Abstract
Background
We hypothesized that select patients undergoing planned soft tissue sarcoma (STS) excision with anticipated skin and soft tissue deficits could be treated with a two stage surgical procedure which would allow some flexibility in coverage options while not significantly increasing local recurrence rate or wound complication rate.
Methods
A retrospective review was undertaken in a series of consecutive patients with a minimum 2-year follow-up treated by a single orthopedic oncologist and a single reconstructive plastic surgeon who were managed with a staged approach STS excision and reconstruction.
Results
There were 73 patients identified over a ten-year period that underwent staged STS excision and soft tissue reconstruction. There were 12 (16%) initial positive margins resected to negative final margins, and a variety of coverage procedures performed. Wound complication rate was 21%. Local recurrence rate was 11%.
Conclusion
Staged STS excision and reconstruction is an acceptable tool in the armamentarium of the orthopedic oncologist for managing major soft tissue deficits without an increase in local recurrence rates.
Introduction
Surgical excision remains the mainstay for local management of STS. Due to the propensity for tumor cell seeding in a wound, en bloc excision has been recommended in order to improve local control1,2. Furthermore, negative margins after excision have been correlated with a reduced likelihood of local recurrence3,4,5. Tumor bed re-excision is considered feasible to attain negative surgical margins if unable to do so at the time of the index procedure6,7. Intraoperative frozen section of tissue margins has proven to be a poor predictor of final negative margins based on subsequent histologic processing8, and as a result, re-excision may take place several days following the index excision procedure.
Adjuvant radiation therapy (RT) has been shown to diminish local recurrence rates following STS excision, including excision with negative margins, and has become the standard of care for the majority of patients following STS excision. Local recurrence rates are reduced from 25% to about 2% with the utilization of postoperative RT9. However, wound complications are common following RT, and include wound dehiscence and infection. It is our standard of practice to perform post-operative radiation when possible because we feel it has a lower wound complication rate compared with pre-operative RT. The development of a chronic draining wound is particularly problematic following soft tissue reconstruction with split thickness skin grafting10. For this reason, flap reconstruction is preferred over skin grafting in this setting whenever possible.
Large skin and soft tissue defects are frequently created following STS excision, especially in cases of subcutaneous lesions. Additionally, nearly half of all patients with STS will undergo an initial surgical procedure prior to referral to a sarcoma center which may compromise outcomes5,11,12. Because of the need to resect skin and subcutaneous tissues, excision of these tumors often requires complex soft tissue coverage13,14.
Reconstructive plastic surgery techniques have evolved for the coverage of large cutaneous defects. The mainstay for coverage for large skin loss traditionally has been split thickness skin grafting or free tissue transfer. More recently, perforator flaps, including keystone V-Y advancement and propeller flaps, have allowed coverage of large cutaneous defects using local or regional tissue14. These techniques have the advantage of allowing primary skin closure without grafting even in patients with relatively large cutaneous loss.
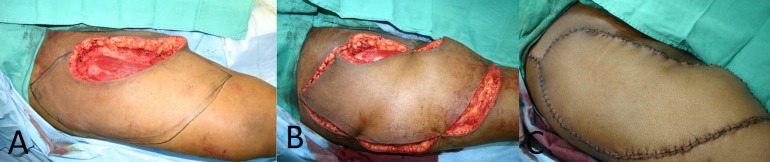
If final pathologic examination of the specimen reveals positive margins after a flap has been done, subsequent management is complicated by several concerns. Reexcision to negative margins may be compromised due to distortion of the local anatomy and large area of the tumor bed as a result of the flap dissection. In addition, the local options for reconstruction may be expended, dictating a more complex and extensive reconstruction after the second excision (Figure 1).
Figure 1.
A) A wide excision of a proximal thigh superficial soft tissue sarcoma (STS) with a planned keystone fasciocutaneous flap marked out. B) Mobilization of the keystone flap for soft tissue defect coverage one week after excision and clearance of tumor margins. Note the large surgical field adjacent to the tumor excision. C) After final wound closure, it is difficult to determine anatomic landmarks of the original resected specimen subsequent to the rotation of soft tissue for coverage.
One way to avoid these potential disadvantages is to stage the surgeries. An initial excision is completed first, with temporary dressing of the wound bed for several days while permanent margins are analyzed. Final coverage is deferred to a subsequent day following complete examination of the resected specimen. Focal positive margins may then be accurately addressed by re-excising appropriate portions of the intact host surgical bed. In the case of extensively positive margins, wide re-excision or other appropriate surgical procedures can be considered. Most importantly, the original tumor bed has not been disturbed by flap reconstruction so the concern of a wider field of tumor seeding is eliminated.
