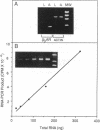
Abstract
During continuous stimulation by agonist, beta 1- and beta 2-adrenergic receptors (ARs) undergo processes that lead to decreases in receptor expression. This receptor down-regulation serves to limit the cellular cAMP response during chronic agonist exposure. In the recently described third subtype of the beta AR, denoted beta 3AR, we found four potential cAMP response elements in the 5' flanking region, suggesting that expression of this receptor might be positively regulated by agonists. These elements were cloned into the vector pA10CAT2, which contains a chloramphenicol acetyltransferase reporter gene, and transiently expressed in VERO cells. Three of these elements, TGACTCCA, TGAGGTCT, and CGAGGTCA (located 518, 622, and 1125 bases upstream of the beta 3AR coding block, respectively) were found to increase transcription of the chloramphenicol acetyltransferase gene in response to cAMP analogues and agents that increase intracellular cAMP. 3T3-F442A cells, when differentiated into the adipocyte phenotype by insulin, expressed beta 3AR, and nuclear runoff studies from such cells confirmed cAMP enhancement of beta 3AR mRNA transcription. In these cells, beta 3AR mRNA increased in response to exposure to the beta 3AR agonist isoproterenol and remained elevated during exposures of up to 24-30 hr. During prolonged exposure to agonist, no downregulation of beta 3AR expression in 3T3-F442A cells occurred. Indeed, beta 3AR expression increased during agonist exposure to approximately 165% of basal expression. In marked contrast, beta 1AR expression declined by approximately 70% in response to chronic agonist exposure. These studies reveal a subtype-specific prolonged transcriptional regulation of a beta AR gene by the end product of its signal transduction pathway. Thus, the beta 3AR undergoes a paradoxical increase in receptor expression during chronic agonist exposure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarons R. D., Nies A. S., Gerber J. G., Molinoff P. B. Decreased beta adrenergic receptor density on human lymphocytes after chronic treatment with agonists. J Pharmacol Exp Ther. 1983 Jan;224(1):1–6. [PubMed] [Google Scholar]
- Arch J. R. The brown adipocyte beta-adrenoceptor. Proc Nutr Soc. 1989 Jul;48(2):215–223. doi: 10.1079/pns19890032. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Collins S., O'Dowd B. F., Campbell P. T., de Blasi A., Kobilka B. K., MacGregor C., Irons G. P., Caron M. G., Lefkowitz R. J. Two distinct pathways for cAMP-mediated down-regulation of the beta 2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J Biol Chem. 1989 Oct 5;264(28):16786–16792. [PubMed] [Google Scholar]
- Collins S., Altschmied J., Herbsman O., Caron M. G., Mellon P. L., Lefkowitz R. J. A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. J Biol Chem. 1990 Nov 5;265(31):19330–19335. [PubMed] [Google Scholar]
- Collins S., Bouvier M., Bolanowski M. A., Caron M. G., Lefkowitz R. J. cAMP stimulates transcription of the beta 2-adrenergic receptor gene in response to short-term agonist exposure. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4853–4857. doi: 10.1073/pnas.86.13.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu Rev Physiol. 1991;53:497–508. doi: 10.1146/annurev.ph.53.030191.002433. [DOI] [PubMed] [Google Scholar]
- Djian P., Phillips M., Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985 Sep;124(3):554–556. doi: 10.1002/jcp.1041240327. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Briend-Sutren M. M., Lasnier F., Strosberg A. D., Pairault J. Differential regulation of beta 1- and beta 2-adrenergic receptor protein and mRNA levels by glucocorticoids during 3T3-F442A adipose differentiation. J Biol Chem. 1990 Sep 25;265(27):16343–16349. [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Hadcock J. R., Malbon C. C. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5021–5025. doi: 10.1073/pnas.85.14.