Abstract
Background
The cannabinoid (CB) system is a rational novel target for treating opioid dependence, a significant public health problem around the world. This proof-of-concept study examined the potential efficacy of a CB1 receptor partial agonist, dronabinol, in relieving signs and symptoms of opioid withdrawal.
Methods
Twelve opioid dependent adults participated in this 5-week, inpatient, double-blind, randomized, placebo-controlled study. Volunteers were maintained on double-blind oxycodone (30mg oral, four times/day) and participated in a training session followed by 7 experimental sessions, each testing a single oral test dose (placebo, oxycodone 30 and 60mg, dronabinol 5, 10, 20, and 30mg [decreased from 40mg]). Placebo was substituted for oxycodone maintenance doses for 21 hours before each session in order to produce measurable opioid withdrawal. Outcomes included observer- and participant-ratings of opioid agonist, opioid withdrawal and psychomotor/cognitive performance.
Results
Oxycodone produced prototypic opioid agonist effects (i.e., suppressing withdrawal and increasing subjective effects indicative of abuse liability). Dronabinol 5 and 10mg produced effects most similar to placebo, while the 20 and 30mg doses produced modest signals of withdrawal suppression that were accompanied by dose-related increases in high, sedation, bad effects, feelings of heart racing, and tachycardia. Dronabinol was not liked more than placebo, showed some impairment in cognitive performance, and was identified as marijuana with increasing dose.
Conclusion
CB1 receptor activation is a reasonable strategy to pursue for the treatment of opioid withdrawal; however, dronabinol is not a likely candidate given its modest withdrawal suppression effects of limited duration and previously reported tachycardia during opioid withdrawal.
Keywords: Dronabinol, opioid withdrawal, abuse liability, treatment
1. Introduction
In 2014, there were 2.5 million community dwelling persons age 12 years and older in the United States (US) meeting criteria for opioid abuse or dependence (prescription opioid and heroin) and an additional 1.7 million initiating use of heroin and prescription opioid analgesics for non-medical purposes (Substance Abuse and Mental Health Services Administration [SAMHSA] 2015). Given the chronic relapsing nature of opioid dependence (McLellan et al., 2000), the continued availability of opioids worldwide, and the substantial number of persons initiating heroin and non-medical opioid analgesic use each year, treatment demand will continue to grow (Cicero et al., 2015; Dart et al., 2015). However, the three currently available FDA-approved medications for opioid dependence treatment all exert their efficacy through action at the mu opioid receptor (i.e., methadone, buprenorphine and naltrexone) and possess significant practical and therapeutic limitations. Methadone and buprenorphine have restrictions that limit their availability, and they are not always effective. Naltrexone, while lacking abuse liability and diversion risk, has had limited utility due to poor patient acceptance, adherence and challenges associated with initial induction.
Finding new medications with novel mechanisms of action to effectively alleviate opioid withdrawal is important because withdrawal relief is a critical aspect of: assisting those who cannot access, do not want, or do not respond to opioid agonist/antagonist medications; and may assist with the transition onto, adherence to, and effectiveness of naltrexone. The endocannabinoid system is a potential novel target for opioid withdrawal treatment. Both cannabinoid (CB)1 receptors and mu opioid receptors are G-protein-coupled receptors, which have downstream effects on adenylyl cyclase enzyme activity, Ca2+ channel activation and neurotransmitter release (Rios et al., 2006). They both have widespread and partially overlapping anatomical distributions in central and peripheral nervous system regions involved with analgesia (e.g., thalamus, spinal cord), drug reward and self-administration (e.g., nucleus accumbens), and opioid withdrawal (e.g., locus coeruleus; Pickel et al., 2004; Welch, 2009; Scavone et al., 2010). Neurochemical and behavioral preclinical studies reveal a significant degree of functional interaction between the opioid and cannabinoid receptor systems. For example, both CB1 and mu opioid receptor activation increase dopamine release in the nucleus accumbens (an effect observed for most abused drugs) and the effect of both can be blocked by infusion of naloxone (Tanda et al., 1997). Other studies have demonstrated that exogenous ∆9-THC decreases signs of opioid withdrawal in morphine-dependent mice and rats (Hine et al., 1975a, 1975b; Bhargava, 1976,; Lichtman et al., 2001; Cichewicz and Welch, 2003; Gamage et al., 2015), and conversely, CB1 antagonists precipitate opioid withdrawal (Scavone et al., 2013).
Notably, there are three CB medications marketed in different countries under the brand names of Sativex®, Marinol®, and Nabilone®. These medications are approved for specific indications, such as chemotherapy-induced nausea and loss of appetite associated with anorexia in acquired immune deficiency syndrome. Importantly, these medications are effective in treating signs and symptoms that are typical of opioid withdrawal, such as pain, nausea, vomiting, and poor appetite. Bisaga and colleagues (Bisaga et al., 2015) recently reported a study in which oral dronabinol (∆9-THC), a Schedule III marketed CB1 partial agonist (Marinol®), was examined in opioid dependent adults undergoing inpatient detoxification and induction onto depot naltrexone. Using a double-blind randomized placebo-controlled design, volunteers received dronabinol 10, 20, and 30mg daily on inpatient days 2, 3, and 4 respectively and 30mg for 5 weeks thereafter as outpatients. Dronabinol was well-tolerated and produced significantly lower scores on the short opioid withdrawal scale (SOWS) on days 2-4 but did not improve rates of induction on naltrexone or treatment retention. While there was a promising signal of withdrawal suppression for dronabinol, detected even in the presence of open-label buprenorphine on day 1 and other non-opioid ancillary medications, it is not yet clear the extent that oral dronabinol alone can relieve opioid withdrawal. This is important because it may spare the use of other medications with abuse potential and safety concerns (e.g., benzodiazepines) particularly for outpatient detoxification protocols.
The current inpatient, placebo-controlled study investigated the effects of a range of acute oral dronabinol doses (5-40mg) compared to oxycodone and placebo among opioid dependent adults experiencing acute opioid withdrawal. We previously reported on the physiologic and safety outcomes from this study whereby dronabinol 40mg was poorly tolerated, producing sinus tachycardia, anxiety and panic, which led to its replacement with a lower 30mg dose (Jicha et al., 2015). Herein, we report on the subjective- and observer-rated opiate withdrawal and agonist outcomes, subjective effects indicative of abuse liability, and cognitive outcomes.
