Abstract
Background
Biomarkers such as cytokines, chemokines, and soluble activation markers can be unstable when processing of blood is delayed. The stability of various biomarkers in serum and plasma was investigated when unprocessed blood samples were stored for up to 24 h at room and refrigerator temperature.
Methods
Blood was collected from 16 healthy volunteers. Unprocessed serum, EDTA and heparinized blood was stored at room (20–25 °C) and refrigerator temperature (4–8 °C) for 0.5, 2, 4, 6, 8, and 24 h after collection before centrifugation and separation of serum and plasma. Samples were batch tested for various biomarkers using commercially available immunoassays. Statistically significant changes were determined using the generalized estimating equation.
Results
IFN-γ, sIL-2Rα, sTNF-RII and β2-microglobulin were stable in unprocessed serum, EDTA and heparinized blood samples stored at either room or refrigerator temperature for up to 24 h. IL-6, TNF-α, MIP-1β and RANTES were unstable in heparinized blood at room temperature; TNF-α, and MIP-1β were unstable in unprocessed serum at room temperature; IL-12 was unstable in unprocessed serum at refrigerator temperature; and neopterin was unstable in unprocessed EDTA blood at room temperature. IL-1ra was stable only in unprocessed serum at room temperature.
Conclusion
All the biomarkers studied, with the exception of IL-1ra, were stable in unprocessed EDTA blood stored at refrigerator temperature for 24 h. This indicates that blood for these biomarkers should be collected in EDTA and if delays in processing are anticipated the unseparated blood should be stored at refrigerator temperature until processing.
Keywords: Biomarker, Stability, Chemokines, Cytokines, Soluble activation marker, Significant relative change
1. Introduction
Cytokines and chemokines are low molecular weight glycoprotein molecules produced by both immune and non-immune cells that can interact with each other and elicit biological activity at extremely low concentrations. Both a physiological or pathological stimulus can result in the increased production and release of these biomarkers into the circulation. Following release, cytokines and chemokines have relatively short half-lives [1].
Variation in the levels of cytokines, chemokines, cytokine/chemokine receptors, and soluble activation markers in various biological fluids are now recognized as a potential and useful tool for evaluating ongoing clinical disorders [2,3]. They can provide valuable information regarding the diagnosis, stage, and prognosis of various diseases. Unfortunately, a number of pre-analytical factors have been shown to influence the validity and quality of cytokine, chemokine, and activation marker measurements [4–7].
Blood levels of cytokines and chemokines can be altered by patient behaviors such as stress-induced psychological or physiological responses, fasting, drugs, diurnal rhythms, physical activity, blood collection techniques, handling, processing, and the storage conditions of unprocessed blood samples [8–13]. Potential problems can be avoided by paying careful attention to pre-analytical, analytical, and post-analytical variables while applying appropriate quality control procedures. Published studies have suggested that the use of various anticoagulants, endotoxin tube contamination, and delays in blood processing (centrifugation) can have a major impact on serum and plasma concentrations of various biomarkers, and can result in falsely increased or decreased biomarker concentration measurements [14–18].
Manual or semi-automated enzyme immunoassays (EIA) and multiplex assays are widely available to measure cytokines, chemokines, and activation markers, although there are known complexities of the various methodologies. Abundant circulating blood proteins such as α-2 macroglobulin, auto-antibodies, as well as assay matrices, cross-reaction of multiple antibodies used in the multiplex format, and lack of assay standardization can greatly impact the reliability and reproducibility of these assays. There are also additional limitations with measurements of those cytokines at the lower limit of detection of the assay [19–22].
In most situations it is not be possible to transport blood samples from remote collection sites to the testing area quickly so blood samples can remain unprocessed for extended periods of time. Based on our assumption, unprocessed blood tubes should be stable for measurement of cytokines and chemokines for up to 24 h as long as blood is drawn into pyrogen-free Vacutainer tubes. This study was undertaken to identify the maximum time delay and ideal handling and storage conditions before processing and separation of serum and plasma in order to obtain reliable concentrations of several important blood immunological biomarkers.
2. Materials and methods
2.1. Specimens
Eleven 10 mL samples of blood were collected from 16 healthy volunteers into either 10 mL glass serum separator tubes (6 donors), 10 mL glass tubes containing EDTA (5 donors), or 10 mL glass tubes containing sodium heparin (5 donors) to obtain either serum or plasma (Becton Dickinson Vacutainer tubes, Franklin Lakes, NJ). One tube of blood from each volunteer was centrifuged within 0.5 h after collection (500 × g for 10 min) and the separated serum and plasma was frozen at −70 °C. The remaining tubes of blood were stored at room temperature (20–25 °C) or in the refrigerator (4–8 °C) for 2, 4, 6, 8, and 24 h (1 tube of blood per time point at each temperature) before centrifugation (500 × g for 10 min) and isolation of serum, EDTA plasma and heparinized plasma. All separated serum and plasma samples were stored at −70 °C until analysis. The study was approved by the institutional review board for human studies at UCLA and the blood samples were obtained from each individual after informed consent.