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadcock J. R., Wang H. Y., Malbon C. C. Agonist-induced destabilization of beta-adrenergic receptor mRNA. Attenuation of glucocorticoid-induced up-regulation of beta-adrenergic receptors. J Biol Chem. 1989 Nov 25;264(33):19928–19933. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hough C., Chuang D. M. Differential down-regulation of beta 1- and beta 2-adrenergic receptor mRNA in C6 glioma cells. Biochem Biophys Res Commun. 1990 Jul 16;170(1):46–52. doi: 10.1016/0006-291x(90)91238-n. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett S. B., Bouvier M., Hausdorff W. P., O'Dowd B., Caron M. G., Lefkowitz R. J. Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant beta 2-adrenergic receptors lacking phosphorylation sites. Mol Pharmacol. 1989 Oct;36(4):641–646. [PubMed] [Google Scholar]
- Liggett S. B., Caron M. G., Lefkowitz R. J., Hnatowich M. Coupling of a mutated form of the human beta 2-adrenergic receptor to Gi and Gs. Requirement for multiple cytoplasmic domains in the coupling process. J Biol Chem. 1991 Mar 15;266(8):4816–4821. [PubMed] [Google Scholar]
- Liggett S. B. Desensitization of the beta-adrenergic receptor: distinct molecular determinants of phosphorylation by specific kinases. Pharmacol Res. 1991 Aug;24 (Suppl 1):29–41. doi: 10.1016/1043-6618(91)90119-i. [DOI] [PubMed] [Google Scholar]
- Liggett S. B. Identification and characterization of a homogeneous population of beta 2-adrenergic receptors on human alveolar macrophages. Am Rev Respir Dis. 1989 Feb;139(2):552–555. doi: 10.1164/ajrccm/139.2.552. [DOI] [PubMed] [Google Scholar]
- Liggett S. B., Ostrowski J., Chesnut L. C., Kurose H., Raymond J. R., Caron M. G., Lefkowitz R. J. Sites in the third intracellular loop of the alpha 2A-adrenergic receptor confer short term agonist-promoted desensitization. Evidence for a receptor kinase-mediated mechanism. J Biol Chem. 1992 Mar 5;267(7):4740–4746. [PubMed] [Google Scholar]
- Liggett S. B., Schwinn D. A. Multiple potential regulatory elements in the 5' flanking region of the beta 3-adrenergic receptor. DNA Seq. 1991;2(1):61–63. doi: 10.3109/10425179109008441. [DOI] [PubMed] [Google Scholar]
- Longabaugh J. P., Didsbury J., Spiegel A., Stiles G. L. Modification of the rat adipocyte A1 adenosine receptor-adenylate cyclase system during chronic exposure to an A1 adenosine receptor agonist: alterations in the quantity of GS alpha and Gi alpha are not associated with changes in their mRNAs. Mol Pharmacol. 1989 Nov;36(5):681–688. [PubMed] [Google Scholar]
- Meier M. K., Alig L., Bürgi-Saville M. E., Müller M. Phenethanolamine derivatives with calorigenic and antidiabetic qualities. Int J Obes. 1984;8 (Suppl 1):215–225. [PubMed] [Google Scholar]
- Myers T. W., Gelfand D. H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991 Aug 6;30(31):7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve K. A., Molinoff P. B. Turnover of beta 1- and beta 2-adrenergic receptors after down-regulation or irreversible blockade. Mol Pharmacol. 1986 Aug;30(2):104–111. [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sakaue M., Hoffman B. B. cAMP regulates transcription of the alpha 2A adrenergic receptor gene in HT-29 cells. J Biol Chem. 1991 Mar 25;266(9):5743–5749. [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. The mRNA encoding a high-affinity cAMP phosphodiesterase is regulated by hormones and cAMP. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8197–8201. doi: 10.1073/pnas.86.21.8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse J., Powell J. R., Baste C. A., Priest R. E., Kuo J. F. Isoproterenol-induced cardiac hypertrophy: modifications in characteristics of beta-adrenergic receptor, adenylate cyclase, and ventricular contraction. Endocrinology. 1979 Jul;105(1):246–255. doi: 10.1210/endo-105-1-246. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaagsma J., Nahorski S. R. Is the adipocyte beta-adrenoceptor a prototype for the recently cloned atypical 'beta 3-adrenoceptor'? Trends Pharmacol Sci. 1990 Jan;11(1):3–7. doi: 10.1016/0165-6147(90)90032-4. [DOI] [PubMed] [Google Scholar]