ABSTRACT
Non-injectable delivery of peptide and protein drugs is hampered by their labile nature, hydrophilicity, and large molecular size; thus limiting their permeation across mucosae, which represent major biochemical and physical barriers to drugs administered via e.g. the oral, nasal, and pulmonary routes. However, in recent years cell-penetrating peptides (CPP) have emerged as promising tools to enhance mucosal delivery of co-administered or conjugated peptide and protein cargo and more advanced CPP-cargo formulations are emerging. CPPs act as transepithelial delivery vectors, but the mechanism(s) by which CPPs mediate cargo translocation across an epithelium is so far poorly understood; both due to the fact that multiple factors influence the resulting uptake and trafficking mechanisms as well as to the complicated nature of sensitive studies of this. In addition to a proper mechanistic understanding, documentation of CPP-mediated delivery in higher animal species than rodent as well as extensive toxicological studies are necessary for CPP-containing non-injectable DDSs to reach the clinic.
KEYWORDS: cell-penetrating peptides, delivery vectors, drug delivery, epithelium, mucosal barriers, non-injectable delivery, peptide and protein drugs
Introduction
The increasing number of drug molecular entities based on peptides or proteins1 are of particular interest as therapeutics due to their potency and specific mode of action; resulting in predictive responses and fewer side-effects when compared to conventional small molecule synthetic drugs. A major drawback is, however, that biopharmaceuticals are currently mainly administered by injection, which by the patient is associated with pain and discomfort; hence often resulting in poor patient compliance. On the contrary, the oral route of administration is widely accepted among patients. The costs associated with production, storage, and use of oral dosage forms are usually lower than for injectable formulations as there is no need for sterile manufacturing, cold storage, or assistance from health care personnel etc. For people suffering from chronic diseases requiring frequent injections, such as diabetes and osteoporosis, the latter aspect is of particular importance and oral dosage forms as substitutes to injectable drugs would inevitably increase the quality of life for millions of people worldwide. However, oral delivery of peptide and protein drugs constitutes a great challenge in drug delivery due to their inherent chemical instability throughout the gastrointestinal (GI) tract and large molecular size limiting epithelial permeation. Unless the target site of action is the liver, as for e.g., insulin and glucagon-like peptide 1 (GLP-1), first-pass metabolism may moreover significantly reduce the systemic drug concentration. Thus, multiple factors must be taken into consideration when developing drug delivery systems (DDSs) intended for oral administration of peptide and protein drugs. For biopharmaceuticals with target sites to be reached systemically after passing the liver, other administration routes, such as the nasal, pulmonary, and sublingual route, may represent better alternatives, as both the harsh milieu of the stomach as the well as first pass metabolism in the liver will be avoided. However, the costs associated with production and storage of drugs applied to the nasal and pulmonary routes are generally higher than for drugs applied to the oral route, as the administration of these requires use of unique administration devices and, for some patient groups (e.g. children and elderly), also personal assistance.
The obstacles connected with the inherent physicochemical properties of peptide and protein drugs, i.e. enzymatic lability and large molecular size, are essential to consider when aiming for non-injectable administration via both the oral, sublingual, nasal, and pulmonary routes. A pH-sensitive enteric coating may assure protection of the peptide or protein drug against degradation during transport through the gastric lumen to the intestinal lumen, but the absorption of macromolecular drugs across both the intestinal, sublingual, nasal, and pulmonary mucosa requires co-administration or conjugation of the drug with or to safe and functional delivery vectors.
In recent years, a number of studies have demonstrated the potential of peptides belonging to the continuously growing family of cell-penetrating peptides (CPPs) to facilitate permeation of peptide and protein drugs across the intestinal epithelium.2,3 In 1988, the ability of the 86-amino acid residue Human Immunodeficiency Virus (HIV) Trans-activator of Transcription (Tat) protein to translocate across cell membranes was demonstrated.4 Subsequently, truncated peptide sequences (e.g., residues 47–57) have been demonstrated to contain the same cell-penetrating propensity as the full length Tat protein.5 The potential of Tat acting as vector for the delivery of protein into cells was demonstrated in vitro and in vivo by Dowdy et al. as early as in 19986 and 1999,7 respectively. In 2005, Tat was moreover the first CPP successfully employed as a vector to enhance insulin permeation across an intestinal epithelium in vitro.8 Since then, additional CPPs have shown potential as vectors for transmucoal delivery of therapeutic peptides and proteins,9-11 and, more recently, the potential of several more advanced CPP-containing oral DDSs have been reported in literature.12-14
The present review will focus on this relatively new – yet promising – field exploiting CPPs to improve non-injectable drug delivery, and more specifically as tools to enhance the permeation of peptide and protein drugs across the intestinal, nasal, and pulmonary mucosae. Historic as well as recent studies important for the field will be highlighted and the mechanisms will be discussed along with reflections on approaches to study the transport mechanisms. In addition, strategies exploited for optimizing the delivery efficiency of the CPP, as well as their safety, and the implementation of CPPs in advanced DDSs intended for non-injectable delivery, will be described.
Mucosae as Barriers to Non-Injectable Delivery of Peptide and Protein Drugs
Mucosae constitute major physical and biochemical barriers to non-injectable drug delivery - the epithelial cell layer surface-lined by a mucus layer makes up the physical barrier, whereas enzymes present in the mucus and on the surface as well as inside the epithelium represent the biochemical barrier.
Epithelia are composed of polarized cells and especially the intestinal, sublingual, nasal, and pulmonary epithelia are target sites for non-injectable drug delivery. From Nature, the function of epithelia is to serve as protective barriers toward invading pathogens and other foreign molecules, including drug entities. To add further protection, specialized Goblet cells secrete mucus, a hydrogel-like layer composed mainly of negatively charged glycoprotein and water, which acts as a filter barrier, thereby hindering vertical passive diffusion of macromolecules to reach the epithelial surface, combined with an interactive barrier effect, related to the interaction with exogenously administered molecules via the formation of non-covalent bonds.15 The presence of functional enzymes in the mucus matrix as well as the continuous removal of the mucus from the surface of the epithelium adds other levels to the barrier function of the mucus. In addition, a major function of especially the intestinal epithelium is the selective absorption of nutrients from the diet to a large extent via passive diffusion and receptor-mediated uptake of amino acids as well as di- and tripeptides. Despite the absorptive function of the intestinal epithelium, a major obstacle to transepithelial delivery of intact and functional peptide and protein drugs relates to their physicochemical properties, which hinder their unaided absorption from the lumen to the systemic circulation. First, peptides and proteins are inherently prone to degradation when facing the proteases in the intestinal lumen, in the mucus as well as the enzymes presented at the surface of and inside the epithelium. Secondly, their hydrophilic nature as wells as large molecular size limits their permeation across the tight and highly uptake selective epithelium. Thus, epithelia act both as a biochemical barrier via its high protease activity, and as a physical barrier represented by the individual cells tightly interconnected by especially tight junction (TJ) proteins resulting in a highly impermeable barrier.
Nevertheless, a number of transepithelial pathways for uptake of large molecules do exist; ready to be exploited in order to obtain successful transepithelial delivery of peptide and protein drugs. These potential routes for transepithelial permeation are divided into energy-independent direct translocation or paracellular diffusion, and energy-dependent endocytic uptake16 followed by transcytosis,17 carrier-mediated transport,18 or receptor-mediated transport.19 Only some lipophilic molecules are able to passively diffuse across epithelia via direct translocation through the cells and permeation through the paracellular cleft is limited to water and small solutes, whereas the passage of macromolecules, such as the peptide and protein drugs, is highly restricted.
Within recent years several research groups have reported on the successful use of CPPs as delivery vectors for transepithelial delivery of peptide and protein drugs. However, which route(s) the CPPs exploits is highly debated.
Cell-Penetrating Peptides as Vectors for Transepithelial Delivery of Peptide and Protein Drugs
The Tat sequence and the 16-mer penetratin sequence, the latter corresponding to residues 43–58 of the third helix of the Drosophila antennapedia homeodomain protein,20 are to date the most widely studied CPPs. Tat is most commonly employed to enhance intracellular delivery of various drug compounds, such as oligonucleotides21 and proteins,22 and was the first CPP demonstrated to facilitate insulin delivery across the intestinal epithelium in vitro.8 The application of Tat as a vector to enhance transepithelial permeation of cargo peptides or proteins is, however, questionable due to its previously demonstrated inability to internalize into and permeate monolayers of the epithelial cell culture models Caco-2 and MDCK23 and to enhance intestinal delivery of insulin in rats.9 Thus, for transepithelial delivery purposes, polyarginines (R8–10) and, especially, the penetratin 16-mer sequence are often the CPPs of choice (Table 1).
