Introduction
The delivery of macromolecules via the gastrointestinal (GI) tract is one the most exciting, yet challenging frontiers in drug delivery. Over 90 y ago, the same year Banting and Macleod were awarded the Nobel Prize for the discovery of insulin—founded on the classic experiments by Banting and Best at the University of Toronto—Geoffrey Harrison at King's College London conducted the first experiments with an orally delivered insulin in a human patient.1 It is worth noting that the first human patient had been dosed with injectable insulin only 1-year prior. Harrison's experiments represent one of the first documented cases of an orally delivered macromolecule with confirmation of systemic absorption supported by a biomarker measurement, in this case, the onset of hypoglycemia. This seminal experiment highlighted both the promise but also challenge in reliable oral delivery of a macromolecule. Indeed, only 1 out of 4 trials demonstrated a significant decrement in glucose.1
It is clear to the authors and the community that the oral route is the preferred route for drug delivery. This manifests in significant differential usage patterns between injectable and oral formulations of medications with similar mechanisms of action. In type 2 diabetes, for example, it is recognized that initiation of an insulin regimen is delayed by approximately 8 y in those patients requiring escalation of therapy due to patient aversion for injectables. This manifests in poor glycemic control and progression of microvascular complications.1,2 From a market perspective, if we examine the current sales for two first-in-class drugs with the same indication and in the same molecular pathway, the GLP-1 analogs (injectable) and the DPP-4 antagonists (oral), we find that sitagliptin (DPP-4 antagonist) has sales in excess of $2B/year over its injectable counterpart, exenatide (GLP-1 analog) in spite of exenatide being recognized as a superior drug in part due to its weight loss benefit. These strong market pressures are likely rooted in the patient and physician preference for orally administered medication and has stimulated significant efforts in establishing systems to enable delivery of macromolecules across the GI tract. The scientific community has pursued the oral delivery of a broad set of macromolecules applying both formulation-based solutions as well as physical modes of enhancement of GI uptake to the challenge. Here we will briefly review ongoing work in the areas of two physical modes of delivery, specifically microneedles and ultrasound.
The GI tract has evolved as a protective barrier to the organism as well as the site of nutrient digestion to enable absorption. These two functions pose significant challenges to the delivery of molecules, which are made of similar constituents as nutrients requiring breakdown and digestion (e.g. nucleic acids and amino acids). Moreover, the environment in the GI tract faces pH fluctuations between 1–7, fluctuating bacterial loads, degradative enzymes and the variability of dietary intake. Formulation-based solutions have been applied with several products in various phases of clinical development. To design a platform that enables more facile adoption of delivery via the GI tract, several groups have begun actively investigating physical modes of delivery. From our perspective, these are largely founded on the recognition of two essential clinical observations surrounding the incredible tolerance of the GI tract to potential injury from a variety of insults including the ingestion of sharp objects as well the near immediate systemic bioavailability of drugs that are administered for a local effect in the GI tract, specifically epinephrine (adrenaline).
Microneedles
With the above in mind, microneedles have recently been hypothesized to enable oral delivery of drugs and biologics. As a result, there has been an explosion of interest in this area, as evident by the range of issued and pending patent applications in this space (Table 1).
Table 1.
Selected patent applications describing the use of microneedles in the GI tract.
| US. Application Number | Filing Date | Title | Assignee |
|---|---|---|---|
| US13063236 | September 14, 2009 | Painless injector | Medimop Medical Projects Ltd. |
| US12978233 | December 23, 2010 | Swallowable drug delivery device and methods of drug delivery | Rani Therapeutics, LLC |
| US13728300 | December 27, 2012 | Microneedle devices and uses thereof | Massachusetts Institute of Technology, General Hospital Corp. |
| US14620827 | February 12, 2015 | Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device | Rani Therapeutics, LLC |
Microneedles have been studied extensively for transdermal drug delivery.3 There, they have been utilized to painlessly overcome the barrier posed by the stratum corneum, the outermost layer, without eliciting pain receptors in the deeper layers of the skin. The GI tract, unlike the skin, lacks a stratum corneum-like barrier and, instead, is coated by mucus with the GI epithelium immediately accessible underneath.
The use of microneedles for oral delivery is motivated by: 1) the potential for a platform capable of delivering orally a wide range of therapeutics with minimal requirement for formulation, 2) the insensate nature of the GI tract enabling painless microinjection, 3) the capacity of the GI tract to tolerate the passage of sharp objects and mucosal disruption as supported by the low rate or lack of complications associated with small sharp body ingestion and polypectomy (Fig. 1).
Figure 1.