In this study, we examine our experience with staged soft tissue reconstruction following en bloc STS excisions with large cutaneous defects, assessing local recurrence rate and wound complications. We hypothesized that patients treated with this regimen would not have a higher local recurrence or wound complication rate than those treated with a more conventional approach of definitive coverage at the time of excision.
Methods
During an 11-year period from June 2000 through June 2011 at a single institution, selected patients who had anticipated cutaneous defects following excision of STS from the appendicular and axial skeleton were considered for staged excision and subsequent soft tissue reconstruction. All excision procedures were performed by a single orthopedic oncologist (JSB), and all soft tissue reconstructions were performed by a single reconstructive plastic surgeon (WMK) at a tertiary referral center. Patients underwent an index excision with the specimen sent for permanent processing. Titanium clips were placed at the margin of tumor excision to direct subsequent postoperative radiation.
The excision bed was covered provisionally with either a skin allograft or vacuum assisted closure device. If allograft was used, it was meshed and petroleum impregnated gauze was placed over the graft and a dense foam bolster stapled into place to reduce shear stress. The reconstructive surgery was completed at a median 7 days (range 3-32 day) after pathologic analysis of the specimen. Re-excision for positive margins was undertaken prior to definitive soft tissue coverage.
Using an IRB approved protocol, patients were identified from the institutional surgical database. A retrospective chart review was conducted, using the institutional records as available for follow-up. Only patients with a minimum two years of follow-up were considered for inclusion in the study. Generalized statistical comparisons were performed using chi-squared analysis, and all computations were performed in Microsoft Excel.
Results
A total of 425 patients with STS were identified as having undergone excision by the senior orthopedic oncologist during the study period. Of these, 107 had planned two-stage soft tissue reconstructions. Of these patients, 73 patients were identified with minimum 2 year follow-up. Nine patients were lost to follow-up, 1 was incarcerated, and 24 patients had deferred follow-up to their local physicians, and as such, did not have further follow-up beyond the perioperative period available for review.
Of the 73 patients, 50 had high grade sarcomas, 14 low grade sarcoma, 5 dermatofibrosarcoma protuberans, and 4 ungraded sarcomas. Of these, 51 (70%) patients had prior unplanned positive margin excision at outside facilities and 22 had biopsy only prior to presentation at our institution. Postoperative RT was performed in 53 (73%) patients. There were no wound healing complications in 58 (79%) patients while 7 (10%) patients developed wound infection, and 8 (11%) patients developed other wound complications. Positive margins were found in 12 patients (16%) after their first procedure at our institution, and a total of 13 re-excisions to achieve negative margins were performed (a single patiens had two re-excisions). There were 61 (84%) patients who had negative margins at the time of the index tumor excision. A list of demographics and results are summarized in Table I.
Table I.
Demographics and Results
| Demographics | No. (%) of Patients |
|---|---|
| Male | 42 (58%) |
| Female | 31 (42%) |
| Average Age (Years) | 52.0 (17.7-86.3) |
| Average BMI | 30.2 (19.4-45.2) |
| Average follow-up (Months) | 57.1 (24.5-125.9) |
| Tumor and Treatment Characteristics | |
| High Grade lesion | 48 (66%) |
| Average Defect Size (Range) | 135 cm2 (14-528) |
| Previous Positive Margin Resection | 51 (70%) |
| Preop Chemotherapy | 14 (19%) |
| Postop Chemotherapy | 4 (5%) |
| Postop Radiation | 53 (73%) |
| Wound infections | 7 (9.5%) |
| Wound healing complications | 15 (21%) |
| Local Recurrence | 8 (11%) |
| Amputation | 4 (5%) |
| Death | 7 (9.5%) |
The average soft tissue defect was 135 ± 141 cm2 after initial excision. Temporary coverage was performed with allograft skin in 57 (78%) patients and by a negative pressure wound device in 16 (22%) patients. Use of the negative pressure device was discontinued following a bleeding episode with one patient in the post anesthesia care unit, necessitating a return to the operating suite (no active bleeding was identified). Aside from this one patient, no wound complications were noted in the interim period between the index STS excision procedure and definitive soft tissue coverage.
Patients most frequently had a period of 7 days (range 3 - 32) between procedures. Definitive coverage was achieved by various methods as shown in Table II, with the most common modes of coverage being keystone flap (41%), split thickness skin graft (STSG) (22%), and myocutaneous local flap with STSG (21%). Twelve of the 73 patients (16%) required a total of 23 revision surgeries to achieve closure. These surgeries included wound debridement, skin grafting, and delayed closures.
Table II.