2.2. Quantification of biomarkers
All serum and plasma samples were tested in duplicate and the acceptable variability for our laboratory between replicates was required to be <15% [23]. Samples with replicate measurements differing by >15% were retested. The immunoassays that were used and the performance characteristics of each immunoassay were as follows:
2.2.1. Interleukin-6 (IL-6) concentrations were measured using a high sensitive sandwich enzyme immunoassay from R&D Systems (Minneapolis, MN, USA)
The lower limit of detection was 0.025 pg/mL and the intra-assay coefficient of variation (CV) was determined to be 2.5% and 3.3% for control samples at mean concentrations of 1.176 pg/mL (n = 10), and 2.101 pg/mL (n = 10), respectively.
2.2.2. Interleukin 12 (IL-12) concentrations were measured using a sandwich enzyme immunoassay from Pierce Biotechnology (Rockford, IL, USA)
The lower limit of detection was 2.70 pg/mL and the intra-assay CV was determined to be 6.0%, 6.6%, and 2.8% for control samples with mean concentrations of 5.8 pg/mL (n = 10), 86.8 pg/mL (n = 10), and 146.0 pg/mL, (n = 10) respectively.
2.2.3. Interferon gamma (IFN-γ) concentrations were measured using a sandwich enzyme immunoassay from Beckman Coulter (Brea, CA, USA)
The lower limit of detection was 10 U/L and the intra-assay CV was determined to be 16.5%, 6.4%, and 11.1% for control samples with mean concentrations of 4.7 U/L (n = 10), 249.7 U/L (n = 10), and 3665.0 U/L (n = 10) respectively.
2.2.4. Tumor Necrosis Factor alpha (TNF-α) concentrations were measured using a solid phase enzyme amplified sensitivity immunoassay from BioSource Europe SA (Nivelles, Belgium)
The test is based on the use of multiple monoclonal antibodies directed against distinct epitopes on the TNF-α molecule. The lower limit of detection was 3.0 pg/mL and the intra-assay CV was determined to be 8.1% and 5.5% for control samples with mean concentrations of 8.39 pg/mL (n = 10) and 37.8 pg/mL (n = 10) respectively.
2.2.5. Macrophage inflammatory protein-1β (MIP-1β) concentrations were measured using a sandwich enzyme immunoassay from R&D Systems
The lower limit of detection was 9.0 pg/mL and the intra-assay CV was determined to be 14.2% and 3.4% for control samples with mean concentrations of 57.3 pg/mL (n = 12) and 279.0 pg/mL (n = 12) respectively.
2.2.6. Regulated upon Activation Normal T cell Expressed and Secreted (RANTES) or CCL5 concentrations were measured using a sandwich enzyme immunoassay from R&D Systems
The lower limit of detection was 7.8 pg/mL and the intra-assay CV was determined to be 9.7% and 5.8% for control samples with mean concentrations of 25.3 pg/mL (n = 12) and 34.5 pg/mL (n = 12) respectively.
2.2.7. Interleukin-1 receptor antagonist (IL-1ra) concentrations were measured using a sandwich enzyme immunoassay technique from R&D Systems
The lower limit of detection was 22.0 pg/mL and the intra-assay CV was determined to be 20% and 11% for control samples with mean concentrations of 143.0 pg/mL (n = 11) and 668.0 pg/mL (n = 15) respectively.
2.2.8. Interleukin-2 receptor alpha (IL-2Rα) concentrations were measured using a sandwich enzyme immunoassay from R&D Systems
The lower limit of detection was 6.0 pg/mL and the intra-assay CV was determined to be 2.6% and 3.3% for control samples with mean concentrations of 681.0 pg/mL (n = 10) and 1955.0 pg/mL (n = 10) respectively.
2.2.9. Soluble Tumor Necrosis Factor receptor II (sTNF-RII) concentrations were measured using a sandwich enzyme immunoassay from R&D Systems
The lower limit of detection was 3.0 pg/mL and the intra-assay CV was determined to be 3.5% and 4.0% for control samples with mean concentrations of 2.69 ng/mL (n = 10) and 6.97 ng/mL (n = 10) respectively.
2.2.10. β2-Microglobulin (β2M) concentrations were measured by a microparticle enzyme immunoassay using the Abbott IMx autoanalyzer (Abbott Laboratories, Abbott Park, IL)
The lower limit of detection was 3.0 μg/L and the intra-assay CV was 4.6% and 2.4% for control samples with mean concentrations of 0.69 mg/L (n = 10) and 2.62 mg/L (n = 10) respectively.
2.2.11. Neopterin (NPT) concentrations were measured using a competitive enzyme immunoassay from BRAHMS (Berlin, Germany)
The lower limit of detection was 1 nmol/L and the intra-assay CV was 4.1% and 6.6% for control samples with mean concentrations of 7.93 nmol/L (n = 12) and 23.8 nmol/L (n = 12) respectively.
2.3. Statistical analysis
A four-parameter curve-fitting program (Bio-Rad Laboratories, Irvine, CA) was used for generation of calibration curves and calculation of biomarker concentrations. Sigma Plot software (Jandel Scientific, San Rafael, CA, USA) was used to generate figures and Stata statistical software (1StataCorp, College Station, TX, USA) packages were used for descriptive statistics. The rate of change in biomarker concentrations over 24 h was evaluated using the generalized estimating equation (SAS Institute Inc, Cary, NC).
