Abstract
Background
Pulmonary surveillance protocols following sarcoma excision based on clinical evidence and outcomes are limited in current literature. The purpose of this study was to determine the method, frequency, and reasoning behind pulmonary surveillance strategies in patients treated for sarcoma among members of the Musculoskeletal Tumor Society (MSTS).
Methods
SurveyMonkey, an online survey tool, was used to create and distribute a questionnaire to 211 members of the MSTS in 2011. The 16 questions focused on current pulmonary surveillance algorithms and their reasoning.
Results
Of the surveyed members of the MSTS, 65% follow high-grade sarcoma with routine chest CT scans. Most disagreement involved low-grade sarcomas, where radiographs (34%), routine CT (33%), or selective CT scans (31%) were evenly distributed. Selective CT scans in low-grade lesions were warranted with an indeterminate nodule on prior CT (81%), local recurrence (40%), or large/ deep tumor characteristics (31%). Most protocols were based on continuation of training protocols (46%), clinician’s interpretation of the current literature (23%), or personal experience (14%).
Conclusions
Significant clinician variability exists in terms of pulmonary surveillance of sarcomas, most notably in low-grade lesions. The results of this study represent an area in need of further study to develop an evidence-based protocol for sarcoma pulmonary surveillance.
Introduction
Following excision of primary musculoskeletal sarcoma, patients are routinely monitored for evidence of distant metastasis, which is the most common cause of cancer-related mortality, affecting 30-50% of patients with high-grade sarcoma1. Most metastases occur in the first two years following treatment of the primary tumor2,3, with the lungs representing the most common site of distant disease. The early identification of pulmonary metastatic disease is thought to be important as surgical removal of limited disease can result in a survival benefit. For example, 25-40% of patients undergoing complete resection of metastatic disease confined to the lungs will survive long-term, compared to 17% who do not have a complete resection4-7.
The two most commonly used imaging techniques for pulmonary surveillance are chest radiographs (CXR) and computed tomography (CT). Radiographs are quick, accessible, and inexpensive but cannot unequivocally detect subcentimeter pulmonary nodules. While CT scans provide greater detail and information, they are more expensive and expose the patient to two orders of magnitude higher doses of radiation than plain radiographs8. Recent reports have raised concerns about a causative effect with excessive radiation exposure from CT scans and subsequent development of malignancy8-10. Therefore, the elimination of unnecessary CT scans may be beneficial to both patient safety and healthcare costs. The National Comprehensive Cancer Network (NCCN) provides guidelines for follow-up and surveillance of extremity sarcoma, but these guidelines do not differentiate between chest radiographs and CT scans in terms of pulmonary surveillance11,12. For low-grade soft tissue sarcoma (American Joint Committee on Cancer [AJCC] stage IA and IB), the NCCN12 simply recommends to “consider chest imaging every 6-12 months.” For AJCC stage II, III, and IV disease, the NCCN recommends “chest imaging [plain radiograph or chest CT] every 3-6 months for 2-3 years, then every 6 months for next 2 years, then annually.” Several authors have reported on surveillance protocols and strategiesfollowing excision of primary sarcoma13-23, with no clear consensus obtained. Given the large disparities among musculoskeletal oncologists in both method of pulmonary surveillance and frequency, we designed a survey to determine the scope of the disagreement and identify potential questions for further investigation.
Materials and Methods
We used SurveyMonkey (www.surveymonkey.com), an online survey tool, to create and distribute a questionnaire to 211 members of the Musculoskeletal Tumor Society (MSTS). Sixteen questions were created focusing on current algorithms, patient concerns regarding radiation exposure, personal interest in further research, and clinical experience.
The survey was designed by the senior author (BJM) and reviewed prior to distribution by two fellowshiptrained musculoskeletal oncologists (MTS, CPG) (Appendix A). The questions were intended to be hypothesis-generating, and focused on the current preferences of individual surgeons in the means and timing of chest imaging, the reasoning behind their personal protocol, concerns about radiation from medical imaging expressed by their patients, and their perceptions on the overuse or underuse of imaging for pulmonary surveillance. The current membership of the MSTS was then sent two emails in February 2011 with a link to the survey and explanation of the project. The results were compiled and percentages calculated according to responses of the participating surgeons.