Final Wound Coverage
| Local Rearrangement no STSG | 5 (6.8%) |
| Local Rearrangement + STSG | 15 (20.5%) |
| STSG Alone | 16 (21.9%) |
| Keystone with V-Y Advancement No STSG | 23 (31.5%) |
| Keystone with V-Y Advancement + STSG | 7 (9.6%) |
| Free Flap No STSG | 4 (5.5%) |
| Free Flap + STSG | 3 (4.1%) |
| Total | 73 |
| STSG = split thickness skin graft |
There were 8 (11%) local recurrences in the study group overall, including 5 in the positive margins excision group and 3 in the negative margin group. Two of the 8 patients went on to amputation, 2 died, and 4 underwent re-excision of their local recurrences and were subsequently disease free at an average follow-up of 50 months. There were seven deaths in the group overall (10%), including five patients who died of disease, and 2 who died from causes unrelated to their malignancy. Therefore, overall oncologic survival was 93%.
Discussion
A majority of the patients included in this study had prior suboptimal surgical excisions. Despite continued efforts at education, the inadvertent initial surgical misadventure remains a consistent problem in sarcoma management11,12. This, unfortunately, puts patients at increased risk for local recurrence and metastatic disease15,16. Large volume excisions frequently require more complex coverage needs in sarcoma surgery17, and in our series, the majority of patients had undergone excisions prior to referral. Managing this high-risk patient population led to our hypothesis that a two-staged approach would allow more coverage options while not affecting wound complication or local recurrence rates.
Patients who have a surgical excision with positive margins prior to referral to a sarcoma center have a 20-34% chance at local recurrence if the specimen is completely re-excised, and as high as a 60% risk in high-grade tumors7,16,18,19,20. The rate of local recurrence in planned wide-margin excisions ranges from 6-25% in the literature2,4,18,19,20,21. Metastasis occurs in 13.7-27% of patients after undergoing unplanned excision4,7,16,20.
Metastasis occurred in 8.3-24% of patients undergoing planned wide-margin excision4,20. In our study, the overall local recurrence rate of 11% is not different from published series of STS, suggesting that this treatment approach does not adversely affect the local recurrence rate. Overall survival was 90% and disease free survival was 79%.
The wound complication rate of 21% in the present study included infections and other wound complications. The majority of patients received postoperative RT to decrease the risk of wound complications. Wound complication rates after pre-operative RT are 35% compared with 17% in post-operative RT22, which is not significantly different from our study. Additionally, our patients had a relatively high average body mass index (BMI), a factor also associated with higher wound complications.
This study is limited in that it is a single arm, retrospective review with a short follow up from a single institution. Patients were selected for inclusion by the senior author based on clinical experience and were not randomized. However, like most sarcoma studies, due to the rarity of the disease, the protean presentations, and the wide variety of treatment options, Level 1 randomized controlled studies are exceedingly difficult to perform for surgical management in this disease. We felt that comparing a cohort of patients undergoing a single-stage surgical approach would not be possible in our institution owing to the underlying bias in choosing these treatment protocols considering the differences in these populations. As such, we elected to provide our experience with the staged excision and reconstruction approach, as it has provided a suitable treatment option in our hands.
Several non-tumor related advantages led to the incorporation of this staged surgical treatment. Patients are able to understand their reconstructive procedure prior to surgery and as such, are more knowledgeable about the operation at hand. Additionally, while no official cost-analysis was run, the surgeons anecdotally noted that staging results in more efficient scheduling and use of operating room time, surgeon time, and hospital resources. We recognize that this is an additional surgical procedure, and attempt to select our patients with that in mind. As these patients typically go home between surgeries, we feel this does not significantly negatively impact their quality of life.
In conclusion, for select patients in whom large cutaneous defects are anticipated, delayed soft tissue coverage offers the potential to revise unplanned positive margins while still availing the patient of the benefits of local tissue rearrangements. In our small single arm study, the local recurrence rate of 11% in this relatively high risk group was consistent with previously published local recurrence rates, and reoperations for wound complications were only 16%. This technique is an acceptable addition to the armamentarium of the orthopedic oncologist and reconstructive plastic surgeon in achieving wound closure in this difficult clinical situation.
