1016/j.spinee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Fredericks DC, Petersen EB, Sahai N, Corley KG, DeVries N, Grosland NM, Smucker JD. Evaluation of a novel silicate substituted hydroxyapatite bone graft substitute in a rabbit posterolateral fusion model. Iowa Orthop J. 2013;33:25–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Smucker JD, Petersen EB, Nepola JV, Fredericks DC. Assessment of Mastergraft® Strip with Bone Marrow Aspirate as a Graft Extender in a Rabbit Posterolateral Fusion Model. Iowa Orthop J. 2012;32:61–68. [PMC free article] [PubMed] [Google Scholar]
- 20. ISO 19333-6: Biological evaluation of medical devices – Part 6: Tests for local effects after implantation. International Standards Organization. 2007.