Results
Of the 211 active members of the MSTS in 2011, 118 members (55.9%) completed the survey. The complete survey questionnaire and associated results are displayed in Appendix A.
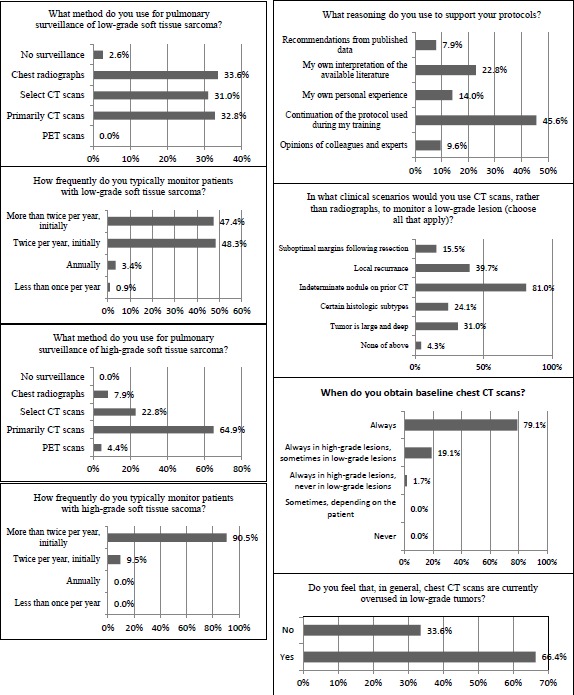
The most apparent disagreement involved surveillance of low-grade sarcomas. In terms of surveillance method, there was a nearly equivalent distribution among chest radiographs, selective use of CT scans (generally chest radiographs with CT scans reserved for particular clinical scenarios), and routine use of CT scans (Figure 1). There was also an equivalent distribution in the frequency of monitoring, with half of respondents electing to monitor more frequently than twice per year and half choosing to monitor every 6 months initially and decreasing over time.
Figure 1.
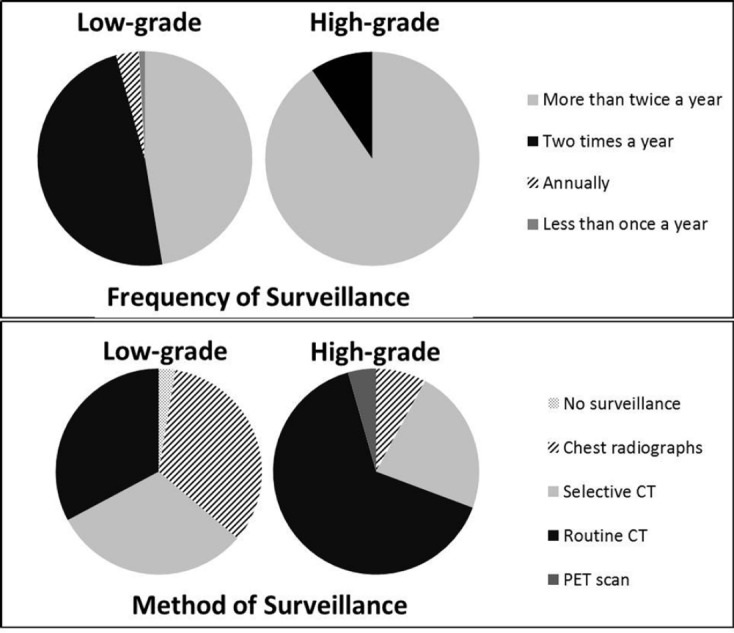
Results illustrating the difference in surveillance methods and frequency between low-grade and high-grade sarcomas.
In contrast, there was less disagreement in both the method and frequency of surveillance for high-grade sarcoma (Figure 1). Nearly 65% of respondents indicated preference for chest CT scans for surveillance, while nearly 23% use primarily chest radiographs with select CT scans for certain patients. Frequency of monitoring high-grade sarcoma was more consistent, with over 90% of MSTS members electing to monitor more than twice a year initially, with decreasing frequency over time.