2. Methods
2.1 Participants
Adult volunteers were screened on an outpatient basis over several visits. Eligible volunteers were between 18-50 years old, self-reported use of short-acting opioids on ≥21 days of the last 30, produced a positive urine opioid test, and had good general health as determined by routine medical screening that included a history and physical exam, 12-lead electrocardiogram, serum chemistry and hematology, and urinalysis testing. Volunteers were excluded if they were pregnant or breastfeeding, seeking substance abuse treatment, or using CYP3A4/2D6 inducing or inhibiting medications in the last 30 days. Other exclusion criteria included: ongoing major medical (diabetes) or psychiatric illness (schizophrenia), current physiologic dependence on alcohol or a sedative/hypnotic requiring medical detoxification, self-report of >15 days of the last 30 of cannabis use (to prevent inclusion of persons who may have tolerance to dronabinol) and buprenorphine or methadone as the primary drug of abuse. Volunteers provided written informed consent and were paid for their participation. The protocol was approved by the University of Kentucky (UK) Institutional Review Board and was conducted in accordance with the Declaration of Helsinki guidelines.
2.2 Study setting
Participants resided for ∼5 weeks on the residential research unit at UK, maintained on a caffeine-free diet. Urine was collected and tested for non-study drugs daily, and females were tested for pregnancy weekly. Cigarette smoking was allowed ad libitum under direct supervision, but restricted starting 30 min before and throughout sessions. Medications for common ailments (e.g., acetaminophen, ibuprofen, colace, bismuth subsalicylate, alumina/magnesia/simethicone, and saline eye and nose drops) were available to volunteers as needed, but restricted on session days from midnight through session completion.
2.3 Study design and procedures
A double-blind, randomized, within-subject, placebo-controlled design was employed. Once admitted to the research unit, participants were stabilized on oral oxycodone 30 mg given at 8:00, 12:00, 18:00, 22:00 hours to produce a stable level of opioid physical dependence throughout the study. After at least 5 days of oxycodone stabilization, a double-blind placebo training session (data excluded from analyses) was conducted to ensure that volunteers were demonstrating opioid withdrawal and comprehended testing procedures.
Seven double-blind experimental sessions were completed, each separated by ≥72 hours. Oxycodone doses scheduled for 08:00 hours on session days and at 18:00 and 22:00 the preceding day were substituted with double-blind placebo to elicit spontaneous opiate withdrawal. Therefore, 21 hours had passed since the last active maintenance dose when sessions began. The 6-hr sessions began at 09:00 with drug administration at 10:00. Oral test conditions were placebo, oxycodone (30 or 60mg), and dronabinol (5, 10, 20 or 40mg). The first two subjects who received dronabinol 40mg experienced sustained sinus tachycardia accompanied by anxiety and panic. This dose was then reduced to 30mg thereafter, and the dose order was fully randomized except that dronabinol 20mg always preceded 30mg. The 40mg dose was excluded from all analyses, and those data are presented elsewhere (Jicha et al., 2015).
2.4 Drugs
Study medications were prepared by the UK Investigational Pharmacy under an Investigational New Drug Application (#69,214). Oxycodone, placebo, and dronabinol doses were prepared with oxycodone HCl 30mg tablets (Mallinckrodt Inc., Hazelwood, MD), lactose monohydrate powder N.F. (Medisca Pharmaceuticals, Plattsburgh, NY), and dronabinol 5 and 10mg capsules (PAR Pharmaceutical, Spring Valley, NY), respectively. All active doses were over-encapsulated and loose-filled with lactose in order to maintain the blind. Active and placebo oral maintenance doses were prepared in size 00 blue/white gelatin capsules (Health Care Logistics, Circleville, OH). Experimental session doses were prepared in size 00 dark green gelatin capsules (Capsugel, Morristown, NJ).
2.5 Outcome measures
Table 1 shows the study timeline for data collection in each session. Subjects completed: 1) a 16-item opioid agonist and 21-item antagonist adjective scale (each item scored from 0 [not at all] to 4 [extremely]) (Preston and Bigelow, 1998); 2) VAS items scored from “not at all” (0) to “extremely” (100); 3) a 10-item short opiate withdrawal scale (SOWS; each item scored from 0 (none) to 3 (severe) (Gossop, 1990); 4) a modified drug class identification questionnaire (Jasinski, 1977, Lofwall et al., 2007); 5) a street value estimate (“What is the street value of the dose you just received?”). The VAS items were: “Do you feel any DRUG EFFECT?”; “How HIGH are you?”; “Does the drug have any GOOD…BAD effects?”; “How much do you LIKE the drug?….DESIRE OPIATES right now?”; and “How severe is your OPIOID WITHDRAWAL?” Because THC can affect cognitive function (Sewell et al., 2013), volunteers also completed a time estimation task requiring subjects to estimate the duration of a 5-, 20- and 80-second time interval (outcome is estimated time in seconds; Mintzer et al., 1997) and a 3-minute continuous performance task (CPT), which measures multiple aspects of attention (Halperin et al., 1991; Conners et al., 2003). CPT outcomes included correct responses (hits), missed responses (misses), incorrect responses (false hits), correct misses, and reaction time to both hits and correct misses.
Table 1. Timeline for data collection in each session.
| Outcome measure: | BL | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 135 | 150 | 165 | 180 | 195 | 210 | 225 | 240 | 255 | 270 | 285 | 300 | 315 | 330 | 345 | 360 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual analog scales | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||
| Subject opioid adjectives | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| SOWS | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| Time estimation | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| CPT | x | x | x | x | x | x | x | ||||||||||||||||||
| Observer opioid adjectives | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| OOWS | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||
| Street value | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||
| Drug Identification | x |
BL indicates baseline. Numbers indicate minutes since drug adminstration. SOWS=Short Opiate Withdrawal Scale, CPT=Continuous Performance Task, and OOWS=Objective Opiate Withdrawal Scale. Physiologic (vital signs and pupil diameter) also completed and results reported in Jicha et al., 2015.