Table 1.
Overview of cell-penetrating peptides successfully employed as vectors for in vitro transepithelial translocation or in vivo transmucosal delivery of peptide and protein cargos.
| Cell-penetrating peptide | Sequence | Peptide or protein cargo drug | Test model | Read-out |
|---|---|---|---|---|
| Tat47-57 | YGRKKRRQRRR | Insulin8 | Caco-2 cell culture model | Insulin in the basolateral compartment |
| R6 | RRRRRR | Insulin26 | Rat intestinal loop injection | Plasma insulin, blood glucose |
| R8 | RRRRRRRR | Insulin,26 gastrin, GLP-1(A)37 | Rat intestinal loop injection | Plasma insulin,26 gastrin, and GLP-1,37 blood glucose26 |
| R9 | RRRRRRRRR | PTH(1-34)(B)36 | Caco-2 cell culture model | PTH(1-34) in the basolateral compartment |
| Penetratin | RQIKIWFQNRRMKWKK | Insulin,9-11,29,30 exendin-4, GLP-1, IFN-β(C)28 | Rat intestinal loop injection,9,11 intraintestinal injection,29 oral gavage,30 nasal administration,10,28 | Plasma insulin,9-11,30 exendin-4, GLP-1, and IFN-β,28 blood glucose10,29,30 |
| pVEC | LLIILRRRIRKQAHAHSK | Insulin9 | Rat intestinal loop injection | Plasma insulin |
| RRL helix | RRLRRLLRRLRRLLRRLR | Insulin9 | Rat intestinal loop injection | Plasma insulin |
| Shuffle | RWFKIQMQIRRWKNKK | Insulin10,11,29 | Nasal administration,10 intestinal loop injection,11 intraintestinal injection,29 Caco-2 cell culture model29 | Plasma insulin,10,11 blood glucose,10,29 insulin in the basolateral compartment29 |
| Penetramax | KWFKIQMQIRRWKNKR | Insulin11 | Intestinal loop injection | Plasma insulin, blood glucose |
(A)Glucagon-like peptide-1
(B)Parathyroid hormone residues 1–34
(C)Interferon-β.
Polyarginines (Rx) are efficient CPPs; possibly due to their high degree of positive charge as presented by the guanidinium side-groups facilitating bidentate binding with the negatively charged proteoglycan constituents (e.g. glycosaminoglycans (GAGs) and sialic acids) integrated in the plasma membrane.24 There is, however, both a minimal and optimal peptide length as demonstrated in a study investigating the effect of sequence length with respect to their internalization into RAW264.7 cells as visualized by confocal microscopy and detection by terminal conjugated fluorescein.25 In that study, no internalization was observed for R4, whereas increasing the chain length, obtaining R6 or R8, markedly enhanced the membrane translocation efficiency. However, further prolonging the chain length to R10, R12, and R16, caused a length-dependent decrease with regards to cellular internalization. Moreover, conjugation of a cargo, carbon anhydrase, to R8 resulted in efficient cargo internalization, whereas R16-carbon anhydrase conjugates did not internalize into cells, but instead resided at the surface of the plasma membrane. The polyarginine-mediated delivery of peptide or protein cargo across epithelia is similarly highly dependent on the length of the polyarginine sequence. Morishita et al. showed that simple co-administration of insulin with R6 and R8 to an intestinal loop in rats resulted in insulin absorption as well as lowering of blood glucose being dependent on the length of the polyarginine sequence.26 However, increasing the sequence length to R10 resulted in less insulin absorption when compared to co-administration of insulin with R8; thus suggesting an optimal sequence length of less than 10 when employing polyarginines as CPPs to enhance intestinal absorption of insulin.
Though the initial promising studies exploiting the use of CPPs as vectors for transepithelial delivery of peptide and protein drugs were carried out using Tat and polyarginies, penetratin is today the most widely studied CPP for that purpose. As Tat and polyarginines, penetratin (pI 12.3) is positively charged at physiological pH, categorizing it as belonging to the group of amphipatic CPPs, whereas Tat and polyarginies belongs to the class of CPPs being cationic – but not amphiphatic. The potential of penetratin as a vector for transepithelial delivery of peptide and protein drugs was first demonstrated by Kamei et al. in 2008, in a study demonstrating successful penetratin-mediated transmucosal delivery of insulin when co-administered with penetratin to the intestine of rats.9 In that study, a number of sequences belonging to the classes of cationic and amphipathic CPPs were tested. Only one of the cationic CPPs (R8) positively affected the delivery of insulin after intestinal administration, whereas 3 of the amphipathic CPPs (penetratin, pVEC,27 RRL helix) enhanced the insulin absorption; thus indicating that amphipathic CPPs may be more suitable as vectors for transmucosal delivery of peptide and protein drugs than non-amphipathic, yet cationic, CPPs. A subsequent study moreover demonstrated the potential of penetratin as a vector for exendin-4 delivery across the nasal mucosa in rats.28 Comparing the efficiency of the penetratin-mediated exendin-4 delivery across the rat nasal and intestinal mucosa, a higher plasma exendin-4 concentration was obtained following nasal administration; even though a 5-fold higher exendin-4 concentration was administered to the intestine than to the nasal cavity.28 This difference is most likely due to loss of exendin-4 during first pass metabolism following intestinal loop administration and differences in the enzymatic activity at the 2 administration sites. Since then, additional studies have demonstrated penetratin-mediated delivery of cargo across rat intestinal11,29,30 and rat nasal10,31,32 mucosa.
Due to the impressive ability of the penetratin sequence to facilitate transmucosal delivery of peptide and protein drugs, Khafagi et al. draw the attention toward the specific amino acids and their positioning being decisive for its potential as a delivery vector.10 Twenty analogs of penetratin were thus synthesized and a shuffled sequence, in which all residues but R and lysine (K) were shuffled, demonstrated to significantly enhance the delivery of co-administered insulin across the rat nasal mucosa when compared to the effect of the parent penetratin sequence. The improved delivery potential of “shuffe,” when compared to penetratin, is likely a result of the rearrangement of the hydrophobic tryptophan (W) residues. Recently, the presence of W residues, rather than the overall hydrophobicity,33 in an amphipathic CPP sequence has been demonstrated to positively affect the resulting propensity of the CPP to internalize into cells.34 The study furthermore suggested that the enhanced W-dependent cell-penetrating propensity of the CPP was due to an increased affinity for GAGs, thereby resulting in GAG-mediated endocytosis. This observation may also explain the enhanced potential of ‘shuffle’ as a delivery vector, when compared to penetratin. In addition, the rearrangement of W may likely affect the CPP interaction with the cargo molecule, which was later demonstrated as an important factor for successful CPP-mediated delivery of co-administered cargo across the nasal mucosa in rats.31 Recently, “shuffe” moreover demonstrated its potential as a delivery vector for co-administered insulin across the intestinal mucosa in rats.11,29 The aim of one study was to further optimize the ‘shuffle’ sequence and 14 analogs of ‘shuffle’ were synthesized.11 Out of those, a sequence (‘penetramax’), having the N-terminal R residue and the C-terminal K residue interchanged, particularly stood out by significantly improving the intestinal delivery of co-administered insulin when compared to the delivery propensity of ‘shuffle’. Thus, the specific amino acid positioning of both the hydrophobic W residues as well as the hydrophilic and positively charged R and K residues, is of great importance for the ability of the resulting CPP to act as a vector for the transmucosal delivery of a co-administered peptide or protein cargo.