Most respondents (45.6%) indicated that their surveillance protocols were a continuation of the practices used during training (Figure 2). Less common reasons for an individual’s surveillance protocols were the physician’s own interpretation of the literature (22.8%), personal experience (14.0%), and opinions of colleagues and experts (9.6%). Interestingly, only 7.9% of respondent’s based their surveillance protocols on recommendations from published data. Two-thirds of respondents felt that chest CT scans are currently overused for monitoring of low-grade sarcomas, while one-third felt chest CT scans were overused for monitoring of high-grade sarcomas.
Figure 2.
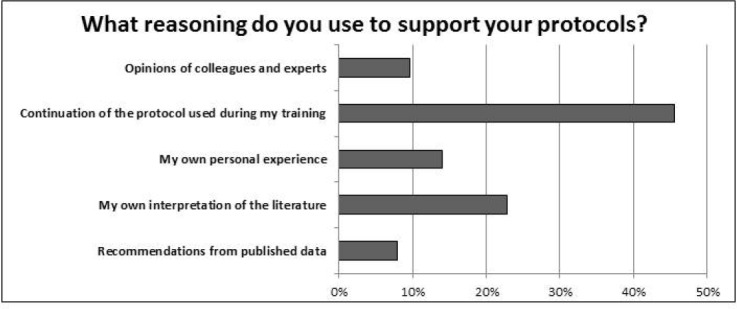
Most respondents reported that their current surveillance protocols were a continuation of the methods used during training. Less than 10% based their protocols on recommendations from published data.
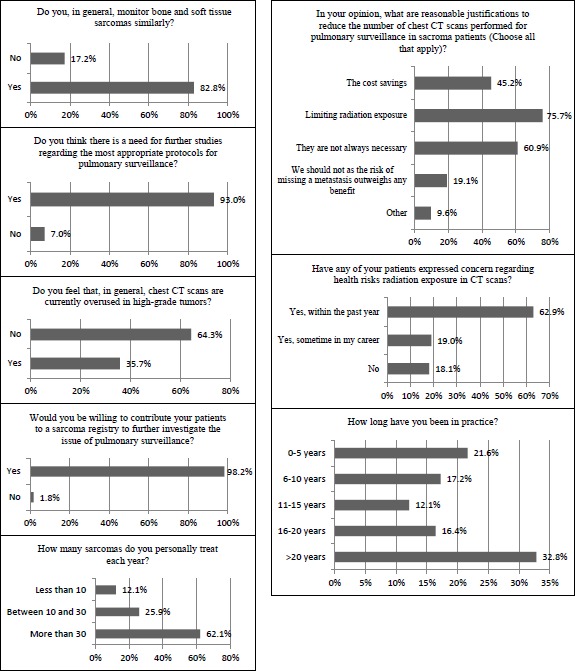
The presence of an indeterminate nodule found on a previous chest CT scan was the most common clinical scenario for monitoring a low-grade lesion using CT scans (81.0%) (Figure 3). Less frequently cited reasons for use of CT scan for surveillance of low-grade lesions included local recurrence (39.7%), large and deep tumors (31.0%), certain histologic subtypes (24.1%), and suboptimal resection (15.5%). Most MSTS members indicated similar monitoring protocols for both bone and soft tissue sarcomas (82.8%).
Figure 3.
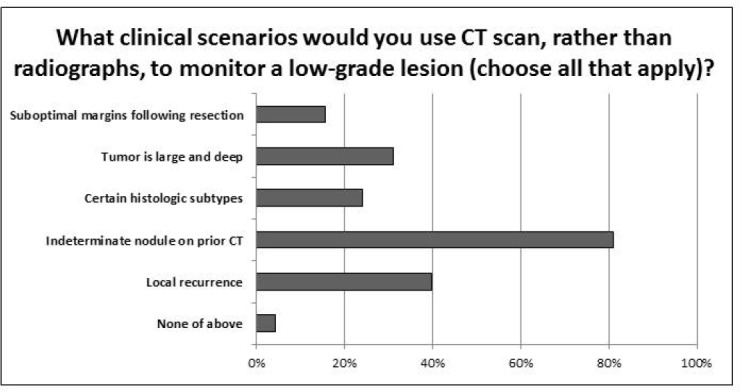
The most common clinical scenario in which respondents elect to monitor low-grade lesions with CT is an indeterminate nodule from a previous CT scan (81%), followed by local recurrence (39.7%) and if the tumor is large and deep (31%).