Trained research assistants completed a 11-item opioid agonist and 21-item antagonist adjective scale (each item scored from 0-4) and a 13-item objective opiate withdrawal scale (OOWS: each item scored 0 (absent) or 1 (present) (Handelsman et al., 1987). Physiologic measures (heart rate, blood pressure, oxygen saturation, respiratory rate, end-tidal CO2, pupil diameter) were repeatedly collected before and after drug administration; these outcomes are reported elsewhere (Jicha et al., 2015).
2.6 Data analysis
Demographic and drug use characteristics and drug identification results are reported descriptively. Time course analyses of raw data from all other measures were completed employing 2-factor within-subject models (dose [placebo; oxycodone 15, 30mg; dronabinol 5, 10, 20, 30mg] × time [variable]). Peak maximum or minimum values after drug administration were calculated and analyzed with one-factor (dose) models. Dunnett post-hoc comparisons of placebo to active doses were completed when models showed significant results. Because the first two volunteers in the protocol did not tolerate the 40mg dronabinol dose, the protocol was modified to reduce the high dose to 30mg. One subsequent volunteer experienced sustained sinus tachycardia and anxiety/panic after 20mg and did not receive 30mg; thus, n=12 for each dose condition except for 30mg where n=9. Analyses were run with Proc Mixed in SAS 9.3 software (SAS Institute, Inc., Cary, NC). Statistical significance was set at p<0.05. Means (standard errors) are reported unless otherwise indicated.
3. Results
3.1 Participants
Forty-nine volunteers screened for the study. Eighteen met eligibility criteria and were admitted to the inpatient research unit. Six left early for the following reasons: violating protocol rules (n=1), personal/family reasons (n=4), and inability to tolerate the withdrawal during the placebo-training session (n=1). Twelve participants (6 females, all Caucasian) age 31.3 (±1.5) years old with 11.8 (±0.6) years of education completed the study. They reported non-medical use of prescription opioids and heroin on 26.0 (±1.0) of the past 30 days; six reported injecting opioids and for five of these, injecting was the primary route of use. Nine were using both heroin and prescription opioids, three were using only prescription opioids, and one was using only heroin. Eleven participants smoked cigarettes (Fagerstrom: 4.4 [±0.7]). Other substances used infrequently in the past 30 days included marijuana (n=2), alcohol (n=4), benzodiazepines (n=2), and buprenorphine (n=4).
3.2 Visual Analog Scale Outcomes
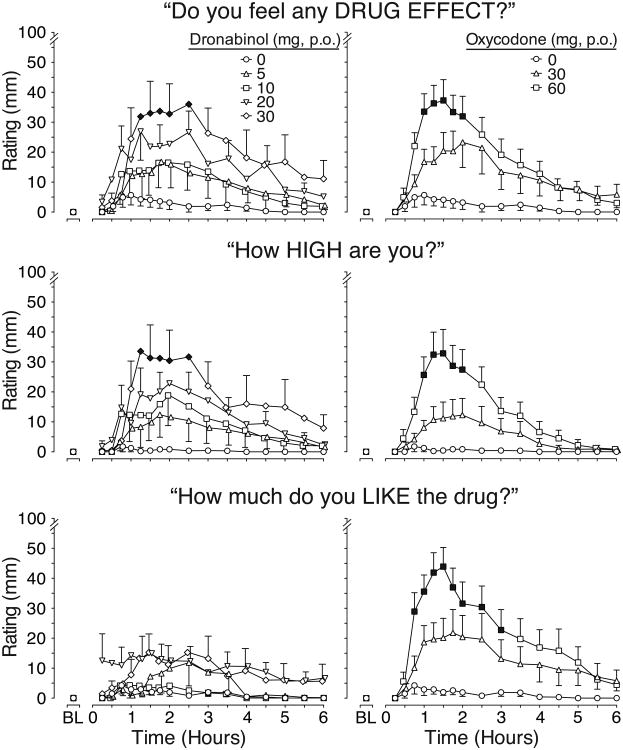
Time course VAS analyses showed significant main effects of time and main effects of drug and/or drug × time interactions except for “bad effects.” However, there were only two VAS items, “drug effect” and “high” (Fig.1, top and center rows), whereby dronabinol, specifically the 30mg dose, produced effects statistically different from placebo. These effects, evident within 75 min after drug administration, were similar in magnitude and duration as those produced by oxycodone 60mg. While dronabinol produced-dose related effects that appear different from placebo on ratings of drug “liking” (Fig.1, bottom row), “good effects,” “sedation,” and “desire for opiates”, post-hocs were not statistically significantly. In contrast, oxycodone 60mg produced effects that were significantly different from placebo for “drug effect,” “high,” “good effects,” and “liking.” There was a main effect of dose on “desire for opiates,” but post-hoc tests were not significant for any active dose condition.
Figure 1.
Time course data for visual analog scale (VAS) ratings of drug effect, high and liking drug.
Time action curves for VAS ratings of drug effect, high and liking are shown as mean data (n=12 for each drug condition except n=9 for dronabinol 30 mg). BL indicates baseline. P.O. indicates oral route. Data from dronabinol dose conditions are shown in the left figures while the placebo and positive oxycodone control condition are shown in the right figures. Standard error bars are shown at alternating time points for dronabinol conditions in order to preserve figure clarity. Black symbols indicate a significant difference from placebo (p<0.05). There was a significant main effect of dose (F(6,63)=2.4, p=0.042) and a dose × time interaction (F(96,1005)=1.3, p=0.045) for drug effect and drug liking (main effect of dose: F(6,63)=5.7, p<0.001; dose × time interaction: F(96,1005)=1.9, p<0.0001). There was a significant dose × time interaction for high (F(96,1005)=1.4, p=0.010).