Changing the amino acid stereochemistry from l to d constitute a viable approach to enhance the CPP-mediated delivery of cargo peptides and proteins for some, but not all CPPs. One study documented that among the CPPs R8, RRL helix, pVec, and penetratin, only the d-form of R8 was able to enhance insulin absorption from the intestine of rats when compared to its less metabolically stable l-form counterpart.9 Earlier, it was moreover shown that also d-R6 was superior to the l-R6 with respect to the ability to enhance insulin absorption across the rat mucosa.26 Another study demonstrated that d-penetratin enhanced insulin absorption to a greater extent than l-penetratin;30 however, this effect was documented in mice instead of rats, thus showing dependence of the animal species. Surprisingly, the choice of cargo influences whether changing stereochemistry of penetratin from the l-form to the d-form is a suitable approach to enhance the absorption enhancing effect of penetratin. This was demonstrated in a study including GLP-1, exendin-4, and interferon-β (IFN- β) as cargos, with only IFN- β being absorbed to a greater extent across rat intestinal or nasal mucosa when co-administered with d-penetratin as compared to when co-administration with l-penetratin.28
In addition to sequence-specific properties of the CPP, it is of importance whether the CPP is covalently conjugated to or simply co-administered with its cargo. It was demonstrated that covalent conjugation of d-R9 to insulin was necessary to obtain d-R9-mediated insulin permeation across rat pulmonary alveolar epithelial monolayers, which was not observed using co-administration.35 The d-R9-conjugated insulin was moreover demonstrated to lower blood glucose levels in diabetic rats subsequent to pulmonary administration. On the contrary, co-administration has proven to be highly suitable to obtain R9-mediated insulin delivery across the rat intestinal mucosa.26 Thus, translating conclusions drawn from studies employing e.g., pulmonary epithelia or mucosa to studies employing the intestinal mucosa should be done with great care though the different barriers do share some physiological features in terms of representing a tight epithelium.
A recent study moreover reported on the CPP effect on permeation of the biologically active part of parathyroid hormone (PTH(1–34)) across Caco-2 cell monolayers, by evaluating the effect of covalently conjugating various CPPs to the N- or C-terminal of PTH(1–34) and comparing with the effect of co-administration of the CPPs with PTH(1–34).36 Firstly, this study demonstrated that N-terminal conjugation was to be preferred over C-terminal conjugation and, secondly, that the co-administration approach was generally more effective than covalent conjugating of the CPPs to PTH(1–34).
Mechanisms of CPP and Cargo Translocation Across Epithelia
The majority of studies reporting on the mechanism of CPP membrane translocation have been conducted using non-polarized cells with respect to studying cellular CPP and/or cargo internalization and thus not transepithelial CPP-mediated translocation of a cargo, where both an apical and basolateral membrane must be crossed. However, despite differences in application, it is likely that similar mechanisms to some extent govern the early CPP-membrane interactions leading to subsequent membrane translocation.
Previously, direct translocation was believed to be the main mechanism of CPP membrane translocation. However, studies leading to this conclusion were commonly carried out using fixation of cells, which in 2003 was demonstrated to cause CPP-independent changes in the membrane permeability.38 By avoiding fixation, later studies have shown that not only direct membrane translocation, but also endocytosis is commonly exploited by the CPPs.39,40 The mechanisms by which CPPs translocate membranes are, however, still widely debated and drawing common conclusions is further complicated by the fact that multiple factors influence the resulting mechanisms responsible for cellular uptake. These include the specific CPP41 and its concentration,42 the cell type43 and their state of differentiation, 44 as well as the specific cargo of interest.45,46 Moreover, it is likely that the formulation approach, being covalent conjugation of the CPP to its cargo or co-administration of the CPP with its cargo, affects the overall mechanism of membrane translocation. In this context, one could speculate that covalently conjugating a highly positively charged CPP to a therapeutic peptide cargo would result in a molecule bearing longitudinal amphipathicity, thereby leading to direct membrane translocation in a detergent-like manner. On the other hand, by using the co-administration approach, the CPP and its cargo may interact and form complexes consisting of many or few molecules, and with specific physical structures depending on the CPP, the cargo, and the local environment at the cell surface - complexes, which due to their larger size and structure may preferably be taken up by an endocytic mechanism.
Though mechanistic studies have primarily been reported for cellular CPP uptake into non-polarized cells, few studies have addressed the cellular mechanistics involved in CPP or CPP-mediated cargo translocation across epithelial barriers. An early study from 2004 demonstrated that biotinylated transportan (TP), its analog TP10,47 and penetratin were taken up in a temperature-independent manner by Caco-2 cells grown to 50 % confluence on a coverslips; thus suggesting direct translocation being the main mechanism responsible for permeating the plasma membrane.48 In the same study, incubation of the Caco-2 cell monolayer with an inhibitor of endocytosis did not affect the CPP-uptake, thereby confirming the mechanism of TP, TP10, and penetratin uptake into the Caco-2 cells being energy-independent. However, the efficiency by which these CPPs translocated across the Caco-2 monolayers when grown on permeable filter inserts differed markedly. Up to 7% and 2.8% of the apically added TP and TP10, respectively, translocated across the monolayers, whereas only 0.6% of the applied penetratin was detected on the basolateral side after a 2 hour incubation period. Nevertheless, multiple studies have demonstrated the potential of the penetratin sequence to act as a vector to enhance the transepithelial delivery of peptide and protein drugs.9,29,30 Thus, the presence of penetratin, and possibly other CPPs, may only be important for permeation across the apical membrane, whereas passage across the basolateral membrane is mediated in a CPP-independent manner. Alternatively, one or more degradation products of the penetratin sequence may contain full cell-penetrating activity,49,50 and thus are competent in enhancing cargo translocation across the basolateral membrane. Finally, the fact that little penetratin escapes to the basolateral side of the epithelium may relate to the fact that penetratin represents a better substrate for intracellular enzymes than e.g. TP and TP1047 or a cargo drug. Thus, penetratin in itself may compete with the degradation of its cargo drug, thereby leaving more intact cargo for permeation across the epithelium.
In 2008 Kamei et al. aimed to elucidate the mechanism by which fluorescein-labeled d-R6 translocates across excised rat ileal membranes.51 That study demonstrated that lowering the experimental temperature to 4°C significantly hindered the translocation of d-R6 from the apical to the basolateral side of the epithelial membrane. Hence, as opposed to the direct translocation mechanism previously proposed for TP, TP10, and penetratin,48 the transepithelial translocation of d-R6 was energy-dependent. This likely involves electrostatic interactions between the positively charged d-R6 and negatively charged proteoglycans sitting in the membrane leading to subsequent endocytic uptake. However, d-R6 was not able to facilitate translocation of leuprolide across the ileal membrane; neither when co-administered with d-R6 nor as covalently conjugated to d-R6. This observation was a surprise as d-R6 had previously demonstrated some potential in enhancing absorption of co-administered insulin in a rat intestinal loop experiment.26 The authors suggested that electrostatic interactions between the CPP and its cargo may be a necessity to obtain CPP-mediated transepithelial delivery of a co-administered cargo. If so, this explains why d-R6 was able to enhance the transepithelial permeation of insulin, but not the much smaller molecule leuprolide, as only insulin is negatively charged at the relevant pH and thus able to electrostatically interact with the positively charged d-R6. Subsequently, this hypothesis was supported by studies demonstrating a positive correlation between the strength of the CPP-cargo interaction, analyzed using surface plasmon resonance (SPR), and the propensity of the CPP to enhance cargo absorption across the intestinal37 as well as the nasal31 mucosa of rats. The outcome of a more recent study, however, questions that simply the ability of the CPP to interact electrostatically with a co-administered cargo governs whether or not a CPP is able to enhance cargo permeation across an epithelium.29 This study examined the effect of changing formulation pH (5, 6.5, and 7.4) on the resulting insulin permeation across Caco-2 monolayers when co-administered with the CPPs penetratin or its analogs “shuffle,” “PenArg” (all K residues substituted with R residues), and “PenLys” (all R → all K). The effect of co-administering insulin with penetratin, “shuffle,” “PenArg” was highly pH-dependent and only at pH 5, an enhanced transepithelial insulin permeation was observed when compared to application of insulin alone. Mixing the CPPs with insulin at pH 5 did not favor electrostatic interactions and the size distributions of CPP-insulin complexes as a function of increasing pH, analyzed by dynamic light scattering (DLS), clearly showed a lack of complex formation at pH 5. Importantly, it should be underlined, that both the SPR31,37 and DLS29 experiments were performed in simple buffers, whereas the interaction pattern between CPPs and co-administered cargos likely changes when applied to the complex biological intestinal fluid and in the vicinity of the intestinal mucosa or epithelial surface. However, studying the CPP-cargo complexation and its potential importance for successful cargo delivery in a more in vivo-like setup, requires more advanced methodologies than presently available, and the development of such would contribute significantly to future understanding of formulation factors important for CPP-mediated transmucosal delivery of co-administered cargo drugs.
Successful transepithelial permeation subsequent to endocytic uptake relates to the ability of the cargo (and the CPP) to avoid segregation into lysosomes for degradation, but instead being transcytosed. Transcytosis is a mechanism commonly exploited by enterotoxins52-54 in order to permeate the intestinal epithelium, but may also be the mechanism involved in CPP-mediated transepithelial translocation of a therapeutic peptide or protein cargo. Studies are, however, needed to confirm this hypothesis, as well as whether the CPP is actually taken up and transcytosed together with its cargo or degraded intracellularly and therefore only necessary for mediating permeation across the abluminal membrane.