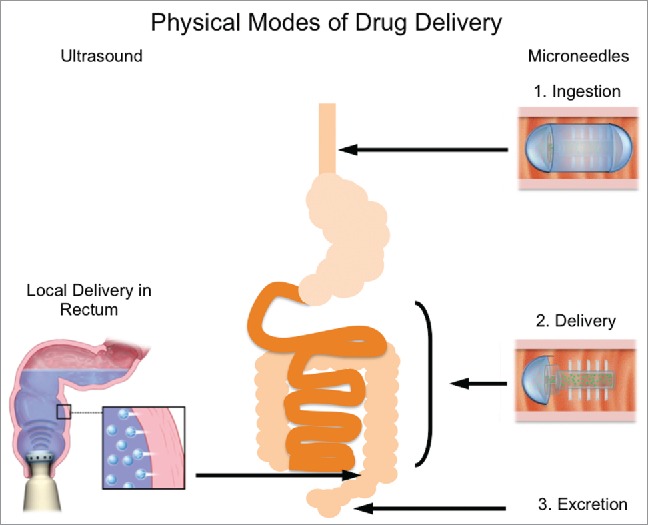
Physical modes of GI-based drug delivery. Ultrasound (left) is a technology recently explored for its ability to enable localized drug delivery in the GI tract. The mechanism of action is transient cavitation, whereby small voids in solution collapse as a result of large pressure gradients, sending microjets of medicated solution into the tissue (inset). This technology could be used at home by patients for more convenient administration. Microneedles (right) have also been explored. Initially, a capsule would be coated to aid ingestion. When the capsule reaches the appropriate location (depending on the drug to be delivered), the coating dissolves, revealing the needles, which can then inject the medication directly into the tissue.
In vivo proof of concept studies surrounding the use of needle-based systems in the GI tract of pigs have recently been carried out.4 The kinetics of delivery of a model biologic, insulin, were explored as a result of microinjections in various locations in the GI tract and compared to traditional subcutaneous administration. These experiments were performed by microinjection under direct observation through a colonoscope. Microinjection administration was tested in the stomach, duodenum, and the colon. The hypoglycemic response was monitored through serial blood sampling from the femoral vein. Onset time to observe a hypoglycemic effect was significantly reduced when insulin was administered via microinjection in the stomach and duodenum as compared to the skin. Specifically, onset time was reduced by almost 20 minutes compared to subcutaneous injection.4 There is also considerable commercial interest in this space. Rani Therapeutics LLC is a commercial entity developing an ingestible microneedle pill. This device relies on actuators to drive drug-loaded needles out of the body of the pill and into the tissue. Recently released data has demonstrated the oral delivery of insulin as well as adalimumab. In the case of the latter, their data demonstrates significantly enhanced kinetics of serum-level detection compared to subcutaneous administration.
Safety of such a technology is paramount for its successful adoption and there are also preliminary results demonstrating the tolerability of passage of such a device. The safety and passage time of a model device has also been explored in pigs. A model device 2 cm in length and 1 cm in diameter with 25G stainless steel needles protruding radially was endoscopically deployed in the stomach of Yorkshire pigs and its progress monitored radiographically. Passage time was found to range from 7 to 56 d More importantly, no adverse events were noted while the pill was inside the animal. After passage, gross and histological examination of the tissue showed no evidence of damage. The range of passage times noted was consistent with other studies examining passage time of objects in pigs and is hypothesized to be due to their quadrupedal nature.4,5
While this is the first study to the best of our knowledge describing an ingestible device, microneedle patches in the oral cavity have been explored for vaccinations. Indeed, recent reports have demonstrated the ability of hollow microneedles to deliver liposomes loaded with model antigens, including hepatitis B antigen and bovine serum albumin, into the oral mucosa and induce a robust immunological response.6,7 Other groups have also explored the use of coated microneedles for oral vaccination.8 Oral administration of these patches coated with ovalbumin induced robust IgA responses in saliva. Further, microneedle administration in the oral cavity of model HIV antigens elicited comparable responses to antigen administered intramuscularly.8
Deployment of microneedles in the GI tract and mucosal surfaces is a burgeoning field with the potential for significant clinical impact. Indeed, there remain many aspects to investigate before this technology may be translated to the clinic. Interestingly, needle geometry may play an important role in determining residence time inside the GI tract and might enable extended release, reducing the required dosing frequency of many treatments, for example.9 Further pre-clinical development in pigs as well as other large animal models will be valuable in guiding the safety and feasibility of these approaches. Also the exploration of novel formulations including polymer-based dosage systems including sugars to maximize the stability of the active pharmaceutical ingredients and maintain the desired mechanical properties will be essential in ensuring safety and efficacy.