The dependent variable was measured blood biomarker concentrations and was modeled as a linear function based on the delay in centrifugation in hours. Since EDTA plasma levels of IL-1ra at room temperature exhibited a significant difference in the rate of increase after 8 h, a linear spline with a knot at 8 h was used for the rate of change and was calculated as follows: If time was ≤8 h then the concentration = intercept + (coefficient 1) × (time). If time was >8 h) then the concentration = intercept + (coefficient 1) × (time) + (coefficient 2) × (time − 8). Coefficients are the rate of change in concentration with time being measured in hours. To account for the correlation between repeated measures in a given subject, generalized estimating equations with a first-order autoregressive covariance pattern were specified to reduce the number of parameters in the model. A p value <0.05 was considered to be statistically significant. In addition to a p value of <0.05, the predicted concentration change per hour was required to be at least 10% or more in order to be considered significant to account for measurement uncertainty of the immunoassays.
3. Results
3.1. General observations
The stability of cytokines, chemokines, and soluble activation markers was evaluated when unprocessed blood was stored at room temperature (20–25 °C) and in the refrigerator (4–8 °C) for up to 24 h before centrifugation and isolation of serum, EDTA plasma, and heparinized plasma. The concentrations of IL-6, IL-12, IFN-γ, TNF-α, MIP-1β, RANTES, IL-1ra, sIL-2Rα, sTNF-RII, β2 microglobulin, and neopterin were measured using commercially available immunoassays. Intercepts (mean baseline concentrations), coefficients (predicted changes in the measured concentration per hour), SE (standard errors associated with the coefficients), and p values (p > |z|) for each of the cytokines, chemokines, and soluble activation markers are presented in Tables 1–3, based on whether the blood samples were kept at room temperature (20– 25 °C) or in the refrigerator (4–8 °C).
Table 1.
Stability of cytokines in unprocessed blood stored at different temperatures.a
| Biomarker | Unprocessed blood stored at room temperature (20–25 °C)
|
Unprocessed blood stored in the refrigerator (4–8 °C)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Coefficient | SE | p > |z| | Intercept | Coefficient | SE | p > |z| | |
| IL-6 (pg/mL) | ||||||||
| Serum | 1.52 | 0.0044 | 0.0069 | 0.519 | 1.51 | −0.0040 | 0.0024 | 0.094 |
| EDTA plasma | 1.08 | −0.0011 | 0.0017 | 0.533 | 1.10 | 0.0016 | 0.0016 | 0.312 |
| Heparin plasma | 1.03 | 0.0128 | 0.0062 | 0.038 | 1.03 | −0.0025 | 0.0017 | 0.158 |
| IL-12 (pg/mL) | ||||||||
| Serum | 30.23 | 0.1686 | 0.1445 | 0.244 | 30.83 | −0.1229 | 0.0563 | 0.029 |
| EDTA plasma | 32.36 | 0.0284 | 0.1613 | 0.860 | 30.58 | 0.2140 | 0.1822 | 0.240 |
| Heparin plasma | 22.80 | 0.1865 | 0.2938 | 0.525 | 23.84 | −0.0638 | 0.1259 | 0.612 |
| IFN-γ (U/L) | ||||||||
| Serum | 72.25 | 0.1840 | 0.1710 | 0.2819 | 69.91 | 0.1140 | 0.1514 | 0.451 |
| EDTA plasma | 63.48 | −0.2411 | 0.2075 | 0.2454 | 60.44 | −0.0346 | 0.1752 | 0.8433 |
| Heparin plasma | 63.54 | 0.3792 | 0.4480 | 0.3973 | 64.49 | 0.0297 | 0.1616 | 0.8543 |
| TNF-α (pg/mL) | ||||||||
| Serum | 22.06 | 0.0938 | 0.0185 | <0.0001 | 22.07 | 0.0814 | 0.0458 | 0.076 |
| EDTA plasma | 15.65 | 0.0316 | 0.0814 | 0.698 | 17.13 | −0.0443 | 0.0969 | 0.647 |
| Heparin plasma | 15.80 | 0.1680 | 0.0345 | <0.0001 | 14.83 | 0.0086 | 0.1295 | 0.947 |
The intercept is the mean concentration at baseline; coefficient is the predicted change in concentration each hour for 24 h; SE is the standard error of the coefficient; and p > |z| is the calculated p value using the generalized estimating equation. p values ≤0.05 are considered statistically significant and are bolded.
Table 3.