Though CPPs are commonly believed to be translocated across cell membranes via direct membrane translocation or via interaction with cell surface proteoglycans leading to endocytic uptake, other potential mechanisms for transepithelial permeation include scavenger-mediated and paracellular translocation.
Translocation across cell membranes via interaction with scavenger receptors is dependent on an overall negative charge55 and was recently demonstrated for non-covalent CPP-oligonucleotide complexes bearing a net negative surface charge.56-58 Though this mechanism of uptake may be most relevant for intracellular delivery of the inherently negatively charged oligonucleotides, some CPP-peptide or protein complexes may likewise bear an overall negative charge, thereby exploiting membrane translocation via interaction with scavenger receptors.
Finally, the CPP-mediated transepithelial transport of peptide and protein drugs may occur through the hydrophilic cleft between the epithelial cells. In such cases one could speculate that local high CPP concentrations directly affect the TJ integrity, thereby facilitating translocation of the cargo drug through the paracellular space. In fact, a previous study demonstrated that incubation of Caco-2 monolayers with the CPPs TP and TP10 caused an up to 53% decrease in the transepithelial electrical resistance (TEER),48 which is commonly used as a measure for the integrity of an epithelium. Penetratin was also included in that study, but did not affect the epithelial integrity. However, despite the dramatic increase in TP- and TP10-mediated paracellular ion flux, no marked increase in co-administered 4.4 kDa dextran was observed. Thus, for some CPPs, paracellular delivery may contribute to the net transepithelial delivery of cargo peptide or protein drugs, whereas transcellular translocation likely accounts for the majority of permeated cargo (Fig. 1). This moreover relates to the fact that a significantly larger intestinal surface area is accessible for transcellular translocation when compared to the area accessible for paracellular translocation. Nevertheless, a recent study has demonstrated the potential of a membrane penetrating peptide acting indirectly on the paracellular barrier via intracellular action on the myosin light chain protein controlling the opening of the TJs.59
Figure 1.
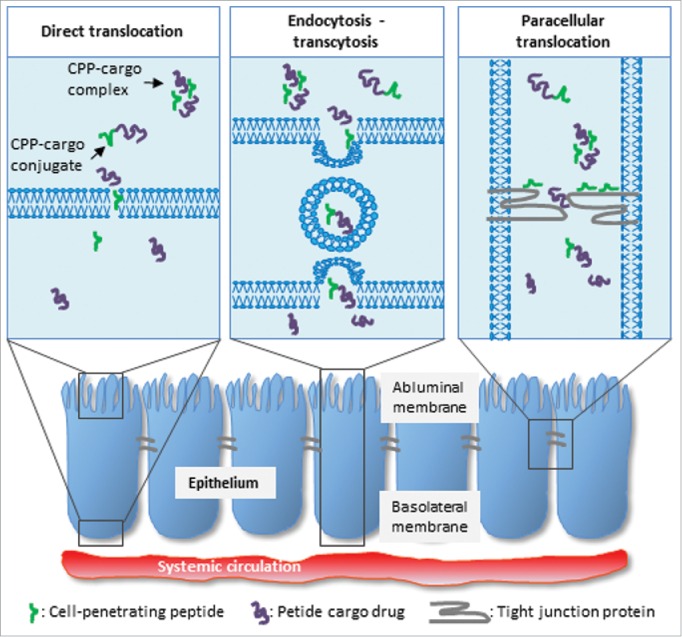
Simplified illustration representing suggested mechanisms for CPP-mediated delivery of peptide and protein cargos across epithelia. These include direct translocation, endocytosis followed by transcytosis, and paracellular translocation.
Studying the mechanisms of membrane interaction and transepithelial translocation
The mechanisms by which CPPs translocate across cell membranes are still widely discussed; both due to the fact that the resulting mechanism is dictated by multiple factors and to a large extent also due to the fact that carrying out such mechanistic studies are rather complicated. Several methodologies are, however, applicable for studying the mechanisms by which CPPs interact with the plasma membrane, and its constituents, leading to cellular uptake as well as for studying the interaction between a CPP and a co-administered cargo drug. Liposomes are commonly employed as simplified mimics of the plasma membrane in order to study the affinity of the CPP for a lipid membrane, which can then be analyzed via tryptophan fluorescence60 or by employing methodologies such as isothermal calorimetry (ITC),61 quartz crystal microbalance with dissipation (QCM-D) monitoring,62 and plasmon waveguide resonance spectroscopy.63 Differential scanning calorimetry (DSC) may be employed to study effects on lipid reorganization as a result of incubation with CPPs64 In addition, the propensity of the CPP to obtain a well-defined secondary structure in the vicinity of lipid membranes can be studied using circular dichroism (CD) spectroscopy65 and the extent to which a CPP is able to disrupt lipid membranes may be assessed by e.g., a calcein release assay.66 For studying the interaction between the CPP and membrane constituents, such as the GAGs, ITC67 and nuclear magnetic resonanceb (NMR) spectroscopy34 are examples of relevant methods to be applied. Finally, the CPP-cargo drug interaction can be assessed according to affinity using e.g. SPR37 or ITC,13 and the resulting complex size and morphology using e.g., DLS and electron microscopy,29 respectively. Advanced fluorescence microscopy such as 2-photon confocal laser scanning microscopy may also be employed for imaging of some CPP-cargo complexes in bulk or in contact with membranes, but require the presence of fluorescent molecules, which – especially if conjugated to the CPP or cargo – alters the physicochemical properties of these molecules. For detailed structural information about the complex, more advanced scattering techniques may be applied, e.g. small angle x-ray or neutron scattering (SAXS or SANS, respectively), may be applied.68,69
On the contrary, studying the mechanisms responsible for intracellular transport to and across the basolateral membrane is more complicated due to the higher degree of complexity of cell culture models employed in such studies, when compared to the more simplified systems listed above. Nevertheless, various approaches are commonly taken in order to draw conclusions on the cellular mechanisms involved in CPP or cargo drug translocation across an epithelial barrier. For the assessment of whether energy-independent or paracellular epithelial translocation is involved, lowering the experimental temperature to 4° C or inclusion of a small hydrophilic marker, such as radio-labeled mannitol, respectively, may be implemented during transport studies. In addition, measuring the TEER across the epithelial monolayer during and after a transport study is commonly employed to monitor changes in the TJ integrity and well-defined molecules of various sizes can be included for studying size-dependent paracellular permeation59 The addition of specific chemical inhibitors70 or siRNA inhibition71 of individual endocytic mechanisms, can be included to point out the endocytic uptake mechanisms in play. In addition, the trafficking into early and late endosomes can be studied using sucrose gradient centrifugation with subsequent western blotting analysis to detect potential co-localization of the peptide or protein cargo with specific endosomal markers.72 This, however, requires access to a specific antibody directed toward the cargo peptide or protein and do not allow for detection of the CPP as no antibodies toward these are commercially available. If the CPP and its therapeutic cargo are labeled with a fluorophore, confocal microscopy can be employed to study the endosomal trafficking in live cells by co-immunostaining of endosomal marker proteins. However, regarding the attachment of bulky and often hydrophobic fluorophores to the CPP and potentially a cargo, one must keep in mind that the physicochemical characteristics of the CPP and the cargo are altered. Hereby, the cell-penetrating propensity of the CPP73 as well as potentially how the cargo interacts with the CPP will be altered, thereby ultimately affecting the net amount of permeated cargo.
Safety of Cell-Penetrating Peptides as Vectors to Enhance Peptide and Protein Absorption
The CPPs have demonstrated promising potential as enhancers for transmucosal delivery of therapeutic peptides and proteins in a pre-clinical setting. However, to advance the field to clinical development thorough toxicological short and long term studies must be conducted; including testing for adverse effects due to unwanted immune responses elicited by the CPP, CPP-cargo conjugates, or CPP-cargo complexes.