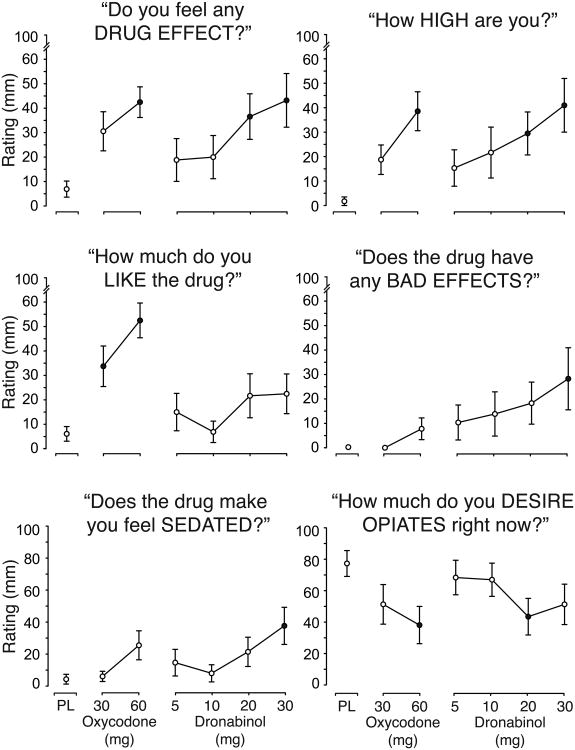
Peak maximum (minimum for opiate desire) values for all VAS items had significant dose effects. Dronabinol 20 and 30mg significantly increased peak maximum ratings of “drug effect” and “high” compared to placebo and to a similar extent as oxycodone (Figure 2, top row). Dronabinol 30mg produced significantly higher ratings than placebo on “good effects” (32.1 ± 7.2 vs. 5.5 ± 3.8; F(6,63)= 7.4; p<0.0001); these were smaller in magnitude compared to oxycodone 30mg (31.8 ± 7.9) and 60mg (48.0 ± 6.0). Dronabinol 30mg also increased “bad effects” (Fig. 2, middle row right panel) and “sedation” ratings (Fig. 2, bottom row left panel), which were not significantly increased with oxycodone. Ratings of “liking” for dronabinol were not greater than for placebo (Fig. 2, middle row left panel) in contrast to oxycodone which significantly elevated ratings (p<.05). Interestingly, dronabinol 20mg significantly decreased “desire for opiates” compared to placebo and similarly to oxycodone 60mg (Fig. 2, bottom row right panel).
Figure 2.
Mean peak VAS ratings of drug effect, high, drug liking, bad drug effects, drug sedation and opiate desire.
Data are shown as peak maximum (minimum for desire opiates) means (n=12 for each drug condition except n=9 for dronabinol 30 mg) with standard error bars. PL indicates placebo. All doses were administered orally. Black symbols indicate a significant difference from placebo (p<0.05). All outcomes had significant main effects of drug condition (Drug effect: F(6,63) =4.2, p=.001; High: F(6,63)= 4.7, p=0.001; Like(6,63): F=7.4, p<0.0001; Bad effects: F(6,63)=2.5, p=0.032; Sedated: F(6,63)=2.6, p=0.028; Desire opiates: F(6,63)=2.8, p=0.017).
3.3 Drug identification and Street Value
Table 2 shows the number of participants identifying each drug class for each drug condition. Placebo was identified correctly by 7 of 12 subjects (58.3%). Both oxycodone conditions were each identified correctly as an opiate agonist by 11 of 12 subjects (91.7%). As dronabinol dose increased, participants increasingly identified it as marijuana and less often as placebo. For example, 5 of 12 (41.2%) participants identified dronabinol 10mg as marijuana and 5 identified it as placebo (41.2%), while 6 of 9 (66.6%) participants identified dronabinol 30mg as marijuana and none identified it as placebo. The maximum street values of dronabinol 5 and 10mg were similar to placebo (Table 3), but were 3-fold higher for dronabinol 20 and 30mg (p>0.05) and 6- to 9-fold higher for oxycodone 30 and 60mg, respectively (p values <0.05).
Table 2. Drug class identifications.
| Drug condition (mg, p.o.) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Placebo | Oxycodone | Dronabinol | |||||
|
|
|
||||||
| 30 | 60 | 5 | 10 | 20 | 30 | ||
| Placebo | 7 | 1 | 0 | 4 | 5 | 1 | 0 |
| Opiate agonist | 1 | 11 | 11 | 2 | 1 | 0 | 1 |
| Opiate antagonist | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Marijuana | 2 | 0 | 1 | 3 | 5 | 8 | 6 |
| Benzodiazepine | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| Barbiturate | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Antipsychotic | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hallucinogen | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Other | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
Values shown are the number of subjects identifying each drug class for each drug condition. “Other” represents a drug effect that the volunteer could not identify as belonging to a specific drug class. For each drug condition, n=12 except n=9 for dronabinol 30 mg. P.O. indicates oral route.
Table 3. Peak values from opioid agonist scale, street value and cognitive measures.
| Drug condition (mg, p.o.) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Placebo | Oxycodone | Dronabinol | ||||||
|
|
|
|||||||
| F (6, 63) | 30 | 60 | 5 | 10 | 20 | 30 | ||
| Maximum values | ||||||||
| Opioid agonist adjective scale | ||||||||
| Participant-rated total score | 6.3 | 4.8 (0.7) | 9.5 (1.2) | 13.5 (2.0) | 6.9 (1.4) | 5.2 (1.2) | 8.8 (1.8) | 9.1 (2.8) |
| Coasting/spaced out | 3.3 | 0.1 (0.1) | 0.2 (0.1) | 0.4 (0.2) | 0.8 (0.4) | 0.8 (0.3) | 1.2 (0.3) | 1.1 (0.4) |
| Dry mouth | 3.3 | 0.7 (0.2) | 1.1 (0.3) | 1.4 (0.3) | 0.9 (0.3) | 1.0 (0.2) | 1.3 (0.3) | 1.8 (0.3) |
| Drunken | 3.5 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.5 (0.3) | 0.8 (0.5) |
| Observer-rated total score | 9.4 | 1.8 (0.5) | 4.8 (0.7) | 7.9 (0.7) | 3.8 (0.9) | 2.8 (0.5) | 3.9 (0.6) | 4.2 (1.1) |
| Coasting | 4.9 | 0.1 (0.1) | 0.2 (0.1) | 0.5 (0.2) | 0.4 (0.2) | 0.3 (0.1) | 1.0 (0.2) | 0.8 (0.2) |
| Good mood | 14.9 | 0.1 (0.1) | 0.9 (0.2) | 1.6 (0.1) | 0.2 (0.1) | 0.4 (0.1) | 0.5 (0.2) | 0.7 (0.2) |
| Street value | 17.3 | 2.5 (1.4) | 15.4 (2.9) | 20.8 (2.9) | 2.3 (1.0) | 1.5 (0.9) | 7.9 (3.3) | 6.1 (2.2) |
| Continuous performance task | ||||||||
| Incorrect responses | 2.9 | 13.5 (2.7) | 15.8 (3.2) | 14.8 (4.1) | 30.3 (11.0) | 17.3 (3.5) | 15.8 (3.2) | 22.1 (6.1) |
| Reaction time for hits | 2.4 | 365.5 (7.7) | 375.8 (11.4) | 366.8 (8.6) | 374.8 (7.5) | 379.2 (9.8) | 381.8 (10.4) | 390.2 (9.8) |
| Reaction time for false hits | 2.5 | 264.5 (15.7) | 279.0 (18.2) | 252.2 (7.7) | 226.7 (19.5) | 220.3 (13.9) | 256.9 (17.3) | 284.8 (27.0) |
| Minimum values | ||||||||
| Time estimation - 80 seconds | 3.5 | 48.7 (6.3) | 50.4 (6.5) | 45.1 (7.5) | 41.6 (6.7) | 43.2 (7.0) | 38.5 (6.9) | 21.5 (6.7) |
| Continuous performance task | ||||||||
| Correct misses | 2.8 | 260.3 (2.5) | 256.3 (2.8) | 258.1 (3.5) | 243.0 (10.3) | 257.0 (3.3) | 256.0 (3.6) | 249.4 (5.8) |
Bolded F values indicate p<0.05. Bold mean (SE) values indicate significant (p<0.05) post-hoc compared to placebo. Only individual adjective items with significant post-hoc results are shown. Only cognitive test outcomes with significant dose effects are shown. For each drug condition, n=12 except n=9 for dronabinol 30mg. P.O. indicates oral route.