Stability of various soluble activation markers in unprocessed blood stored at different temperatures.a
| Biomarker | Unprocessed blood stored at room temperature (20–25 °C)
|
Unprocessed blood stored in the refrigerator (4–8 °C)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Coefficient | SE | p > |z| | Intercept | Coefficient | SE | p > |z| | |
| sIL-2Rα (pg/mL) | ||||||||
| Serum | 943 | 0.5476 | 1.434 | 0.703 | 955 | −0.2086 | 1.059 | 0.844 |
| EDTA plasma | 603 | 2.9243 | 2.104 | 0.165 | 611 | −1.0638 | 2.216 | 0.631 |
| Heparin plasma | 715 | −0.0292 | 0.835 | 0.972 | 715 | −0.2133 | 1.837 | 0.908 |
| sTNF-RII (ng/mL) | ||||||||
| Serum | 2.35 | 0.0051 | 0.005 | 0.261 | 2.38 | −0.0063 | 0.006 | 0.330 |
| EDTA plasma | 1.76 | 0.0030 | 0.006 | 0.584 | 1.71 | 0.0031 | 0.005 | 0.560 |
| Heparin plasma | 1.87 | −0.0023 | 0.005 | 0.611 | 1.78 | 0.0035 | 0.002 | 0.032 |
| β2-microglobulin (mg/L) | ||||||||
| Serum | 1.29 | 0.0015 | 0.002 | 0.472 | 1.31 | 0.0008 | 0.003 | 0.779 |
| EDTA plasma | 1.04 | 0.0032 | 0.002 | 0.077 | 1.05 | −0.0022 | 0.002 | 0.338 |
| Heparin plasma | 1.14 | −0.0029 | 0.002 | 0.210 | 1.19 | −0.0033 | 0.003 | 0.296 |
| Neopterin (nmol/L) | ||||||||
| Serum | 5.79 | 0.0143 | 0.008 | 0.065 | 5.78 | 0.0039 | 0.004 | 0.275 |
| EDTA plasma | 6.18 | 0.0710 | 0.007 | <0.0001 | 6.10 | 0.0132 | 0.013 | 0.307 |
| Heparin plasma | 5.12 | −0.0057 | 0.012 | 0.637 | 5.05 | −0.0013 | 0.008 | 0.861 |
The intercept is the mean concentration at baseline; coefficient is the predicted change in concentration each hour for 24 h; SE is the standard error of the coefficient; and p > |z| is the calculated p value using the generalized estimating equation. p values ≤0.05 are considered statistically significant and are bolded.
3.2. Cytokines
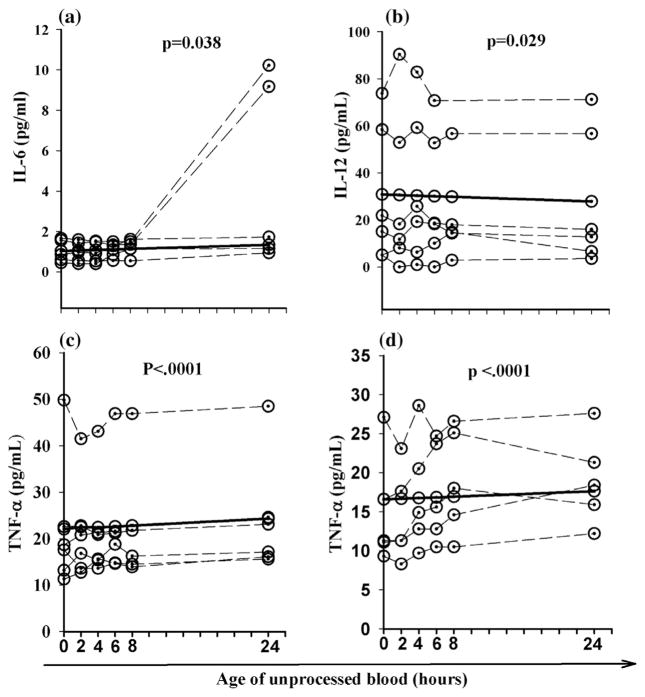
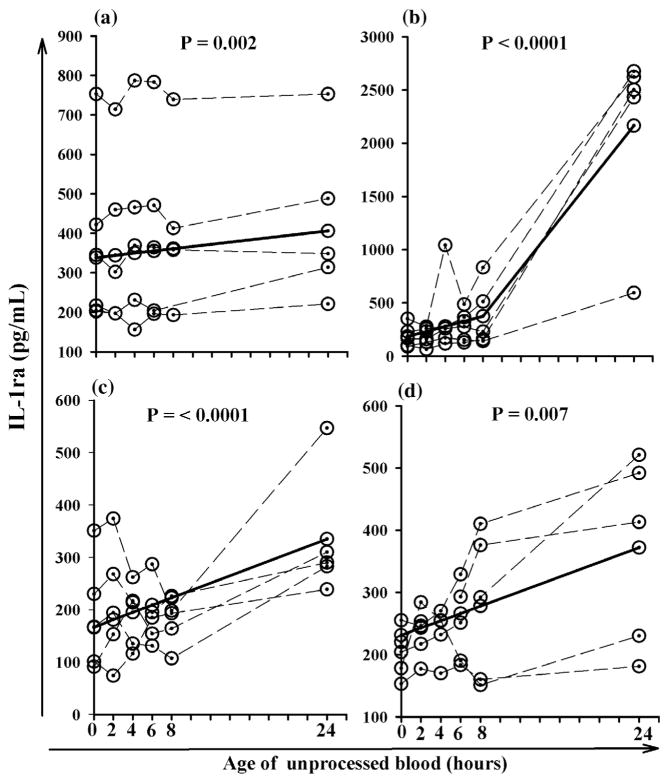
When cytokines were evaluated there were statistically significant changes in the concentrations of IL-6, IL-12, TNF-α, and IL-1ra after 24 h compared to the baseline concentrations. The concentration of IL-6 increased 30% per hour in unseparated heparinized plasma stored at room temperature whereas serum concentrations of IL-12 decreased 10% at refrigerator temperature (Table 1). Serum concentrations of TNF-α increased 10% and heparinized plasma samples increased 26% when stored at room temperature (Table 1). Changes in IL-6, IL-12 and TNF-α concentrations over time for individual blood samples are presented in Fig. 1. IL-1ra appeared to be the least stable cytokine and increased significantly when EDTA plasma (1071% increase) and heparinized plasma (61% increase) was stored at room temperature, and when serum (20% increase), EDTA plasma (101% increase) and heparinized plasma (38% increase) was stored in the refrigerator (Table 2 and Fig. 2).