The effect of the CPPs (with or without a co-administered or conjugated cargo) on the cellular viability can be determined in vitro using e.g., the MTS/PMS,29,36 MTT,13 or lactate dehydrogenase (LDH) leakage74 assays. It is, however, questionable whether the outcome of such in vitro assays translates directly to an in vivo situation in animal or man. The Caco-2 cell culture model is a widely accepted in vitro model representing the intestinal epithelium, but is a less robust system as compared to the in vivo intestinal mucosa, partly due to the absence of surface-lining mucus. Therefore, the CPP dosing concentrations necessary for obtaining both mucosal permeation and a physiological effect in vivo may be toxic to the cells in the in vitro model, but not causing adverse effects in vivo. Moreover, is the in vivo system much more complex than a rather simple cell culture model, and adverse effects due to e.g. off-target distribution of the CPP are thus not disclosed from in vitro experiments. However, despite the obvious drawbacks connected to the use of in vitro models, they are valuable tools for comparative and mechanistic studies related to drug interaction, uptake, and transport across an epithelium. In addition, the widely applied rodent in vivo models do possess shortcomings. Off-target effects resulting in adverse side-effects are not obvious from the outcome of the, often short-term, animal experiments currently carried out in which potential toxicity is commonly evaluated by histological examination at the local site of administration10,32 or by the assessment of LDH leakage from the exposed epithelial segment.9,26,32 Also, the degree of translation of the experimental outcome from rodents to higher animal species is so far not known.
Within the field of CPP-mediated transepithelial absorption of peptide and protein drugs, the number of studies including toxicological and immunogenicity tests are limited – but promising. In a study employing penetratin and its analogs “shuffle,” “PenArg,” and “PenLys” to enhance the permeation of co-administered insulin across Caco-2 monolayers, only incubation with the “shuffle” analog10 gave rise to decreased cellular viability and that solely at pH 5, whereas no adverse effect on the cellular viability was observed at pH 6.5 and 7.4.29 Also effective concentrations of R8 co-administered with insulin did not give rise to any undesired effects in Caco-2 cells.74 Another in vitro study documented safe use of R9 to enhance the permeation of co-administered PTH(1–34) across Caco-2 monolayers, whereas a R9-PTH(1–34) conjugate negatively affected the cellular viability,36 thus, demonstrating that the co-administration approach may, for some CPP-cargo systems, be the safer alternative to the covalent conjugation approach. Also, in that study no adverse immunogenic responses were detected for the R9-PTH(1–34) conjugate or a number of other CPP-PTH(1–34) conjugates when evaluated by the Toll-like receptor and the dendritic maturation assays.36
In vivo, no signs of toxicity were observed for subsequent successful penetratin-, R8-, pVEC-, or RRL helix-mediated insulin delivery across the rat intestinal mucosa when assessed by the LDH leakage assay.9 Also, the penetratin analogs “shuffe”10 and “penetramax”32 did not cause any adverse effects when administered to the rat nasal mucosa; neither when assessed by the LDH leakage assay nor by histological examination of the epithelium. In addition, the latter study reported on the potential immunogenicity resulting from systemic exposure to penetratin and “penetramax” via detection of the inflammatory biomarkers interleukin-1α and tumor necrosis factor-α, demonstrating that the plasma levels of the biomarkers in rats receiving both “penetramax” and insulin did not differ from the levels in rats receiving insulin without a CPP.32 Importantly, no change in neither release of inflammatory biomarkers nor in histology of the epithelium was observed after nasal administration of penetratin and “penetramax” for 30 consecutive days. More recent studies evaluated the safety of penetratin75 and a bis-β-cyclodextrin-conjugated penetratin (penetratin-bis-CD)12 as a consequence of continuous oral administration over a period of 7 days. The aim of these studies was to examine whether intestinal exposure to penetratin would cause unwanted co-absorption of enterotoxins, thus questioning the safe use of orally administered penetratin, especially for use in the treatment of chronic diseases, such as diabetes, which requires frequent administration of insulin. Lipopolysaccharide (LPS) was included as an example of an enterotoxin, which causes hepatic necrosis when continuously exposed systemically. LPS was orally administered to mice immediately before oral administration of different concentrations of CPP and plasma levels of 2 biomarkers for liver damage were analyzed. No increase in any of the biomarkers was observed in the mice receiving LPS and penetratin75 or LPS and penetratin-bis-CD12 when compared to mice dosed with an equal volume of buffer, thus supporting earlier studies documenting safe use of CPPs as transmucosal delivery vectors.
Advanced Non-Injectable Peptide and Protein Formulations Exploiting Cell-Penetrating Peptides
Previous attempts to optimize the potential of CPPs to function as vectors for transmucosal delivery of peptide and protein drugs include change of the amino acid stereochemistry from l to d9,26,28,30 or interchanging the positioning of the individual amino acids within the sequence.10,11 Recently, however, more advanced strategies have been implemented to improve the potential of the CPP-cargo formulation. Zhu et al. conjugated penetratin to bis-β-cyclodextrin (bis-CD), a cyclic oligosaccharide with a hydrophobic core facilitating self-assembly with insulin into nanocomplexes.12 Penetratin-bis-CD significantly enhanced the transepithelial permeation of co-administered insulin in vitro and in vivo when compared to penetratin. The effect was explained by stronger intermolecular interactions within the nanocomplex due to the hydrophobicity added by bis-CD to penetratin enabling both hydrophobic and electrostatic interactions with insulin. Hence, supporting the results of previous studies, these data suggest that the strength of intermolecular CPP-cargo interactions dictate the delivery propensity of the CPP.31,37 In addition, the enzymatic degradation of insulin was slightly slower in the presence of penetratin-bis-CD than in the presence of penetratin. Also, the mechanism by which the 2 nanocomplexes internalized into Caco-2 cells differed; insulin complexed with p-bis-CD internalized via endocytosis, whereas, in addition to endocytic uptake, direct translocation contributed to cellular internalization of insulin complexed with penetratin. Another study reported on optimizing the potential of R8 for delivery across rat intestinal mucosa via single amino acid modifications at position 7 (R → glutamic acid (E)) and 8 (R → W) as well as N-terminal conjugation to stearic acid (SA-R6EW).13 SA-R6EW significantly improved insulin delivery across rat intestinal mucosa when compared to R8; an effect also in that study explained by stronger intermolecular interactions in the SA-R6EW-insulin complex as compared to the R8-insulin complex. Different mechanisms of cellular insulin internalization were also observed in that study. Both direct translocation and endocytosis were responsible for the R8-mediated insulin uptake into Caco-2 cells, whereas only endocytosis seemed to be involved in the cellular internalization of insulin when complexed with SA-R6EW. Moreover, different endocytotic mechanisms were activated subsequent to incubation with the R8-insulin complexes and the SA-R6EW-insulin complexes.
In order to further advance the CPP-insulin formulation to consider not only transepithelial insulin delivery, but also mucus adhesion and mucus permeation, Shan et al. recently developed N-(2-hydroxypropyl) methacrylamide polymer (pHPMA)-coated penetratin-insulin complexes.14 The pHPMA-coated penetratin-insulin nanoparticles (NPs) with near neutral surface charge exhibited better mucus diffusion as well as improved insulin delivery in diabetic rats, than the NPs bearing an overall negative surface charge. This observation supports earlier studies demonstrating that neutral NPs are preferred over charged NPs in order to avoid repulsion by or adhesion to mucus constituents.76 Nevertheless, other studies have demonstrated successful use of NPs coated with the positively charged chitosan for mucosal drug delivery, as chitosan then provides positive charges responsible for mucus adhesion as wells as acts as a paracellular permeation enhancer.77 Another mucoadhesive formulation was recently proposed by Huining et al., suggesting the use of alginate/silica NPs for oral delivery of CPP-insulin conjugates.78 Alginate shrinks and swells in a pH-dependent manner. In the low pH of the stomach fluid the alginate/silica NPs will shrink and thereby hinder the release of the CPP-insulin conjugates, whereas at the higher pH of the intestinal lumen fluid alginate swells, whereby the CPP-insulin conjugates are believed to be released for translocation across the epithelium. However, the potential of this delivery strategy is yet to be demonstrated.
A variety of formulation approaches are pursued for delivery of biopharmaceuticals after non-injectable administration and it is evident that the promising results of CPPs to improve transmucosal delivery likely need further improvement of the formulation design and must be combined with other promising excipients and/or technologies for the development of a suitable final dosage form.