3.4 Cognitive measures
All drug conditions, including placebo, underestimated the 80-sec time interval in time course analyses; this was most marked for dronabinol 20 and 30mg doses (dose: F(6, 63) =2.7; p=0.021). Analysis of minimum time estimates (Table 3) show that dronabinol 30mg produced estimations of the 80-sec interval as only 21.5 ± 6.7 compared to placebo estimations at 48.7 ± 6.3 sec (p=0.002). Oxycodone condition estimates were not different from placebo (p>0.05). There were no significant effects on analyses from the other time estimation intervals.
On the CPT, there were no significant main effects of dose, but there were significant dose × time interactions for the number of correct misses (F(36, 375)=1.8; p=0.005) and incorrect responses (F(36, 375)=1.6; p=0.023) that were mostly due to dronabinol. For instance, Table 3 shows that, for analysis of minimum values, dronabinol 5mg produced approximately 17 fewer correct misses than placebo (p= 0.003) and on analysis of maximum values, this dose produced 17 more incorrect responses than placebo (p=0.004). While the maximum number of correct responses and reaction time to false hits were not different between active and placebo doses, dronabinol 30mg produced maximum reaction hit times that were significantly longer than placebo.
3.5 Opioid agonist adjective scale
Time course analyses of participant and observer-rated opioid agonist adjective scales showed significant dose effects (participant: F(6,63)=3.3; p=0.007, observer: F(6,63)=8.5; p<0.0001). On the participant-rated scale, only oxycodone 60mg (7.3 ± 0.4) produced a higher total score than placebo (3.5 ± 0.2; p=0.008) across time. Observers reported higher agonist scores across time after dronabinol 20mg (2.4 ± 0.1) and 30mg (2.0 ± 0.2) and oxycodone 30mg (2.1 ± 0.1) and 60mg (3.4 ± 0.2) compared to placebo (0.8 ± 0.09; all post-hoc p values <0.05).
Results from maximum value analyses are shown in Table 3 along with individual scale items that had significant post-hoc results for dronabinol. While both dronabinol and oxycodone produced higher total scores on both the participant- and observer-rated adjective scales, only oxycodone produced results that were statistically different from placebo. On individual items, dronabinol 20 and 30mg produced significantly higher ratings than placebo on participant-rated coasting/spaced out, dry mouth (30mg only) and drunken (30mg only) and observer-rated coasting and good mood (30mg only); however, the intensity of these effects was modest with mean peak scores always less than 2.0.
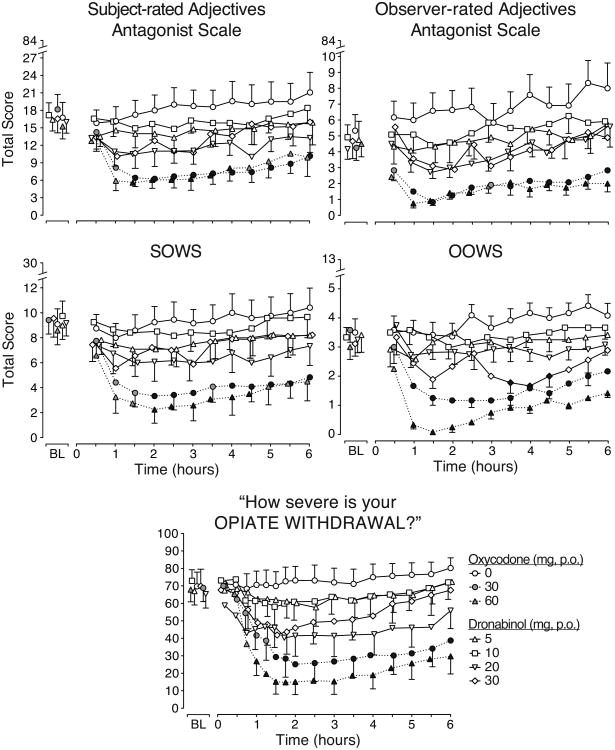
3.6 Opioid withdrawal measures
Figure 3 displays the time course of the total scores from the five withdrawal measures employed, all of which had significant main effects of dose and time and demonstrated that opioid withdrawal was present prior to drug administration. Oxycodone 30 and 60mg diminished withdrawal on all five measures. Dronabinol 5 and 10mg produced results most similar to placebo, while the 20 and 30mg doses produced some evidence of withdrawal suppression (i.e., on the observer-rated adjectives antagonist scale [upper row, right panel] and OOWS [middle row, right panel)]). On the former, dronabinol 20 and 30mg produced significantly lower total scores (4.0 ± 0.23 and 4.2 ± 0.24, respectively) than placebo (6.7 ± 0.33) across all time points, and on the latter (middle row, right panel), dronabinol 30mg significantly decreased withdrawal compared to placebo from 3.5 to 4.5 hours post-drug administration.