Of the participating respondents, 62.9% indicated that they have had patients express concerns regarding the health risks from radiation exposure in CT scans within the past year, while 19.0% have had patients express this concern at some point in their career (Figure 4). Greater than 75% of clinicians cited limiting radiation exposure as a reasonable justification to reduce the number of CT scans performed for pulmonary surveillance. In fact, 60.9% felt that CT scans are not always necessary for routine surveillance and 45.2% reported cost savings as reasonable justifications for reduction in CT scans for pulmonary surveillance of sarcomas. In contrast, 19.1% of respondents felt that the number of chest CT scans should not be reduced as the risk of missing metastatic disease outweighs any potential benefit of reducing the number of CT scans performed.
Figure 4.
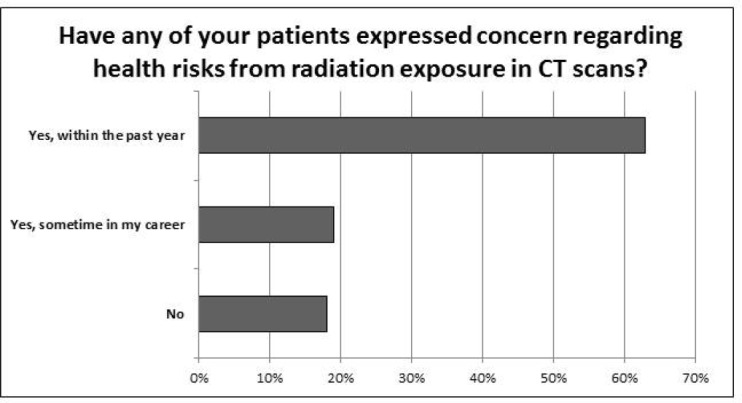
Nearly two-thirds of clinicians had patients express concerns in the last year over health risks due to radiation exposure from CT scans.
Lastly, most MSTS members felt there is a need for further studies regarding appropriate protocols for pulmonary surveillance (93.0%) and nearly all were willing to contribute their patients to research efforts to further investigate the issue of pulmonary surveillance for sarcomas (98.2%).
Discussion
Current guidelines for pulmonary surveillance of soft tissue sarcoma (STS) do not specify a specific imaging modality or periodicity, leading to controversy regarding both the method and frequency of pulmonary surveillance among practicing orthopedic oncologists. With concerns about secondary effects of radiation from CT scans, many have questioned the need for advanced imaging for routine surveillance purposes, especially for lower-grade lesions with minimal risk of metastases. Additionally, reports from other solid tumor types challenge the usefulness of multiple follow-up imaging and laboratory studies in terms of cost-effectiveness, efficacy, and survival benefit18,24-27. The results of this survey of MSTS members highlight the lack of evidence-based recommendations for pulmonary surveillance strategies, with the surveillance of low-grade sarcomas representing the largest area of disagreement.
The lungs are the most common site of STS metastatic disease, and most new metastases occur within two years following treatment of the primary tumor, although current guidelines recommend surveillance for at least 5-10 years or longer. However, these guidelines do not specify a specific imaging modality or periodicity, and controversy exists among practicing orthopedic oncologists regarding both the method and frequency of pulmonary surveillance.
While chest CT scans provide greater detail and information, concerns over excessive radiation, healthcare costs, and unnecessary interventions prompted by incidental discovery of benign lesions have led many to question the necessity of advanced imaging for routine surveillance purposes, specifically in lower-grade lesions with a minimal risk of metastatic spread. Additionally, reports from other solid tumor types challenge the usefulness of multiple follow-up imaging and laboratory studies in terms of cost-effectiveness, efficacy, and survival benefit (18, 24-27). Given the disagreement and lack of widely-accepted guidelines regarding appropriate surveillance strategies for STS, this survey was designed to define the scope of the problem and determine the current state of practice and controversy for pulmonary imaging.