Future Perspectives
The application of CPPs as tools to enhance the mucosal delivery of biopharmaceuticals by non-injectable formulations is still in its infancy and so far the majority of studies report on applications for overcoming the intestinal, nasal, and pulmonary mucosae. Until now, many studies within the field of CPP-mediated transepithelial delivery of peptide and protein drugs document merely efficiency studies with little or no focus on the cellular mechanisms involved. However, a thorough mechanistic understanding is indeed needed for the development of future efficient and specifically acting CPPs. A specific mode of action relates to the safe use of CPPs as delivery vectors for some cargo drugs as off-target delivery and activation, due to e.g., unspeific translocation across the blood-brain barrier, may likely result in severe side-effects. Mechanistic studies are primarily carried out using cell culture models, which constitute simple representations of the complex in vivo system and will therefore not reveal all details of importance for improving non-injectable delivery of peptide and protein drugs. Nevertheless, using representative cell culture models allow for mechanistic studies at the level of single cells, which is not feasible in an in vivo model. Rodent animal models are widely used and applicable to predict the delivery propensity of the CPPs in vivo, but no studies have so far reported on the efficiency of CPPs in other species. However, studies in higher animal species, such as dog or monkey, are necessary in order to translate to man.
The use of CPPs as vectors for transmucosal delivery of peptide and protein drugs is still relatively new and the major focus often relates to documenting their efficiency and searching for new amino acid sequences and CPP analogs qualifying as delivery vectors. Nevertheless, more and more reports from mechanistic and safety studies appear in literature and even more advanced formulations providing protection against the high enzymatic activity as well as attempting to overcome the mucus barrier are emerging. Thus, design of final dosage forms containing a CPP for delivery of peptide or protein drugs to human patients may at some point be feasible when promising results from experiments in larger animal species begin to appear in the scientific literature along with reports from clinical trials.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115363 resources, which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007–2013) and EFPIA companies' in kind contribution.
References
- [1].Biotech products in big pharma clinincal pipelines have grown dramatically. Tufts CSDD impact report 2013; 15:1-4. [Google Scholar]
- [2].Kristensen M, Nielsen HM. Cell-penetrating peptides as carriers for oral delivery of biopharmaceuticals. Basic Clin Pharmacol Toxicol 2016; 118:99-106; PMID:26525297; http://dx.doi.org/ 10.1111/bcpt.12515 [DOI] [PubMed] [Google Scholar]
- [3].Khafagy E-S, Morishita M. Oral biodrug delivery using cell-penetrating peptide. Adv Drug Deliv Rev 2012; 64:531-9; PMID:22245080; http://dx.doi.org/ 10.1016/j.addr.2011.12.014 [DOI] [PubMed] [Google Scholar]
- [4].Frankel A, Pabo C. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988; 8:1189-93; http://dx.doi.org/ 10.1016/0092-8674(88)90263-2 [DOI] [PubMed] [Google Scholar]
- [5].Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem 1997; 272:16010-7; http://dx.doi.org/ 10.1074/jbc.272.25.16010 [DOI] [PubMed] [Google Scholar]
- [6].Nagahara H, Vochero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27 Kip1 induces cell migration. Nat Med 1998; 4:1449-52; PMID:9846587; http://dx.doi.org/ 10.1038/4042 [DOI] [PubMed] [Google Scholar]
- [7].Schwarze SR. In Vivo Protein Transduction: Delivery of biologically active protein into the mouse. Science 1999; 285:1569-72; PMID:10477521; http://dx.doi.org/ 10.1126/science.285.5433.1569 [DOI] [PubMed] [Google Scholar]
- [8].Liang JF, Yang VC. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun 2005; 335:734-8; PMID:16115469; http://dx.doi.org/ 10.1016/j.bbrc.2005.07.142 [DOI] [PubMed] [Google Scholar]
- [9].Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J Control Release 2008; 132:21-5; PMID:18727945; http://dx.doi.org/ 10.1016/j.jconrel.2008.08.001 [DOI] [PubMed] [Google Scholar]
- [10].Khafagy E-S, Morishita M, Ida N, Nishio R, Isowa K, Takayama K. Structural requirements of penetratin absorption enhancement efficiency for insulin delivery. J Control Release 2010; 143:302-10; PMID:20096319; http://dx.doi.org/ 10.1016/j.jconrel.2010.01.019 [DOI] [PubMed] [Google Scholar]
- [11].Kamei N, Kikuchi S, Takeda-morishita M, Terasawa Y, Yasuda A, Yamamoto S, Ida N, Nishio R, Takayama K. Determination of the optimal cell-penetrating peptide sequence for intestinal insulin delivery based on molecular orbital analysis with self-organizing maps. J Pharm Sci 2013; 102:469-79; PMID:23160942; http://dx.doi.org/ 10.1002/jps.23364 [DOI] [PubMed] [Google Scholar]
- [12].Zhu X, Shan W, Zhang P, Jin Y, Guan S, Fan T, Yang Y, Zhou Z, Huang Y. Penetratin derivative-based nanocomplexes for enhanced intestinal insulin delivery. Mol Pharm 2014; 11:317-28; PMID:24255985; http://dx.doi.org/ 10.1021/mp400493b [DOI] [PubMed] [Google Scholar]
- [13].Zhang Y, Li L, Han M, Hu J, Zhang L. Amphiphilic lipopeptide-mediated transport of insulin and cell membrane penetration mechanism. Molecules 2015; 20:21569-83; PMID:26633348; http://dx.doi.org/ 10.3390/molecules201219771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shan W, Zhu X, Liu M, Li L, Zhong J, Sun W, Zhang Z, Huang Y. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano 2015; 9:2345-56; PMID:25658958; http://dx.doi.org/ 10.1021/acsnano.5b00028 [DOI] [PubMed] [Google Scholar]
- [15].Boegh M, García-Díaz M, Müllertz A, Nielsen HM. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur J Pharm Biopharm 2015; 95:136-43; PMID:25622791; http://dx.doi.org/ 10.1016/j.ejpb.2015.01.014 [DOI] [PubMed] [Google Scholar]
- [16].Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2001; 2:149-159; PMID:11260520; http://dx.doi.org/ 10.1034/j.1600-0854.2001.020301.x [DOI] [PubMed] [Google Scholar]
- [17].Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev 2003; 83:871-932.; PMID:12843411; http://dx.doi.org/ 10.1152/physrev.00001.2003 [DOI] [PubMed] [Google Scholar]
- [18].Tsuji A, Tamia I. Carrier mediated intestinal transport of drugs. Pharm Res 1996; 7:963-977; http://dx.doi.org/ 10.1023/A:1016086003070 [DOI] [PubMed] [Google Scholar]
- [19].Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev 2003; 55:1439-66; PMID:14597140; http://dx.doi.org/ 10.1016/j.addr.2003.07.004 [DOI] [PubMed] [Google Scholar]
- [20].Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 1994; 269:10444-50; PMID:8144628 [PubMed] [Google Scholar]
- [21].Brown SD, Graham D. Conjugation of an oligonucleotide to Tat, a cell-penetrating peptide, via click chemistry. Tetrahedron Lett 2010; 51:5032-4; http://dx.doi.org/ 10.1016/j.tetlet.2010.07.101 [DOI] [Google Scholar]
- [22].Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res 2002; 62:2013-8; PMID:11929818 [PubMed] [Google Scholar]
- [23].Violini S, Sharma V, Prior JL, Dyszlewski M, Piwnica-Worms D. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well differentiated epithelial cells: lack of correlation with heparan sulfate. Biochemistry 2002; 41:12652-61; PMID:12379107; http://dx.doi.org/ 10.1021/bi026097e [DOI] [PubMed] [Google Scholar]