Ultrasound
There has been considerable interest for over half a century in using ultrasound to enable transdermal drug delivery.10 Initial work focused on the use of high frequency (≥1 MHz) ultrasound to enhance drug delivery through the skin.11 Only in the late 1980s did investigations begin to focus on low frequency (<100 kHz) ultrasound.12,13 This transition came about through the discovery that transient cavitation plays a significant role in disrupting the skin barrier and could facilitate the delivery of biologics, such as insulin (∼5.8kDa), interferon γ (∼17kDa) and erythropoietin (∼48kDa).14 Recent developments have focused on the synergistic use of simultaneous low and high frequency ultrasound to maximize permeabilization while reducing the required treatment time and device development and miniaturization.15-17 These technologies are unique in that they represent the direct application of ultrasound to a tissue surface through a coupling agent for the purposes of modulating the permeability of that tissue. This is in contrast to high-intensity focused ultrasound (HIFU), which is another technology used for thermal ablation or drug release at a specific site within the body utilizing focused ultrasound fields.18 HIFU typically employs systemically-administered microbubbles either with or without a therapeutic payload to achieve either local release of the therapeutic only at the site of the HIFU or enhanced localized heating, respectively.19 The methods described in this review are distinct to the method of HIFU that has been previously reported.
The power of the direct application of ultrasound is in its safe modulation of tissue barriers to allow for drugs to be delivered without the need for bulky equipment and focusing of ultrasound fields. Harnessing the incredible capacity of tunable transient cavitation for drug delivery was recently extended to its application in the GI tract (Fig. 1).20 This study explored the fundamental use of short, one-minute ultrasound treatments in ex vivo porcine GI tissue and in vivo in both small and large animal models. There are many GI-based diseases that could greatly benefit from ultra-rapid drug delivery. Inflammatory bowel disease is one such disease where the standard of care is the administration of medicated enemas containing anti-inflammatory medication.21 However, the disease results in diarrhea and the need for frequent bowel movements, making the extended retention of a medicated enema almost impossible.
In the study, low-frequency ultrasound was found to increase absorption of model therapeutics 2–10-fold in ex vivo tissue utilizing the Franz diffusion cell, depending on location within the GI tract. Specifically, fluorescently labeled dextrans between 3–70 kDa in size could be efficiently delivered into porcine tissue as a result of a one-minute treatment. No fluorescent signal was visible in the absence of ultrasound.20 The capability of delivering macromolecules in such a short exposure time is unexpected and quite remarkable.
Further, a model device was tested in vivo in Yorkshire pigs. A model device was inserted superficially into the rectum and a medicated enema instilled. The same one-minute treatment was found to be safe and well tolerated and the generation of transient cavitation was confirmed. The ability for this treatment to deliver unencapsulated biologics was also demonstrated. Insulin was chosen as a model biologic as it is recognized to not have any significant bioavailability in the GI tract. Indeed, a robust hypoglycemic effect was achieved when insulin was co-administered as an enema in the colon with ultrasound. As expected, there was no effect on the animals' blood-glucose when ultrasound was not utilized.20 Finally, the preclinical efficacy of this technology was tested in a chemically-induced murine model of colitis. Simultaneous administration of rectal ultrasound with mesalamine, a common drug given topically for the treatment of ulcerative colitis, demonstrated superior efficacy compared to administration of mesalamine alone.20 Safety and tolerability was also confirmed in healthy animals. Cytokine profiling from colonic tissue samples demonstrated no increase in pro-inflammatory cytokine levels and minimal histological disruption was noted. These are encouraging results suggesting the potential safety and efficacy of this technology even in the setting of inflamed mucosa. Further safety studies evaluating the effects of US on mucosal integrity both in inflamed and healthy mucosa will be required as part of the pre-clinical development toward human application.
The delivery of a wide-range of molecules and compounds with such a short treatment time is exciting and is indicative of the potential power of this new technology. Indeed, ultrasound-mediated gastrointestinal delivery could have far-reaching impact from the delivery of small molecules and biologics, to the potential delivery of vaccines to modulate the mucosal response.22 Even more exciting is the prospect of delivering DNA and RNA therapeutics, the delivery of which requires overcoming several biologic barriers.23
Physical modes of delivery have the potential to rapidly expand the repertoire of drugs that can be delivered via the GI tract and greatly improve treatment efficacy of currently available treatments.20 As the hypodermic needle transformed drug delivery by expanding the range of therapeutics that could be delivered to the organism, we view the development of delivery technologies specifically for macromolecules via the GI tract as one of the next transformative sets of technologies.
Disclosure of potential conflicts of interest
The authors declare U.S. Provisional Patent applications no. 62/144,842 filed on 8 April 2015 and 13/728,300 filed on December 27, 2012 covering ultrasound and microneedle applications in the gastrointestinal tract respectively.