Figure 3.
Time course data for opioid withdrawal outcome measures.
Time action curves for opioid withdrawal outcomes are shown as mean data (n=12 for each drug condition except n=9 for dronabinol 30 mg). BL indicates baseline. P.O. indicates oral route. Standard error bars are shown at alternating time points in one direction for active drug conditions in order to preserve figure clarity. Black symbols indicate a significant difference from placebo (p<0.05). All outcomes had significant main effects of drug condition (Subject-rated adjectives: F(6,63)=4.5, p=0.001; Observer-rated adjectives: F(6,63)=8.3, p<0.0001; SOWS: F(6,63)=4.4, p=0.001; OOWS: F(6,63)=16.5, p<0.0001; VAS severity of opiate withdrawal: F(6,63)=6.3, p<0.0001). There were also significant dose × time interactions on the OOWS (F(72,753)=1.7, p<0.0001) and VAS severity of opiate withdrawal: F(96, 1005)=1.3, p=0.048).
Table 4 displays the results from analyses of trough withdrawal scores, which are consistent with the time course results. Oxycodone clearly suppresses withdrawal, dronabinol 5 and 10mg are most similar to placebo, and dronabinol 20 and 30mg produce modest opioid withdrawal suppression. On participant-rated scales, dronabinol significantly reduced ratings of backache (30mg), feeling sick (30mg), muscular tension (20mg) and runny eyes (5 and 30mg), while the observer-rated scale items show reductions for dronabinol only on yawning (20 and 30mg), watery eyes (5, 20 and 30mg), and rhinorrhea (10 and 30mg). There were no other significant effects of dronabinol on the remaining individual withdrawal items. To determine if dronabinol was exacerbating withdrawal, analyses of maximum increases on withdrawal measure items were performed. These showed significant results only for the 30mg dronabinol dose whereby there were increased ratings of “heart pounding” on the SOWS and “nervous” on the observer-rated antagonist adjective scale, but the mean maximum values were modest and less than 1.2 for both items (data not shown).
Table 4. Maximum withdrawal suppression values.
| Drug condition (mg, p.o.) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Placebo | Oxycodone | Dronabinol | ||||||
|
|
|
|||||||
| F (6, 63) | 30 | 60 | 5 | 10 | 20 | 30 | ||
| Participant- rated measures: | ||||||||
| Opioid antagonist adjectives total | 8.1 | 13.1 (2.2) | 4.7 (1.7) | 3.8 (1.3) | 9.5 (2.1) | 12.3 (2.3) | 7.5 (2.0) | 6.8 (1.9) |
| Backache | 4.4 | 1.1 (0.3) | 0.5 (0.2) | 0.4 (0.2) | 1.1 (0.3) | 1.3 (0.4) | 0.7 (0.2) | 0.4 (0.2) |
| SOWS total | 10.5 | 7.3 (1.2) | 2.8 (1.2) | 2.0 (1.1) | 5.3 (1.3) | 6.8 (1.5) | 4.8 (1.5) | 3.8 (1.7) |
| Feeling sick | 4.4 | 1.1 (0.3) | 0.3 (0.2) | 0.2 (0.2) | 0.8 (0.3) | 0.8 (0.3) | 0.5 (0.2) | 0.3 (0.2) |
| Muscular tension | 4.9 | 0.8 (0.3) | 0.3 (0.2) | 0.2 (0.2) | 0.7 (0.2) | 0.8 (0.3) | 0.3 (0.2) | 0.4 (0.2) |
| Runny eyes | 3.1 | 0.7 (0.3) | 0.1 (0.1) | 0.1 (0.1) | 0.2 (0.1) | 0.3 (0.1) | 0.3 (0.1) | 0.1 (0.1) |
| VAS opioid withdrawal severity | 10.2 | 64.4 (8.6) | 21.6 (10.2) | 11.1 (6.1) | 52.3 (10.3) | 55.9 (10.2) | 36.3 (11.0) | 36.6 (11.8) |
| Observer-rated measures: | ||||||||
| Opioid antagonist adjectives total | 6.9 | 4.4 (1.0) | 0.5 (0.2) | 0.3 (0.1) | 2.2 (0.8) | 3.2 (0.9) | 2.0 (0.6) | 1.3 (0.6) |
| Yawning | 4.4 | 0.6 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.3 (0.1) | 0.3 (0.1) | 0.2 (0.1) | 0.1 (0.1) |
| Watery eyes | 4.0 | 0.3 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.3 (0.1) | 0.0 (0.0) | 0.0 (0.0) |
| OOWS total | 12.7 | 2.8 (0.4) | 0.9 (0.2) | 0.0 (0.0) | 1.8 (0.4) | 2.4 (0.4) | 2.0 (0.2) | 1.1 (0.2) |
| Rhinorhea | 3.2 | 0.4 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.2 (0.1) | 0.1 (0.1) | 0.3 (0.1) | 0.0 (0.0) |
Bolded F values indicate p<0.05. Bold mean (SE) values indicate significant (p<0.05) post-hoc compared to placebo. Only individual items with significant post-hoc result for dronabinol are shown. P.O. indicates oral route.
4. Discussion
This inpatient human laboratory study evaluated the withdrawal suppression efficacy of a range of acute oral dronabinol doses among opioid dependent adults experiencing opioid withdrawal. Single acute doses of dronabinol were compared to placebo and oral oxycodone (the positive control) on opioid withdrawal measures and physiologic, cognitive and other subjective outcomes, including measures of abuse liability. Oxycodone reliably attenuated withdrawal, increased opioid agonist adjective scale scores, was consistently identified as an opioid, rated with a higher street value than placebo, and produced clear signals of abuse liability (Fig 1). Dronabinol produced more variable results with only the 20 and 30mg doses showing modest evidence of opioid withdrawal suppression and abuse liability.