- [24].Ziegler A, Seelig J. Binding and clustering of glycosaminoglycans: a common property of mono- and multivalent cell-penetrating compounds. Biophys J 2008; 94:2142-9; PMID:18065465; http://dx.doi.org/ 10.1529/biophysj.107.113472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem 2001; 276:5836-40; PMID:11084031; http://dx.doi.org/ 10.1074/jbc.M007540200 [DOI] [PubMed] [Google Scholar]
- [26].Morishita M, Kamei N, Ehara J, Isowa K, Takayama K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J Control Release 2007; 118:177-84; PMID:17270307; http://dx.doi.org/ 10.1016/j.jconrel.2006.12.022 [DOI] [PubMed] [Google Scholar]
- [27].Elmquist A, Lindgren M, Bartfai T. VE-Cadherin-derived cell-penetrating peptide, pVEC, with carrier functions 2001; 244:237-44. [DOI] [PubMed] [Google Scholar]
- [28].Khafagy E-S, Morishita M, Kamei N, Eda Y, Ikeno Y, Takayama K. Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int J Pharm 2009; 381:49-55; PMID:19646515; http://dx.doi.org/ 10.1016/j.ijpharm.2009.07.022 [DOI] [PubMed] [Google Scholar]
- [29].Kristensen M, Franzyk H, Klausen MT, Iversen A, Bahnsen JS, Skyggebjerg RB, Foderá V, Nielsen HM. Penetratin-mediated transepithelial insulin permeation: importance of cationic residues and pH for complexation and permeation. AAPS J 2015; 17:1200-9; PMID:25990963; http://dx.doi.org/ 10.1208/s12248-015-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nielsen EJB, Yoshida S, Kamei N, Iwamae R, Khafagy E-S, Olsen J, Rahbel UL, Pedersen BL, Takayama K, Takeda-Morishita M. In vivo proof of concept of oral insulin delivery based on a co-administration strategy with the cell-penetrating peptide penetratin. J Control Release 2014; 189:19-24; PMID:24973720; http://dx.doi.org/ 10.1016/j.jconrel.2014.06.022 [DOI] [PubMed] [Google Scholar]
- [31].Khafagy E-S, Morishita M, Takayama K. The role of intermolecular interactions with penetratin and its analogue on the enhancement of absorption of nasal therapeutic peptides. Int J Pharm 2010; 388:209-12; PMID:20060451; http://dx.doi.org/ 10.1016/j.ijpharm.2009.12.060 [DOI] [PubMed] [Google Scholar]
- [32].Khafagy E-S, Kamei N, Nielsen EJB, Nishio R, Takeda-Morishita M. One-month subchronic toxicity study of cell-penetrating peptides for insulin nasal delivery in rats. Eur J Pharm Biopharm 2013; 85:736-43; PMID:24060698; http://dx.doi.org/ 10.1016/j.ejpb.2013.09.014 [DOI] [PubMed] [Google Scholar]
- [33].Madani F, Lindberg S, Langel Ü, Futaki S, Gräslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011; 2011:1-10; http://dx.doi.org/ 10.1155/2011/414729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bechara C, Pallerla M, Zaltsman Y, Burlina F, Alves ID, Lequin O, Sagan S. Tryptophan within basic peptide sequences triggers glycosaminoglycan-dependent endocytosis. Biochemistry 2013; 27:1-12 [DOI] [PubMed] [Google Scholar]
- [35].Patel LN, Wang J, Kim K, Borok Z, Crandall ED, Shen W. Conjugation with cationic cell-penetrating peptide increases pulmonary absorption of insulin. Mol Pharm 2009; 6:492-503; PMID:19228019; http://dx.doi.org/ 10.1021/mp800174g [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kristensen M, de Groot AM, Berthelsen J, Franzyk H, Sijts A, Nielsen HM. Conjugation of cell-penetrating peptides to parathyroid hormone affects its structure, potency, and transepithelial permeation. Bioconjug Chem 2015; 26:477-488; PMID:25611217; http://dx.doi.org/ 10.1021/bc5005763 [DOI] [PubMed] [Google Scholar]
- [37].Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem 2003; 278:585-90; PMID:12411431; http://dx.doi.org/ 10.1074/jbc.M209548200 [DOI] [PubMed] [Google Scholar]
- [38].Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med 2007; 11:670-84; PMID:17760832; http://dx.doi.org/ 10.1111/j.1582-4934.2007.00062.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cleal K, He L, Watson PD, Jones AT. Endocytosis, intracellular traffic and fate of cell penetrating peptide based conjugates and nanoparticles. Curr Pharm Des 2013; 19:2878-94; PMID:23140451; http://dx.doi.org/ 10.2174/13816128113199990297 [DOI] [PubMed] [Google Scholar]
- [40].Lundin P, Johansson H, Guterstam P, Holm T, Hansen M, Langel Ü, El-Andaloussi S. Distinct uptake routes of cell-penetrating peptide conjugates. Bioconjug Chem 2008; 19:2535-42; PMID:19012426; http://dx.doi.org/ 10.1021/bc800212j [DOI] [PubMed] [Google Scholar]
- [41].Jones AT, Sayers EJ. Cell entry of cell penetrating peptides: tales of tails wagging dogs. J Control Release 2012; 161:582-91; PMID:22516088; http://dx.doi.org/ 10.1016/j.jconrel.2012.04.003 [DOI] [PubMed] [Google Scholar]
- [42].Tréhin R, Krauss U, Beck-Sickinger AG, Merkle HP, Nielsen HM. Cellular uptake but low permeation of human calcitonin-derived cell penetrating peptides and Tat(47-57) through well-differentiated epithelial models. Pharm Res 2004; 21:1248-56; http://dx.doi.org/ 10.1023/B:PHAM.0000033013.45204.c3 [DOI] [PubMed] [Google Scholar]
- [43].Foerg C, Ziegler U, Fernandez-Carneado J, Giralt E, Merkle HP. Differentiation restricted endocytosis of cell penetrating peptides in MDCK cells corresponds with activities of Rho-GTPases. Pharm Res 2007; 24:628-42; PMID:17334941; http://dx.doi.org/ 10.1007/s11095-006-9212-1 [DOI] [PubMed] [Google Scholar]
- [44].Tünnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J 2006; 20:1775-84; PMID:16940149; http://dx.doi.org/ 10.1096/fj.05-5523com [DOI] [PubMed] [Google Scholar]
- [45].Bárány-Wallje E, Gaur J, Lundberg P, Langel U, Gräslund A. Differential membrane perturbation caused by the cell penetrating peptide Tp10 depending on attached cargo. FEBS Lett 2007; 581:2389-93; http://dx.doi.org/ 10.1016/j.febslet.2007.04.046 [DOI] [PubMed] [Google Scholar]
- [46].Soomets U, Lindgren M, Gallet X, Hällbrink M, Elmquist A, Balaspiri L, Zorko M, Pooga M, Brasseur R, Langel Ü. Deletion analogues of transportan. Biochim Biophys Acta 2000; 1467:165-76; PMID:10930519; http://dx.doi.org/ 10.1016/S0005-2736(00)00216-9 [DOI] [PubMed] [Google Scholar]
- [47].Lindgren ME, Hällbrink MM, Elmquist AM, Langel Ü. Passage of cell-penetrating peptides across a human epithelial cell layer in vitro. Biochem J 2004; 377:69-76; PMID:12968950; http://dx.doi.org/ 10.1042/bj20030760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Palm C, Jayamanne M, Kjellander M, Hällbrink M. Peptide degradation is a critical determinant for cell-penetrating peptide uptake. Biochim Biophys Acta 2007; 1768:1769-76; PMID:17499577; http://dx.doi.org/ 10.1016/j.bbamem.2007.03.029 [DOI] [PubMed] [Google Scholar]
- [49].Tréhin R, Nielsen HM, Jahnke H-G, Krauss U, Beck-Sickinger AG, Merkle HP. Metabolic cleavage of cell-penetrating peptides in contact with epithelial models: human calcitonin (hCT)-derived peptides, Tat(47-57) and penetratin(43-58). Biochem J 2004; 382:945-56; http://dx.doi.org/ 10.1042/BJ20040238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kamei N, Morishita M, Ehara J, Takayama K. Permeation characteristics of oligoarginine through intestinal epithelium and its usefulness for intestinal peptide drug delivery. J Control Release 2008; 131:94-9; PMID:18692532; http://dx.doi.org/ 10.1016/j.jconrel.2008.07.016 [DOI] [PubMed] [Google Scholar]
- [51].Kamei N, Morishita M, Takayama K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J Control Release 2009; 136:179-86; PMID:19250953; http://dx.doi.org/ 10.1016/j.jconrel.2009.02.015 [DOI] [PubMed] [Google Scholar]
- [52].Mrsny RJ. Lessons from nature: “pathogen-mimetic” systems for mucosal nano-medicines. Adv Drug Deliv Rev 2009; 61:172-92; PMID:19146895; http://dx.doi.org/ 10.1016/j.addr.2008.09.009 [DOI] [PubMed] [Google Scholar]