The results from the current report indicate that surveillance of low-grade sarcoma represents the most apparent area of disagreement amongst members of the MSTS, with a nearly even split between chest x-ray, chest CT, and selective CT scans. Additionally, roughly half of respondents prefer to initially monitor these patients twice per year, with the remainder electing for more frequent clinic visits initially (a higher frequency than the current NCCN guidelines12). There was less discrepancy with respect to high-grade sarcomas, as a majority of respondents preferred chest CT scans for routine surveillance, with a lower number electing chest CT scans for select patients. Likewise, over 90% of MSTS respondents elect to initially follow patients with high-grade sarcoma more than twice per year, consistent with the NCCN recommendations.
Previous surveys of surgical oncologists have attempted to better understand practice patterns. In a 1997 survey on surveillance strategies among members of the Society of Surgical Oncology, Beitler et al. reported that office visits and chest radiographs were the most frequently used modalities during each year of follow-up13. While 74% believed routine follow-up testing would result in detection early enough to institute potentially curative treatment, only 26% believed that current literature supported a survival benefit to follow-up testing. Reanalyzing the same survey data, Sakata et al. reported tumor grade and size significantly impacted physician practice patterns in postoperative treatment follow-up19. In another survey, Gerrand et al. found that clinic visits and radiographs were the most commonly used method of surveillance, and most respondents based their follow-up protocol on the perceived risk of local or systemic relapse15.
Previous reports suggest chest radiography may be sufficient for pulmonary surveillance following primary treatment of extremity STS with reported positive and negative predictive values of surveillance chest radiograph of 92% and 97%, respectively7,17,18,20,22. Cool et al. concluded that the vast majority of metastasis detected by routine surveillance/CXR or restaging has proved successful in identifying pulmonary metastases before they became clinically apparent in 67% of cases17. Puri et al. randomized 500 non-metastatic patients to demonstrate non-inferiority with primary end point of overall survival at 3 years and disease free survival at 3 years,22. CXR as an imaging modality did not lead to worsened survival and was not inferior to CT scan in terms of detecting pulmonary metastases. While most studies did not find added benefit with routine chest CT scans for pulmonary surveillance, Cho et al. reported a significant survival advantage at 2 and 4 years in patients followed with routine chest CT after surgical treatment of primary extremity sarcoma14. However, no survival benefit was seen at 5 years. They concluded that serial monitoring with chest CT could give rise to early detection of pulmonary metastases, providing a chance for pulmonary lesion excision and survival advantage.
With most reports suggesting recurrent or metastatic disease occurs within the first two years following primary surgical treatment of soft tissue sarcoma, more aggressive follow-up and surveillance methods should be weighted during this time period2,7,16,18. Several authors advocate risk stratification, with more frequent and intense follow-ups for high-risk patients16,18,21. Another potential area of disagreement among surgical oncologists is length of follow-up, which was not specifically addressed in the current study. Sawamura et al. noted that 95% of metastases developed by 7.3 years and the rate of metastases was extremely high for high-grade tumors during the first two years23. The authors suggest that follow-up beyond 10 years does not yield a sufficient number of local recurrences or metastases to warrant further monitoring.
The health risks secondary to the accumulation of low-dose radiation from medical imaging deserve specific discussion and several reports have addressed these concerns8,28-31. Extrapolating upon data reported in previous studies, Brenner and Hall estimated that 1.5-2% of all cancers in the United States were caused by radiation exposure in medical imaging8. Patients are aware of these risks, with nearly two-thirds of survey respondents having patients express concerns regarding repeated radiation exposure. Patient concerns over accumulated radiation exposure are legitimate, and a discussion of potential risks and benefits from additional imaging studies should be standard practice for treating clinicians.
Inherent limitations are unavoidable with any study based on questionnaire to survey a large group of individuals. There are uncertainties and difficulties in interpreting responses. Inherent to this specific survey is the difficulty in categorizing follow-up protocols into a select few choices, as some clinicians likely perform additional testing based on results of physical examination or previous clinical data. There is also no way of knowing whether the results obtained in this survey actually translate into clinical practice. Additionally, this is a limited sampling of orthopedic oncologists, and should be generalized to other sarcoma specialists with care. Finally, the relatively low rate of MSTS member participation (118/211) was another limitation of this study.