The higher dronabinol doses (20 and 30mg) attenuated some signs and symptoms of opioid withdrawal, although suppression was for a limited duration of time and generally incomplete. For instance, on the OOWS, dronabinol 30mg suppressed withdrawal from 3.5 to 4.5 hours after dosing (Fig 3), and on peak withdrawal suppression analyses (Table 4), 20 and 30mg of dronabinol produced total participant-rated withdrawal scores that were 34-48% lower than placebo, while for oxycodone, these scores were 62-70% lower than placebo showing that the effects of dronabinol are less robust than a full opioid agonist. The two lower dronabinol doses (5 and 10mg) showed scant evidence of withdrawal suppression and subjective drug effects and were more placebo-like.
Dronabinol reduced several specific withdrawal signs and symptoms but also produced potentially concerning stimulatory effects (e.g., sinus tachycardia). With regard to withdrawal, dronabinol 30mg specifically reduced scores on backache, feeling sick, lacrimation (20mg also), rhinorrhea, yawning (20mg also), and the 20mg, but not 30mg, dose suppressed muscular tension (Table 4). However, these potentially therapeutic dronabinol doses also increased heart rate. Dronabinol 20 and 30mg doses increased average maximum heart rates to 107.6 and 112.6 bpm, respectively (Jicha et al., 2015). This was also detected and reported by subjects as “heart racing” suggesting that other dosing strategies, including drug combinations (e.g., with clonidine), may be needed in order to capitalize on the beneficial withdrawal effects while minimizing adverse effects on heart rate. The concomitant use of clonidine may be why dronabinol was well tolerated in the recent clinical trial evaluating chronic dosing of dronabinol (30mg daily; Bisaga et al., 2015).
The higher doses of dronabinol also produced psychoactive effects. There were dose-related increases on subject ratings of high, sedation, coasting, and drunken, as well as bad effects. Subjects also demonstrated impairment with time estimation and had slower reaction times for identifying correct responses on the CPT after dronabinol 30mg (Table 3). Notably, there was no significant drug liking, and street values were not statistically different from placebo. While there are no human laboratory studies evaluating the abuse liability and reinforcing effects of dronabinol in opioid users, published work evaluating oral THC abuse liability among persons who smoke marijuana indicate it has less reinforcing effects and abuse liability compared to smoked marijuana. For instance, of 23 subjects who smoked marijuana and were offered the opportunity to work (pressing a response key 3600 times) for smoked marijuana, oral THC (17.5mg) or nabilone (a CB1 agonist), 18 worked for marijuana, two worked for oral THC and none worked for nabilone (Mendelson and Mello, 1984). This is also consistent with post-marketing surveillance indicating limited diversion and abuse since its initial approval in 1985 (e.g., emergency room visits; Calhoun et al., 1998), which, in part, supported the DEA rescheduling dronabinol from Schedule II to III in 1999.
There also may be alternative pharmacologic endocannabinoid targets to consider for opioid dependence treatment. There is a growing body of pre-clinical research demonstrating that inhibition of endocannibinoid degradation enzymes, such as fatty acid amide hydrolase (FAAH) and/or monacylglyerol lipase (MAGL), can elevate endogenous cannabinoids to promote CB receptor activation potentially without some of the undesirable effects seen with exogenous CB agonists (Ahn et al., 2008). For instance, primates trained to self-administer exogenous ∆9-THC do not self-administer the FAAH inhibitor (URB597), although this compound increases the endogenous cannabinoid anandamide (Justinova et al., 2008). The FAAH inhibitor (PF-3845) suppressed some signs of opioid withdrawal when administered prior to naloxone in morphine-dependent mice and attenuated naloxone-induced contractions in ilea treated with morphine (Ramesh et al., 2011). When a FAAH inhibitor (PF-3845) and a MAGL inhibitor (JZL-184) were combined, all acute opioid withdrawal signs were reduced (Ramesh et al., 2013). While these are promising preclinical results, a recent Phase I first-in-man study conducted in Rennes, France testing the FAAH inhibitor (BIA-10-2474), resulted in six volunteers being hospitalized and one death (French National Agency for Medicines and Health Products [FNAMHP] 2016). Investigation into these unexpected serious adverse events is ongoing by FDA and FNAMHP, but early reports suggest this agent may have effects on other non-cannabinoid targets at high doses. Thus, caution is needed, and there is more to understand about these novel agents (FNAMHP 2016). However, it is worth noting that BIA-10-2474 was well tolerated at lower doses and other investigational FAAH inhibitors have been tested in Phase I, II and III studies without significant adverse consequences (Huggins et al., 2012; Li et al., 2012; Pawsey et al., 2016). Lastly, this study employed a novel oral oxycodone opioid maintenance procedure with subsequent double-blind placebo-dose substitutions, which reliably was well tolerated, produced opioid withdrawal (Fig. 3) and showed sensitivity for detecting withdrawal suppression. It was adapted from the 24-hour substitution procedure employed at the US Public Health Service Addictions Research Center in Lexington, Kentucky, which employed subcutaneous morphine typically four times/daily and then administered double-blind doses of experimental drug to test for their physical dependence potential (Jasinski et al., 1971, 1975). Later work substituted placebo doses for morphine to produce withdrawal, allowing for evaluation of withdrawal suppression (Lofwall et al., 2007). The oral oxycodone maintenance procedure here represents a further adaptation of these methods with an added advantage of avoiding the need for multiple daily injections with morphine.
The study has limitations. Nicotine withdrawal was not systematically assessed, which has overlapping symptoms with opioid withdrawal. It is unlikely that withdrawal from nicotine, however, contributed to or accounted for the opioid withdrawal reported herein because volunteers had low-moderate severity nicotine dependence (mean Fagerstrom Test for Nicotine Dependence <5) and were allowed to smoke before experimental session to avoid acute nicotine withdrawal. The study also cannot address the effects of chronic dronabinol dosing or dronabinol effects on the reinforcing efficacy of opioids. In summary, higher dronabinol doses (20 and 30mg) demonstrated modest opioid withdrawal suppression efficacy and abuse liability but produced significant increases in heart rate. These results do not support the further investigation of acute dronabinol as a monotherapy in the treatment of opioid withdrawal. However, there may be other CB1 agents or medications with different mechanisms of action on the endocannabinoid system that may be useful for treating the various neuropsychological dysfunctions associated with opioid dependence.
Highlights.
Dronabinol, a partial CB1 agonist, was evaluated among adults in opioid withdrawal.