- [53].Ahsan CR, Hajnóczky G, Maksymowych AB, Simpson LL. Visualization of binding and transcytosis of botulinum toxin by human intestinal epithelial cells. J Pharmacol Exp Ther 2005; 315:1028-35; PMID:16144978; http://dx.doi.org/ 10.1124/jpet.105.092213 [DOI] [PubMed] [Google Scholar]
- [54].Lukyanenko V, Malyukova I, Hubbard A, Delannoy M, Boedeker E, Zhu C, Cebotaru L, Kovbasnjuk . Enterohemorrhagic Escherichia coli infection stimulates Shiga toxin 1 macropinocytosis and transcytosis across intestinal epithelial cells. Am J Cell Physiol 2011; 301:1140-9; http://dx.doi.org/ 10.1152/ajpcell.00036.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Platt N, Gordon S. Scavenger receptors: diverse activities and promiscuous binding of polyanionic ligands. Chem Biol 1998; 5:R193-203; PMID:9710567; http://dx.doi.org/ 10.1016/S1074-5521(98)90156-9 [DOI] [PubMed] [Google Scholar]
- [56].Lindberg S, Regberg J, Eriksson J, Helmfors H, Muñoz-Alarcón A, Srimanee A, Figueroa RA, Hallberg E, Ezzat K, Langel Ü. A convergent uptake route for peptide- and polymer-based nucleotide delivery systems. J Control Release 2015; 206:58-66; PMID:25769688; http://dx.doi.org/ 10.1016/j.jconrel.2015.03.009 [DOI] [PubMed] [Google Scholar]
- [57].Ezzat K, Helmfors H, Tudoran O, Juks C, Lindberg S, Padari K, El-Andaloussi S, Pooga M, Langel Ü. Scavenger receptor-mediated uptake of cell-penetrating peptide nanocomplexes with oligonucleotides. FASEB J 2012; 26:1172-80; PMID:22138034; http://dx.doi.org/ 10.1096/fj.11-191536 [DOI] [PubMed] [Google Scholar]
- [58].Lindberg S, Muñoz-Alarcón A, Helmfors H, Mosqueira D, Gyllborg D, Tudoran O, Langel Ü. PepFect15, a novel endosomolytic cell-penetrating peptide for oligonucleotide delivery via scavenger receptors. Int J Pharm 2013; 441:242-7; PMID:23200958; http://dx.doi.org/ 10.1016/j.ijpharm.2012.11.037 [DOI] [PubMed] [Google Scholar]
- [59].Taverner A, Dondi R, Almansour K, Laurent F, Owens S-E, Eggleston IM, Fotaki N, Mrsny RJ. Enhanced paracellular transport of insulin can be achieved via transient induction of myosin light chain phosphorylation. J Control Release 2015; 210:189-97; PMID:25980620; http://dx.doi.org/ 10.1016/j.jconrel.2015.05.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rydberg HA, Matson M, Amand HL, Esbjörner EK, Nordén B. Effects of tryptophan content and backbone spacing on the uptake efficiency of cell-penetrating peptides. Biochemistry 2012; 51:5531-9; PMID:22712882; http://dx.doi.org/ 10.1021/bi300454k [DOI] [PubMed] [Google Scholar]
- [61].Jobin ML, Bonnafous P, Temsamani H, Dole F, Grélard A, Dufourc EJ, Alves ID. The enhanced membrane interaction and perturbation of a cell penetrating peptide in the presence of anionic lipids: toward an understanding of its selectivity for cancer cells. Biochim Biophys Acta 2013; 1828:1457-70; PMID:23462641; http://dx.doi.org/ 10.1016/j.bbamem.2013.02.008 [DOI] [PubMed] [Google Scholar]
- [62].Piantavigna S, McCubbin GA, Boehnke S, Graham B, Spiccia L, Martin LL. A mechanistic investigation of cell-penetrating Tat peptides with supported lipid membranes. Biochim Biophys Acta 2011; 1808:1811-7; PMID:21414289; http://dx.doi.org/ 10.1016/j.bbamem.2011.03.002 [DOI] [PubMed] [Google Scholar]
- [63].Alves ID, Bechara C, Walrant A, Zaltsman Y, Jiao CY, Sagan S. Relationships between membrane binding, affinity and cell internalization efficacy of a cell-penetrating peptide: Penetratin as a case study. PLoS One 2011; 6:24096; http://dx.doi.org/ 10.1371/journal.pone.0024096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Joanne P, Galanth C, Goasdoué N, Nicolas P, Sagan S, Lavielle S, Cassaing G, Amri EL, Alves ID. Lipid reorganization induced by membrane-active peptides probed using differential scanning calorimetry. Biochim Biophys Acta 2009; 1788:1772-81; PMID:19427300; http://dx.doi.org/ 10.1016/j.bbamem.2009.05.001 [DOI] [PubMed] [Google Scholar]
- [65].Rydberg HA, Carlsson N, Nordén B. Membrane interaction and secondary structure of de novo designed arginine-and tryptophan peptides with dual function. Biochem Biophys Res Commun 2012; 427:261-5; PMID:22989747; http://dx.doi.org/ 10.1016/j.bbrc.2012.09.030 [DOI] [PubMed] [Google Scholar]
- [66].Bahnsen JS, Franzyk H, Sandberg-Schaal A, Nielsen HM. Antimicrobial and cell-penetrating properties of penetratin analogs: effect of sequence and secondary structure. Biochim Biophys Acta 2013; 1828:223-32; PMID:23085001; http://dx.doi.org/ 10.1016/j.bbamem.2012.10.010 [DOI] [PubMed] [Google Scholar]
- [67].Yang J, Tsutsumi H, Furuta T, Sakurai M, Mihara H. Interaction of amphiphilic α-helical cell-penetrating peptides with heparan sulfate. Org Biomol Chem 2014; 12:4673-81; PMID:24867193; http://dx.doi.org/ 10.1039/c4ob00673a [DOI] [PubMed] [Google Scholar]
- [68].Mertens HDT, Svergun DI. Structural characterization of proteins and complexes using small-angle X-ray solution scattering. J Struct Biol 2010; 172:128-41; PMID:20558299; http://dx.doi.org/ 10.1016/j.jsb.2010.06.012 [DOI] [PubMed] [Google Scholar]
- [69].Links DA, Choi D, Moon JH, Kim H, Sung BJ, Kim MW, Tae GY, Satija SK, Akgun B, Yu C-J, Lee HW, Lee DR, Henderson JM, Kwong JW, Lam KL, Lee KYC, Shin K. Insertion mechanism of cell-penetrating peptides into supported phospholipid membranes revealed by X-ray and neutron reflection. Soft Matter 2012; 8:8294; http://dx.doi.org/ 10.1039/c2sm25913c [DOI] [Google Scholar]
- [70].Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 2007; 8:848-66; PMID:17587406; http://dx.doi.org/ 10.1111/j.1600-0854.2007.00572.x [DOI] [PubMed] [Google Scholar]
- [71].Al Soraj M, He L, Peynshaert K, Cousaert J, Vercauteren D, Braeckmans K, De Smedt SC, Jones AT. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J Control Release 2012; 161:132-41; PMID:22465675; http://dx.doi.org/ 10.1016/j.jconrel.2012.03.015 [DOI] [PubMed] [Google Scholar]
- [72].Pärnaste L, Arukuusk P, Zagato E, Braeckmans K, Langel Ü. Methods to follow intracellular trafficking of cell-penetrating peptides. J Drug Target 2015; 2330:1-12 [DOI] [PubMed] [Google Scholar]
- [73].Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I, Kobayashi S, Haraguchi T, Futaki S. Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Mol Ther 2012; 20:1-10; PMID:22215048; http://dx.doi.org/ 10.1038/mt.2011.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kamei N, Onuki Y, Takayama K, Takeda-Morishita M. Mechanistic study of the uptake/permeation of cell-penetrating peptides across a caco-2 monolayer and their stimulatory effect on epithelial insulin transport. J Pharm Sci 2013; 102:3998-4008; PMID:23963728; http://dx.doi.org/ 10.1002/jps.23708 [DOI] [PubMed] [Google Scholar]
- [75].Nielsen EJB, Kamei N, Takeda-Morishita M. Safety of the cell-penetrating peptide penetratin as an oral absorption enhancer. Biol Pharm Bull 2015; 38:144-6; PMID:25744470; http://dx.doi.org/Ref74 [DOI] [PubMed] [Google Scholar]
- [76].Samuel K. Lai, Ying-Ying Wang JH. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev 2009; 61:158-71; http://dx.doi.org/ 10.1016/j.addr.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci 2001; 14:201-7; PMID:11576824; http://dx.doi.org/ 10.1016/S0928-0987(01)00172-5 [DOI] [PubMed] [Google Scholar]
- [78].He H, Ye J, Sheng J, Wang J, Huang Y, Chen G, Wang J, Yang VC. Overcoming oral insulin delivery barriers: application of cell penetrating peptide and silica-based nanoporous composites. Front Chem Sci Eng 2013; 7:9-19; http://dx.doi.org/ 10.1007/s11705-013-1306-9 [DOI] [Google Scholar]