Fig. 1.
Cytokines that were significantly altered (p < 0.05) when unprocessed blood was stored at either room or refrigerator temperature for 24 h. Unprocessed heparin plasma stored at room temperature (a); unprocessed serum stored in the refrigerator (b); unprocessed serum stored at room temperature (c); and unprocessed haparin plasma stored at room temperature (d). Dashed lines represent data for each individual subject and the solid line represents the predicated average change for all subjects.
Table 2.
Stability of various cytokines and chemokines in unprocessed blood stored at different temperatures.a
| Biomarker | Unprocessed blood stored at room temperature (20–25 °C)
|
Unprocessed blood stored in the refrigerator (4–8 °C)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Coefficient | SE | p > |z| | Intercept | Coefficient | SE | p > |z| | |
| IL-1ra (pg/mL) | ||||||||
| Serum | 369 | 2.4715 | 3.2806 | 0.451 | 339 | 2.8225 | 0.9150 | 0.002 |
| EDTA plasma | 185 | 88.085 | 19.877 | <0.0001 | 166 | 7.0286 | 1.6032 | <0.0001 |
| Heparin plasma | 231 | 5.8881 | 2.1636 | 0.007 | 163 | 2.8168 | 1.2411 | 0.023 |
| MIP-1β (pg/mL) | ||||||||
| Serum | 72.90 | 0.8197 | 0.4144 | 0.048 | 75.40 | −0.3944 | 0.3132 | 0.208 |
| EDTA plasma | 47.97 | 0.2070 | 0.1359 | 0.128 | 48.80 | 0.0241 | 0.2666 | 0.928 |
| Heparin plasma | 34.44 | 2.7742 | 0.8681 | 0.001 | 38.81 | 0.0822 | 0.1010 | 0.416 |
| RANTES (ng/mL) | ||||||||
| Serum | 3.22 | −0.0090 | 0.0151 | 0.551 | 3.04 | 0.0219 | 0.0169 | 0.197 |
| EDTA plasma | 6.86 | 0.0183 | 0.0431 | 0.672 | 6.93 | −0.0011 | 0.0842 | 0.989 |
| Heparin plasma | 2.63 | 0.0759 | 0.0376 | 0.044 | 2.09 | −0.0006 | 0.0071 | 0.938 |
The intercept is the mean concentration at baseline; coefficient is the predicted change in concentration each hour for 24 h; SE is the standard error of the coefficient; and p > |z| is the calculated p value using the generalized estimating equation. p values ≤0.05 are considered statistically significant and are bolded.
Fig. 2.
Increases in IL-1ra that occur over time when unprocessed EDTA and heparin blood was stored at either room or refrigerator temperature for 24 h. Unprocessed serum stored in refrigerator (a); unprocessed EDTA plasma stored at room temperature (b); unprocessed EDTA plasma stored in the refrigerator (c); and unprocessed heparin plasma stored at room temperature (d); dashed lines represent data for each individual subject and solid line represents the predicted average change for all subjects.
3.3. Chemokines
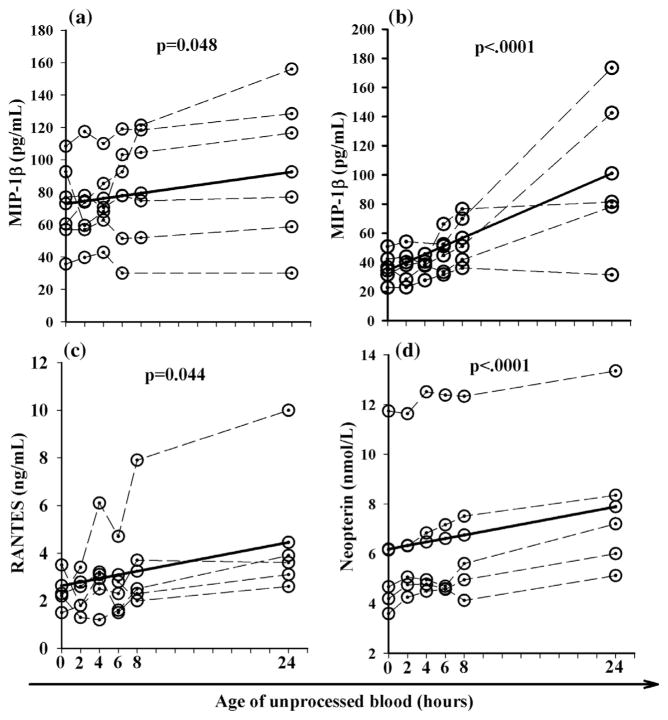
When chemokines were examined serum MIP-1β and heparinized plasma increased 27% and 193%, respectively, when stored at room temperature (Table 2, Fig. 3a and b). RANTES also increased by 69% when heparinized plasma was stored at room temperature (Table 2, Fig. 3c).