References
- 1.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;(153):106–20. [PubMed] [Google Scholar]
- 2.Trovik CSB, Berlin O, Tukiainen E, Erlanson M, Gustafson P, Klepp R, et al. Local recurrence of deep-seated high-grade soft tissue sarcoma 459 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scan. 2001;72(2):160–66. doi: 10.1080/000164701317323417. [DOI] [PubMed] [Google Scholar]
- 3.Fiore M, Casali PG, Miceli R, Mariana L, Bertulli R, Lozza L, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13(1):110–7. doi: 10.1245/ASO.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Sabolch A, Feng M, Griffith K, Rzasa C, Gadzala L, Feng F, et al. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am J Clin Oncol. 2012;35(2):151–7. doi: 10.1097/COC.0b013e318209cd72. [DOI] [PubMed] [Google Scholar]
- 5.Trovik CS. Local Recurrence of soft tissue sarcoma A Scandinavian Sarcoma Group Project. Acta Orthop Scand. 2001;72:1–27. Suppl 300. [PubMed] [Google Scholar]
- 6.Manoso MW, Frassica DA, Gene Deune E, Frassica FJ. Outcomes of re-excision after unplanned excisions of soft-tissue sarcomas. J Surg Oncol. 2005;91(3):153–8. doi: 10.1002/jso.20323. [DOI] [PubMed] [Google Scholar]
- 7.Morii TY, Morioka H, Anazawa U, Suzuki Y, Toyama Y. Clinical Significance of Additional Wide Resection for Unplanned Resection of High Grade Soft Tissue Sarcoma. Open Orthop J. 2008;2:126–9. doi: 10.2174/1874325000802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golouh R. Bracko M. Accuracy of frozen section diagnosis in soft tissue tumors. Mod Pathol. 1990;3(6):729–33. [PubMed] [Google Scholar]
- 9.Beane JD, Yang JC, White D, Steinberg SM, Rosenberg SA, Rudloff U. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21(8):2484–9. doi: 10.1245/s10434-014-3732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emory CL, Montgomery CO, Potter BK, Keisch ME, Conway SA. Early complications of high-doserate brachytherapy in soft tissue sarcoma: a comparison with traditional external-beam radiotherapy. Clin Orthop Relat Res. 2012;470(3):751–8. doi: 10.1007/s11999-011-2106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson PD, Rydholm A. Soft tissue sarcoma should be treated at a tumor center. Acta Orthop Scan. 1994;65(1):47–50. doi: 10.3109/17453679408993717. [DOI] [PubMed] [Google Scholar]
- 12.Siegel HJB, Lopez-Ben R, Siegal GP. Unplanned surgical excision of extremity soft tissue sarcomas patient profile and referral patterns. J Surg Orthop Advances. 2009;18(2):93–8. [PubMed] [Google Scholar]
- 13.Kang S, Han I, Kim S, Lee YH, Kim MB, Kim HS. Outcomes after flap reconstruction for extremity soft tissue sarcoma: A case-control study using propensity score analysis. Eur J Surg Oncol. 2014;40(9):1101–8. doi: 10.1016/j.ejso.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Khouri JS, Egeland BM, Daily SD, Harake MS, Kwon S, Neligan PC, et al. The keystone island flap: use in large defects of the trunk and extremities in soft-tissue reconstruction. Plast Reconstr Surg. 2011;127(3):1212–21. doi: 10.1097/PRS.0b013e318205f36f. [DOI] [PubMed] [Google Scholar]
- 15.Arai E, Sugiura H, Tsukushi S, Nakashima H, Urakawa H, Kozawa E, et al. Residual tumor after unplanned excision reflects clinical aggressiveness for soft tissue sarcomas. Tumour Biol. 2014 Aug;35(6):8043–9. doi: 10.1007/s13277-014-2043-5. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekar CRW, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of unplanned excision of a soft tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90(2):203–8. doi: 10.1302/0301-620X.90B2.19760. [DOI] [PubMed] [Google Scholar]
- 17.Arai E, Nishida Y, Tsukushi S, Wasa J, Ishiguro N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin Orthop Relat Res. 2010;468(11):3028–34. doi: 10.1007/s11999-010-1392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter BK, Adams SC, Pitcher JD, Temple T. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res. 2008;466(12):3093–100. doi: 10.1007/s11999-008-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi YA, Huddy JR, Miller JD, Strauss DC, Thomas JM, Hayes AJ. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol. 2012;19(3):871–7. doi: 10.1245/s10434-011-1876-z. [DOI] [PubMed] [Google Scholar]
- 20.Umer HM, Umer M, Qadir I, Abbasi N, Masood N. Impact of unplanned excision on prognosis of patients with extremity soft tissue sarcoma. Sarcoma. 2013 April 2013;:1–5. doi: 10.1155/2013/498604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis AMK, Wunder JS, Unger R, Meer J, O’Sullivan B, Catton CN, Bell RS. Impact residual disease local recurrence patients treated by unplanned resection for soft tissue sarcoma. J Surg Onc. 1997;66:81–7. doi: 10.1002/(sici)1096-9098(199710)66:2<81::aid-jso2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002 Jun;11(359):2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]