The disagreements amongst members of the MSTS are secondary to variations in personal opinion in combination with lack of high-quality, evidence-based recommendations. As it was evident that most orthopedic oncologists were likely to monitor patients in a similar manner to their fellowship training, it is not surprising that surveillance protocols are not uniform. This issue is complex with theoretically dramatic consequences. Certainly it is a justifiable goal to limit “unnecessary” imaging studies. This would result in cost savings, less radiation from medical imaging, and no consequence for overall survival. However, this decision is based on riskstratification and a judgment of the “likelihood” of metastatic disease, and it is conceivable that a less intensive surveillance protocol would result in delayed detection of treatable metastatic disease in select patients.
What is clear from these data is that the current level of knowledge is not adequate to reflect a consensus opinion of pulmonary surveillance. Encouragingly, the overwhelming majority of those surveyed think that further research would be meaningful, and they would be willing to contribute patients to a society-wide effort. Our hope is that this simple report will stimulate further conversation and thought into determining the ideal protocol that balances metastatic risk, medical imaging radiation dose minimization, cost consciousness, and detection of can be treated for a survival benefit.
In conclusion, evidence-based recommendations on pulmonary surveillance strategies are lacking in the literature and this is highlighted by the results of this survey. The largest disagreement among clinicians involves surveillance of low-grade sarcomas and most strategies are a continuation from protocols used in training. Based upon these results, we believe a prospective, multi-center, comparative study design focusing on low-grade sarcoma would be the most informative and supported effort.
Appendix A – Results of Pulmonary Surveillance Questionnaire
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1998. CA: a cancer journal for clinicians. 1998;48(1):6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(5):1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 3.Gadd MA, Casper ES, Woodruff JM, McCormack PM, Brennan MF. Development and treatment of pulmonary metastases in adult patients with extremity soft tissue sarcoma. Annals of surgery. 1993;218(6):705–12. doi: 10.1097/00000658-199312000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casson AG, Putnam JB, Natarajan G, Johnston DA, Mountain C, McMurtrey M, et al. Five-year survival after pulmonary metastasectomy for adult soft tissue sarcoma. Cancer. 1992;69(3):662–8. doi: 10.1002/1097-0142(19920201)69:3<662::aid-cncr2820690311>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Rehders A, Hosch SB, Scheunemann P, Stoecklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Archives of surgery (Chicago, Ill : 1960) 2007;142(1):70–5. doi: 10.1001/archsurg.142.1.70. discission 6. [DOI] [PubMed] [Google Scholar]
- 6.van Geel AN, Pastorino U, Jauch KW, Judson IR, van Coevorden F, Buesa JM, et al. Surgical treatment of lung metastases: The European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer. 1996;77(4):675–82. doi: 10.1002/(sici)1097-0142(19960215)77:4<675::aid-cncr13>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Billingsley KG, Burt ME, Jara E, Ginsberg RJ, Woodruff JM, Leung DH, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Annals of surgery. 1999;229(5):602–10. doi: 10.1097/00000658-199905000-00002. discussion 10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. The New England journal of medicine. 2007;357(22):2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 9.Huppmann MV, Johnson WB, Javitt MC. Radiation risks from exposure to chest computed tomography. Seminars in ultrasound, CT, and MR. 2010;31(1):14–28. doi: 10.1053/j.sult.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Motaganahalli R, Martin A, Feliciano B, Murphy MP, Slaven J, Dalsing MC. Estimating the risk of solid organ malignancy in patients undergoing routine computed tomography scans after endovascular aneurysm repair. Journal of vascular surgery. 2012;56(4):929–37. doi: 10.1016/j.jvs.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 11. Network NCC. NCCN Clinical Practice Guidelines in Oncology: Bone Cancer. 1.2014.