Fig. 3.
Chemokines and soluble activation markers that were significantly altered (p < 0.05) when unprocessed blood was stored at room temperature for 24 h. Unprocessed serum (a); unprocessed heparin plasma (b); unprocessed heparin plasma (c); and unprocessed EDTA plasma (d); dashed lines represent data for each individual subject and the solid line represents the predicated average change for all subjecs.
3.4. Activation markers
For soluble activation markers, neopterin increased 28% when EDTA plasma was stored at room temperature (Table 3 and Fig. 3d). Although changes in sTNF-RII were statistically significant based on the p value of 0.032, a 5% change per hour failed to exceed the established threshold change of 10%, so this marker was considered to be stable.
4. Discussion
The goal of this study was to determine if significant changes in cytokines, chemokines and soluble activation markers occurs when unprocessed blood samples are stored at room temperature and refrigerator temperature for periods of time up to 24 h. The study was designed to mimic common delays in sample processing that often occur when blood samples are collected at remote sites and need to be transported to a central laboratory for processing and testing. Our data indicate that IFN-γ, sIL-2Rα, sTNF-RII, and β2-microglobulin are stable biomarkers when unprocessed serum, EDTA plasma and heparinized plasma blood samples are stored at either room or refrigerator temperatures for up to 24 h prior to separation and isolation of serum or plasma. IL-1ra was the least stable biomarker and was only stable for 24 h when unseparated blood containing no anticoagulant (serum) was stored at room temperature. If IL-1ra is excluded, our data indicates that EDTA blood is the optimal specimen type since all the other 10 biomarkers were stable when unprocessed blood was stored at refrigerator temperature. In addition, 9 out of the 10 biomarkers (only neopterin was significantly increased) were stable when EDTA blood was stored at room temperature.
It has previously been demonstrated that prolonged contact of serum and plasma with cellular elements and clotting factors can alter the concentration of many routinely ordered chemistry tests [23–29]. We found that when unprocessed blood samples were stored for up to 24 h the concentrations of IL-6, TNF-α, IL-1ra, MIP-1β, RANTES and neopterin increased in a time-dependent fashion based on the storage condition. The only biomarker that decreased was IL-12, and only when unprocessed serum and not plasma was stored in the refrigerator. Activation of monocytes during the clotting process might account for the increases in TNF-α that were observed when unprocessed serum was stored at room temperature. The longer blood samples remain unprocessed, the more likely that certain biomarkers such as IL-6 and TNF-α will increase, and this increase will be more dramatic when the collection tubes contain trace amounts of endotoxin [17,30].
There are only a limited number of studies that have examined the stability of cytokines, chemokines and soluble activation markers in unprocessed blood samples stored under different conditions. Skogstrand et al. examined the stability of serum samples that were left unprocessed for 4, 24 and 48 h at room temperature and found that IL-12, TNF-α, MIP-1β, and RANTES were not stable when stored at ambient temperature for 4 h [31]. These findings are in agreement with ours, except that we found RANTES to be stable in unprocessed serum samples for up to 24 h. This group also found that IFN-γ, TNF-α, MIP-1β and RANTES were not stable in unprocessed EDTA plasma stored at ambient temperature for 4 h [31], which differs from our results since we found these biomarkers to be stable in EDTA plasma up to 24 h. Although this group did not examine heparinized plasma, we found that TNFα, MIP-1β and RANTES were not stable in unprocessed heparinized blood stored at ambient temperature.
A more recent study obtained similar results to ours and found that IL-6, TNF-α, MIP-1β and sIL-2Rα were stable whereas IL-1ra dramatically increased when unprocessed EDTA blood was stored at ambient temperature for 3 days and tested daily [32]. This study also observed a 17% decrease in IL-12 p40 but no change in IL-12 p70 concentrations when unprocessed EDTA blood was stored at ambient temperature [32]. In contrast, we found that total IL-12 concentrations (includes both the p40 and p70 forms of IL-12) were stable in unprocessed EDTA blood stored at ambient temperature for 24 h, but the instability of IL-12 p40 could have been masked by the contribution of IL-12 p70 to the total concentration.