Funding
This work was funded by the NIH (grant nos. EB-00351, EB-000244, and CA014051) and the Max Planck Research Award, Award Ltr Dtd. 2/11/08, Alexander von Humboldt-Stiftung Foundation (to R.L.). G.T. was supported in part by NIH grant T32-DK007191-38-S1.
References
- [1].Harrison GA. Insulin in alcoholic solution by the mouth,” Br Medical J 1923; 2(3286), pp. 1204-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract 2007; 57(539), pp. 455-460; PMID:17550670 [PMC free article] [PubMed] [Google Scholar]
- [3].Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin. Drug Deliv 2014; 11(3) pp:393-407; PMID:24392787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Traverso G, Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Polat BE, Anderson DG, Blankschtein D, Langer R. Microneedles for Drug Delivery via the Gastrointestinal Tract. J Pharmaceutical Sci 2015; 104(2), pp:362-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snoeck V, Huyghebaert N, Cox E, Vermeire A, Saunders J, Remon JP, Verschooten F, Goddeeris BM. Gastrointestinal transit time of nondisintegrating radio-opaque pellets in suckling and recently weaned piglets. J Control Release 2004; 94(1), pp:143-153; PMID:14684278 [DOI] [PubMed] [Google Scholar]
- [6].Wang T, Zhen Y, Ma X, Wei B, Li S, Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids and Surfaces B-Biointerfaces; 126:520-530 [DOI] [PubMed] [Google Scholar]
- [7].Zhen Y, Wang N, Gao Z, Ma X, Wei B, Deng Y, and Wang T, “Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine 2015; 33(35), pp:4330-4340; PMID:25858854 [DOI] [PubMed] [Google Scholar]
- [8].Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm Res 2014; 31(9), pp. 2393-2403; PMID:24623480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Traverso G, Langer R. Perspective: Special delivery for the gut. Nature; 519(7544), pp. S19-S19; PMID:25806494 [DOI] [PubMed] [Google Scholar]
- [10].Newman MK, KILL M, and FRAMPTON G, “Effects of ultrasound alone and combined with hydrocortisone injections by needle or hypo-spray.” Am J Physical Med 1958; 37(4), pp. 206-209 [PubMed] [Google Scholar]
- [11].Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin Drug Deliv 2010; 7(12), pp. 1415-1432; PMID:21118031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J Controlled Release 2011; 152(3), pp. 330-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol 2008; 26(11), pp. 1261-1268; PMID:18997767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science 1995; 269(5225), pp. 850-853; PMID:7638603 [DOI] [PubMed] [Google Scholar]
- [15].Schoellhammer CM, Polat BE, Mendenhall J, Maa R, Jones B, Hart DP, Langer R, Blankschtein D. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J Controlled Release 2012; 163(2), pp. 154-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schoellhammer CM, Srinivasan S, Barman R, Mo SH, Polat BE, Langer R, Blankschtein D. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J Controlled Release 2015; 202, pp. 93-100; http://dx.doi.org/ 10.1016/j.jconrel.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bawiec CR, Sunny Y, Nguyen AT, Samuels JA, Weingarten MS, Zubkov LA, Lewin PA. Finite element static displacement optimization of 20–100 kHz flexural transducers for fully portable ultrasound applicator. Ultrasonics 2012; 53 (2), pp. 511-517; PMID:23040829; http://dx.doi.org/ 10.1016/j.ultras.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kajiyama K, Yoshinaka K, Takagi S, Matsumoto Y. Micro-bubble enhanced HIFU. Int Congress on Ultrasonics Santiago de Chile January 2009 2010; 3(1):pp. 305-314 [Google Scholar]
- [19].Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 2008; 48(4), pp. 260-270; PMID:18096196; http://dx.doi.org/ 10.1016/j.ultras.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schoellhammer CM, Schroeder A, Maa R, Lauwers GY, Swiston A, Zervas M, Barman R, DiCiccio AM, Brugge WR, Anderson DG, et al.. Ultrasound-mediated gastrointestinal drug delivery. Sci Translational Med 2015; 7(310), p. 310ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Seibold F, Fournier N, Beglinger C, Mottet C, Pittet V, Rogler G, “Topical therapy is underused in patients with ulcerative colitis. J Crohn's Colitis JA 2014; 8(1). pp. 56-63; http://dx.doi.org/ 10.1016/j.crohns.2013.03.005 [DOI] [PubMed] [Google Scholar]
- [22].Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler BAR, Klinman DM, et al.. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med 2012; 18(8), pp. 1291-1296; PMID:22797811; http://dx.doi.org/ 10.1038/nm.2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morrow MP, Weiner DB. DNA Drugs Come of Age. Scientific Am 2010; 303(1), pp. 48-53; http://dx.doi.org/ 10.1038/scientificamerican0710-48 [DOI] [PubMed] [Google Scholar]