- 12. Network NCC. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma. Version 2.2014. [Google Scholar]
- 13.Beitler AL, Virgo KS, Johnson FE, Gibbs JF, Kraybill WG. Current follow-up strategies after potentially curative resection of extremity sarcomas: results of a survey of the members of the society of surgical oncology. Cancer. 2000;88(4):777–85. [PubMed] [Google Scholar]
- 14.Cho HS, Park IH, Jeong WJ, Han I, Kim HS. Prognostic value of computed tomography for monitoring pulmonary metastases in soft tissue sarcoma patients after surgical management: a retrospective cohort study. Annals of surgical oncology. 2011;18(12):3392–8. doi: 10.1245/s10434-011-1705-4. [DOI] [PubMed] [Google Scholar]
- 15.Gerrand CH, Billingham LJ, Woll PJ, Grimer RJ. Sarcoma. Follow up after Primary Treatment of Soft Tissue Sarcoma: A Survey of Current Practice in the United Kingdom. 2007;2007:34128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane JM. 3rd. Surveillance strategies for patients following surgical resection of soft tissue sarcomas. Current opinion in oncology. 2004;16(4):328–32. doi: 10.1097/01.cco.0000127879.62254.d3. [DOI] [PubMed] [Google Scholar]
- 17.Cool P, Grimer R, Rees R. Surveillance in patients with sarcoma of the extremities. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2005;31(9):1020–4. doi: 10.1016/j.ejso.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Whooley BP, Gibbs JF, Mooney MM, McGrath BE, Kraybill WG. Primary extremity sarcoma: what is the appropriate follow-up? Annals of surgical oncology. 2000;7(1):9–14. doi: 10.1007/s10434-000-0009-x. [DOI] [PubMed] [Google Scholar]
- 19.Sakata K, Johnson FE, Beitler AL, Kraybill WG, Virgo KS. Extremity soft tissue sarcoma patient follow-up: tumor grade and size affect surveillance strategies after potentially curative surgery. Internationaljournal of oncology. 2003;22(6):1335–43. [PubMed] [Google Scholar]
- 20.Lord HK, Salter DM, MacDougall RH, Kerr GR. Is routine chest radiography a useful test in the follow up of all adult patients with soft tissue sarcoma? The British journal of radiology. 2006;79(946):799–800. doi: 10.1259/bjr/69175634. [DOI] [PubMed] [Google Scholar]
- 21.Miller BJ, Carmody Soni EE, Reith JD, Gibbs CP, Scarborough MT. CT scans for pulmonary surveillance may be overused in lower-grade sarcoma. The Iowa orthopedic journal. 2012;32:28–34. [PMC free article] [PubMed] [Google Scholar]
- 22.Puri A, Gulia A, Hawaldar R, Ranganathan P, Badwe RA. Does intensity of surveillance affect survival after surgery for sarcomas? Results of a randomized noninferiority trial. Clinical orthopaedicsand related research. 2014;472(5):1568–75. doi: 10.1007/s11999-013-3385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawamura C, Matsumoto S, Shimoji T, Okawa A, Ae K. How long should we follow patients with soft tissue sarcomas? Clinical orthopaedics and related research. 2014;472(3):842–8. doi: 10.1007/s11999-013-3076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Investigators TG. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. The GIVIO Investigators. Jama. 1994;271(20):1587-92. [DOI] [PubMed]
- 25.Mooney MM, Mettlin C, Michalek AM, Petrelli NJ, Kraybill WG. Life-long screening of patients with intermediate-thickness cutaneous melanoma for asymptomatic pulmonary recurrences: a costeffectiveness analysis. Cancer. 1997;80(6):1052–64. doi: 10.1002/(sici)1097-0142(19970915)80:6<1052::aid-cncr7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Weiss M, Loprinzi CL, Creagan ET, Dalton RJ, Novotny P, O’Fallon JR. Utility of follow-up tests for detecting recurrent disease in patients with malignant melanomas. Jama. 1995;274(21):1703–5. [PubMed] [Google Scholar]
- 27.Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic followup after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. Jama. 1994;271(20):1593–7. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 28.Lin EC. Radiation risk from medical imaging. MayoClinic proceedings. 2010;85(12):1142–6. doi: 10.4065/mcp.2010.0260. quiz 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of internal medicine. 2009;169(22):2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363(9406):345–51. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 31.Berrington de Gonzalez A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Archives of internal medicine. 2009;169(22):2071–7. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]