In an earlier study, IL-6 was found to decrease 14.3% and TNF-α increased 9.6% when unprocessed EDTA blood samples were stored at ambient temperature for 4 h [15]. In contrast, another investigator found that these two biomarkers were stable in unprocessed EDTA plasma samples stored at 4 °C and ambient temperature for 4 and 24 h [33], which is consistent with our findings. Another group observed changes in IL-6 and TNF-α when unprocessed EDTA blood samples were stored at ambient temperatures, however, blood samples were fortified with cytokines and this approach may not reflect actual changes that occur for endogenous cytokines [16]. Similar to our findings, De Jongh et al. found that IL-6 and sIL-2R were stable in unseparated serum and EDTA plasma stored at 4 °C for 8 and 24 h [14].
IL-1ra was shown to be the most unstable biomarker regardless of the specimen type when unprocessed blood samples were stored at either room temperature or at refrigerator temperature. Studies have shown that monocytes, macrophages, leukemia monocytic cell lines, neutrophils and other cells can produce IL-1ra and that IL-1ra plays a proinflammatory role in various diseases [34]. Neutrophils can produce significant quantities of IL-1ra and monocytes have been estimated to produce 20 times more IL-1ra than neutrophils [35]. The increases in IL-1ra concentrations that we observed within 24 h might be due to the activation of monocytes and neutrophils in unprocessed EDTA and heparinized blood samples. Based on the increases for the various specimen types that were observed it appears that serum might be the most suitable specimen type when testing for IL-1ra. Furthermore, the collected blood should be stored at room temperature if delays in processing are anticipated.
Novel aspects of our study include the use of multiple sample types (serum, EDTA and heparinized plasma), representative biomarkers from multiple classes of immune mediators (cytokines, chemokine, cytokine/chemokine receptors, and soluble activation markers), and 6 time points within a 24 h period for a precise determination of biomarker stability in unprocessed blood samples. Only a few studies have investigated the stability of a large number of biomarkers in unprocessed blood samples, and only 4 and/or 24 h after blood collection [31–33]. The multiple time points that we examined between 0 and 24 h and the modeling of our data to reflect biomarker changes per hour, most likely contribute to differences between our results and other studies.
A limitation of our study was that all three types of blood (serum, EDTA plasma, heparinized plasma) were not collected from the same donors so the study used different donors to evaluate different types of blood samples. Unfortunately, the large blood volume that was required to evaluate all three types of blood given the study design prohibited us from using a single donor for this purpose. This raises the possibility that potential differences in biomarker stability could be influenced by donor variability. However, each specimen type was collected from either 5 or 6 donors, which should minimize the impact of this possibility on our study results.
In conclusion, for quantification of cytokines, chemokines and soluble activation markers in blood, the blood samples should be centrifuged as rapidly as possible after collection and the serum or plasma should be separated and stored at refrigerator temperature until testing. If a delay in processing is expected then the unprocessed blood should be stored at refrigerator temperature. Our results indicate that EDTA blood is the specimen of choice since all of the biomarkers that were examined were stable in unprocessed EDTA blood stored in the refrigerator. This finding is consistent with a guideline document indicating that blood samples should be collected in EDTA and stored at refrigerator temperature [33]. An exception is IL-1ra, which appears to be stable at ambient temperature in unprocessed serum samples. If batch testing of blood samples will occur at a later time, then the separated plasma and serum should be stored at −70 °C until analysis.
Acknowledgments
We thank Megan Detels for data entry, Jason Clague, Benjamin Roger and Priscilla Yen for statistical help and Timothy Ryner for manuscript preparation. This work was supported by grants from the National Institutes of Health, United States (U01-A1-35042); The MACS is funded by the National Institute of Allergy and Infectious Diseases, United States with additional supplemental funding from the National Cancer Institute, United States (U01-AI35042, 5-M01-RR00722 (GCRC), U01-A135043, U01-AI37984, U01-A135039, U01-AI35040, U01-AI37613, and U01-AI35041). This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-CO56000.
Footnotes
Conflict of Interest
The authors declared no conflict of interest.