Dronabinol 20 and 30 mg doses produced modest evidence of withdrawal suppression.
Higher acute oral doses also produced sinus tachycardia.
Dronabinol is not a likely mono-therapy candidate for opioid withdrawal treatment.
However, CB1 receptor activation may be a reasonable strategy to pursue.
Acknowledgments
The authors thank the UK Investigation Drug Service, nursing and staff on inpatient Center for Clinical and Translational Science research unit, and UK Center on Drug and Alcohol research staff.
Role of Funding Source: Funding sources include NIDA DA033932 (PI: Walsh) & NCATS UL TR000117. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Author Disclosures: Conflict of Interest: Dr. Lofwall and Dr. Walsh have received honoraria from PCM Scientific for giving educational talks on opioid dependence and salary support from Braeburn Pharmaceuticals for conducting clinical research at UK. Dr. Lofwall has consulted for Braeburn and CVS Caremark. Mr. Nuzzo was a statistical consultant and project coordinator for the NIDA CTN Clinical Consulting Center and Johns Hopkins Behavioral Pharmacology Research Unit. Dr. Walsh has consulted for Sun Pharma, Camurus, World Meds, Durect, Novartis, Pfizer, Astra Zeneca, Cerecor, and Braeburn Pharmaceuticals. Dr. Babalonis has no conflicts of interest to report.
Contributors: Dr. Walsh designed the study, wrote the protocol, and provided primary study oversight. Dr. Babalonis assisted with recruitment and enrollment as well as study management. Dr. Lofwall helped with study design, provided medical oversight, and assisted in interpretation of data and writing of the manuscript. Mr. Nuzzo arranged randomization, managed data, and completed all analyses. Dr. Elayi assisted in medical oversight. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN. Effect of some cannabinoids on naloxone-precipitated abstinence in morphine-dependent mice. Psychopharmacology (Berl) 1976;49:267–270. doi: 10.1007/BF00426828. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, Raby WN, Levin FR, Carpenter KM, Mariani JJ, Nunes EV. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015;154:38–45. doi: 10.1016/j.drugalcdep.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol) J Psychoactive Drugs. 1998;30:187–196. doi: 10.1080/02791072.1998.10399689. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Harney J. Shifting patterns of prescription opioid and heroin abuse in the United States. N Engl J Med. 2015;373:1789–1790. doi: 10.1056/NEJMc1505541. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- French National Agency for Medicines and Health Products Safety. [accessed March 9, 2016]; http://ansm.sante.fr/content/download/86439/1089765/version/1/file/CR_CSST-FAAH_15-02-2016_Version-Anglaise.pdf.
- Gamage TF, Ignatowska-Jankowska BM, Muldoon PP, Cravatt BF, Damaj MI, Lichtman AH. Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice. Drug Alcohol Depend. 2015;146:7–16. doi: 10.1016/j.drugalcdep.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M. The development of a Short Opiate Withdrawal Scale (SOWS) Addict Behav. 1990;15:487–490. doi: 10.1016/0306-4603(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Halperin J, Sharma V, Greenblatt E, Schwartz S. Assessment of the continuous performance test: reliability and validity in a nonreferred sample. Psychol Assess. 1991;3:603–608. [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Hine B, Friedman E, Torrelio M, Gershon S. Morphine-dependent rats: blockade of precipitated abstinence by tetrahydrocannabinol. Science. 1975a;187:443–445. doi: 10.1126/science.1167428. [DOI] [PubMed] [Google Scholar]
- Hine B, Friedman E, Torrelio M, Gershon S. Tetrahydrocannabinol-attenuated abstinence and induced rotation in morphine-dependent rats: possible involvement of dopamine. Neuropharmacology. 1975b;14:607–610. doi: 10.1016/0028-3908(75)90128-8. [DOI] [PubMed] [Google Scholar]
- Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Griffith JD, Carr CB. Etorphine in man. I. Subjective effects and suppression of morphine abstinence. Clin Pharmacol Ther. 1975;17:267–272. doi: 10.1002/cpt1975173267. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick Jl, Gorodetzky C, Cone El, Kay D. Progress Report From The Clinical Pharmacology Section Of the NIDA Addiction research Center, 39th Annual Meeting, The Committee on Problems of Drug Dependence. National Academy of Sciences; Washington, D.C: 1977. pp. 133–168. [Google Scholar]
- Jasinski DR, Martin WR, Hoeldtke R. Studies of the dependence-producing properties of GPA-1657, profadol, and propiram in man. Clin Pharmacol Ther. 1971;12:613–649. doi: 10.1002/cpt1971124613. [DOI] [PubMed] [Google Scholar]
- Jicha CJ, Lofwall MR, Nuzzo PA, Babalonis S, Elayi SC, Walsh SL. Safety of oral dronabinol during opioid withdrawal in humans. Drug Alcohol Depend. 2015;157:179–183. doi: 10.1016/j.drugalcdep.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GL, Winter H, Arends R, Jay GW, Le V, Young T, Huggins JP. Assessment of the pharmacology and tolerability of PF-04457845, an irreversible inhibitor of fatty acid amide hydrolase-1, in healthy subjects. Br J Clin Pharmacol. 2012;73:706–716. doi: 10.1111/j.1365-2125.2011.04137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–1014. [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl) 2007;194:381–393. doi: 10.1007/s00213-007-0847-3. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology (Berl) 1984;83:351–356. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Pawsey S, Wood M, Browne H, Donaldson K, Christie M, Warrington S. Safety, tolerability and pharmacokinetics of FAAH inhibitor V158866: a double-blind, randomised, placebo-controlled phase i study in healthy volunteers. Drugs R D. 2016:1–11. doi: 10.1007/s40268-016-0127-y. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, Shea-Donohue T, Cravatt BF, Lichtman AH. Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology. 2013;38:1039–1049. doi: 10.1038/npp.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339:173–185. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell R, Schnakenberg A, Elander J, Radhakrishnan R, Williams A, Skosnik PD, Pittman B, Ranganathan M, D'Souza DC. Acute effects of THC on time perception in frequent and infrequent cannabis users. Psychopharmacology (Berl) 2013;226:401–413. doi: 10.1007/s00213-012-2915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) 2014 National Survey on Drug Use and Health: Detailed Tables. SAMHSA; Rockville, MD: 2015. [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]