References
- 1.Whiteside TL. Cytokines and cytokine measurements in a clinical laboratory. Clin Diagn Lab Immunol. 1994;1:257–260. doi: 10.1128/cdli.1.3.257-260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Puri RK. Common and uncommon features of cytokines and cytokine receptors: an overview. In: Aggarwal BB, Puri RK, editors. Human Cytokines: Their Role in Disease and Therapy. Blackwell Science; Cambridge, Mass: 1995. pp. 3–24. [Google Scholar]
- 3.Fahey JL. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5:597–603. doi: 10.1128/cdli.5.5.597-603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aziz N, Nishanian P, Fahey JL. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5:755–761. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon JG, Nrrad JL, Poutsiaka DD, Dinarello CD. Measuring circulating cytokines. J Appl Physiol. 1993;75:1897–1902. doi: 10.1152/jappl.1993.75.4.1897. [DOI] [PubMed] [Google Scholar]
- 6.Mire-Sluis AR. Cytokines-protein structure and biological activity: a complex relationship with implication for biological assays and standardization. Biological. 1993;21:131–144. doi: 10.1006/biol.1993.1062. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe R, Wadhwa M, Bird CR, Mire-Sluis AR. Detection and measurement of cytokines. Blood Rev. 1992;6:133–148. doi: 10.1016/0268-960x(92)90025-l. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13:541–547. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn P, Després JP, Lamarche B, Tremblay A, Bergeron J, Lemieux I, Couillard C. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity. 2006;14:1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 11.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and antiinflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515:287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunter K, Teunissen C, De Jongh R, Bosmans E, Steinbusch H, Maes M. Stability of interleukin 6, soluble interleukin 6 receptor, interleukin 10 and CC16 in human serum. Cytokine. 2002;9(5):228–235. [PubMed] [Google Scholar]
- 13.Koh KB, Lee Y, Beyn KM, Chu SH, Kim DM. Counter-stress effects of relaxation on proinflammatory and anti-inflammatory cytokines. Brain Behav Immun. 2008;22:1130–1137. doi: 10.1016/j.bbi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.De Jongh R, Vranken J, Vundelinckx G, Bosmans E, Maes M, Heylen R. The effects of anticoagulation and processing on assays of IL-6, sIL-6R, sIL-2R and soluble transferring receptor. Cytokine. 1997;9:696–701. doi: 10.1006/cyto.1997.0217. [DOI] [PubMed] [Google Scholar]
- 15.Flower L, Ahuja RH, Humphries SE, Mohamed Ali V. Effect of sample handling on the stability of interleukin-6, tumor necrosis factor alpha and leptin. Cytokine. 2000;12:1712–1716. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- 16.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. J Immunol Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 17.Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6, and TNF-α concentration. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 18.Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm. 2014;2014:545493. doi: 10.1155/2014/545493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg-Hasson Y, Hansmann L, Liedtke M, Herschmann I, Maecker HT. Effects of serum and plasma matrices on multiplex immunoassays. Immunol Res. 2014;58:224–233. doi: 10.1007/s12026-014-8491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peasee AJ, Michael JG. Artifacts and limitations of enzyme immunoassays. J Immunol Methods. 1992;150:111–119. doi: 10.1016/0022-1759(92)90070-a. [DOI] [PubMed] [Google Scholar]
- 21.Petyovka NL, Lyach L, Voitenok NN. Homologous ELISA for detection of oligomeric human TNF: properties of assay. J Immunol Methods. 1995;186:161–170. doi: 10.1016/0022-1759(95)00183-b. [DOI] [PubMed] [Google Scholar]
- 22.Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passey RB. Quality control for the clinical chemistry laboratory. In: Kaplan LA, Pesce JA, editors. Clinical Chemistry: Theory, Analysis, and Correlation. 3. CV Mosby Company; St. Louis: 1996. pp. 385–391. [Google Scholar]
- 24.Boyanton BL, Jr, Blick KE. Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002;48:2242–2247. [PubMed] [Google Scholar]
- 25.Kang HJ, Jeon SY, Park JS, Kil NH, Hong WK, Lee Mee-Hee, Kim Jun-Woo, Jeon Jae-Pil, Han BG. Identification of clinical biomarkers for pre-analytical quality control of blood samples. Biopreserv Biobank. 2013;11:94–100. doi: 10.1089/bio.2012.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson SS, Guillan RA, Hocker EV. Studies of the stability of 18 chemical constituents of human serum. Clin Chem. 1972;18:1498–1503. [PubMed] [Google Scholar]
- 27.Laessig RH, Indriksons AA, Hassemer DJ, Paskey TA, Schwartz TH. Changes in serum chemical values as a result of prolonged contact with the clot. Am J Clin Pathol. 1972;66:598–604. doi: 10.1093/ajcp/66.3.598. [DOI] [PubMed] [Google Scholar]
- 28.Zhang DJ, Elswick RK, Miller WG, Bailey JL. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem. 1998;44:1325– 1333. [PubMed] [Google Scholar]
- 29.Van Vrancken MJ, Briscoe D, Anderson KM, Wians FH., Jr Time-dependent stability of 22 analytes in lithium-plasma specimens stored at refrigerator temperature for up to 4 days. Lab Med. 2012;43:268–275. [Google Scholar]
- 30.Aziz N, Irwin MR, Dickerson SS, Butch AW. Spurious tumor necrosis factoralpha and interleukin-6 production by human monocytes from blood collected in endotoxin-contaminated vacutainer blood collection tubes. Clin Chem. 2004;50:2215–2216. doi: 10.1373/clinchem.2004.040162. [DOI] [PubMed] [Google Scholar]
- 31.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Norgaard-Pedersen B, Hougaard DM. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Jackman RP, Utter GH, Heitman JW, Hirschkorn DF, Law JP, Gefter N, Busch MP, Norris PJ. Effects of blood sample age at time of separation on measured cytokine concentrations in human plasma. Clin Vaccine Immunol. 2011;18:318–326. doi: 10.1128/CVI.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beer L. Guidelines for Measurement of Cytokines in Human Serum and Plasma: Effects of Blood Sample Handling Procedures on Measured Cytokine and Chemokine Concentrations in Human Serum and Plasma. Akademiker Verlag; 2013. Nov 9, [Google Scholar]
- 34.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 35.McColl SR, Paquin R, Ménard C, Beaulieu AD. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med. 1992;176:593–598. doi: 10.1084/jem.176.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]