ABSTRACT
Poly(amidoamine) (PAMAM) dendrimers have been extensively investigated for oral delivery applications due to their ability to translocate across the gastrointestinal epithelium. In this Review, we highlight recent advances in the evaluation of PAMAM dendrimers as oral drug delivery carriers. Specifically, toxicity, mechanisms of transepithelial transport, models of the intestinal epithelial barrier including isolated human intestinal tissue model, detection of dendrimers, and surface modification are discussed. We also highlight evaluation of various PAMAM dendrimer-drug conjugates for their ability to transport across gastrointestinal epithelium for improved oral bioavailability. In addition, current challenges and future trends for clinical translation of PAMAM dendrimers as carriers for oral delivery are discussed.
KEYWORDS: bioavailability, Caco-2 monolayer, complexation, conjugation, cytotoxicity, PAMAM dendrimers, permeability, translocation, ussing chamber
Introduction
Importance of oral delivery
The oral route of administration is most patient compliant, widely used, and readily accepted. It provides additional advantages including minimal intervention and pain during administration, cost-effectiveness, reproducibility and feasibility across a range of patient populations.1 It is estimated that the market for oral drug delivery is growing at a compounded annual growth rate of 10.3 % from 2010 to 2017.2 The total absorptive surface area of the gastrointestinal (GI) tract is around 300–400m2 and is comprised of villi and microvilli that contribute to this high absorptivity of the GI epithelium.3 The GI tract which extends from mouth to colon, consists of 4 layers of architecture: tunica serosa (for abdominal cavity) or tunica adventitia (for esophagus and rectum areas) – the outermost layer, tunica muscularis - next 2 smooth muscle layers, and tunica mucosa – the innermost layer. The tunica mucosa is the layer that faces the gut lumen and contains the gastrointestinal epithelium. The tunica submucosa is the layer that is surrounded by vasculature and vessels of lymphatic systems.4,5 The intestinal epithelial cells (IECs) consist of absorptive enterocytes and secretory IECs including goblet cells, enteroendocrine cells, and Paneth cells. The IECs are organized in the crypts and villi of the intestinal epithelium.6 The enterocytes have absorptive property, whereas goblet cells have mucus secreting property. The lymphoid regions comprising of Peyer's patches are covered with M (microfold or membrane) cells that are specialized for antigen presentation. M cells play a significant role in oral drug delivery because they have high transcytotic activity, and they have relatively less mucus protection.7
The challenges involved with oral drug delivery include the acidic gastric environment, the poor aqueous solubility and chemical stability of many drugs and the presence of digestive enzymes.7 The GI epithelium protects the human body against absorption of unwanted toxic materials by an external barrier system that comprises mucus, an aqueous layer of water, and the glycocalyx. In addition to these layers, there are also abundant undifferentiated crypt cells and mucus-secreting goblet cells, lining the wall. The high enzymatic activity in enterocytes of the GI tract is another challenge involved in oral drug delivery. The enzymatic activity of GI tract often leads to intracellular hydrolysis and degradation of macromolecules after their absorption by endocytosis through enterocytes.4
There are 2 major mechanisms of drug transport across GI epithelium: a) Paracellular, b) Transcellular. Paracellular transport involves passive diffusion of substances through the intercellular spaces in between epithelial cells and is under the control of tight junctions.8 Transcellular transport pathway is further divided into passive diffusion, carrier-mediated transport and the endocytic transport or transcytosis. Passive diffusion is the preferred route of transport of relatively small lipophilic molecules and is dependent on concentration gradient across the GI epithelium. Carrier-mediated transport involves energy-dependent uptake of specific molecules by carriers. For example, β-lactam antibiotics, angiotensin converting enzyme inhibitors, monosaccharides, and amino acids follow carrier-mediated transport processes for absorption across GI epithelium.9 Endocytic transport process involves invagination of the plasma membrane leading to the formation and internalization of vesicles and delivery of internalized molecules into endosomal compartments.10 Mechanisms of endocytic uptake include clathrin-mediated endocytosis, phagocytosis, macropinocytosis and caveolin-mediated endocytosis.11 In addition to being endocytosed, drugs or macromolecules can be transcytosed (transported across cell).
Factors that should be taken into consideration in developing oral drug delivery systems include physicochemical properties of the drug such as lipophilicity and enzyme susceptibility, the modification of epithelial permeability by small molecules (absorption enhancers), the addition of receptor targeted ligands, and the use of particulate carrier systems for transport across the site of absorption. Particularly, the use of particulate carrier systems involving the use of lipoic and polymeric carriers has been highly successful in the field of oral delivery.12,13
Polymeric Oral Drug Delivery Systems
Drug delivery involving either chemical conjugation or physical complexation to polymeric carriers can result in prolonged circulation half-life, increased concentration at the site of action and reduced non-specific toxicity.14,15 For oral drug delivery, polymers provide the potential advantage of protecting drugs and macromolecules against degradation in the gastrointestinal tract and enhanced uptake.16 The absorption of polymer based oral drug delivery systems is dependent on properties such as size, surface chemistry, regiospecificity of a particle in the small intestine, the bio/-mucoadhesive property of the polymer, and permeation enhancement, among other factors.17 Polymer-based oral drug delivery systems primarily follow the following 3 routes for absorption through GI barrier: (1) through M cells; (2) via enterocyte mediated transcytosis; (3) via the paracellular route.17 It was also reported that the uptake and translocation of the polymer-based particles in the small intestine occur rapidly in less than an hour after administration.17
The polymers used in oral drug delivery can be widely classified as either hydrophilic or hydrophobic. The hydrophobic group comprises poly (esters), poly (cyanoacrylate), poly (orthoesters) and poly (phosphazenes). Examples of poly (esters) include poly (lactic acid-co-glycolic acid) (PLGA) and poly(ξ-caprolactone).18 The hydrophilic polymer group comprises of poly(alkyl methacrylates), poly(methacrylates), poly(acrylates), alginates, chitosan, polyphosphazene hydrogels, poly(ethylene glycol)s and poly(amidoamine) (PAMAM) dendrimers.18
PAMAM dendrimers
PAMAM dendrimers belong to a class of hydrophilic and hyperbranched polymers reported by Tomalia in 1979.19 They are synthesized by a divergent synthetic method that starts with an ethylene diamine core followed by amidoamine branching structure. This pattern leads alternatively to amine-terminated full generation or carboxyl-terminated half-generation dendrimers after each addition step in the synthesis.20 As the generation number increases, the number of functional groups doubles, while the diameter of dendrimer increases by about 1 nm.20,21 PAMAM dendrimers because of their highly tunable and controlled synthesis have the unique advantage of having very low polydispersities. A complete generation of PAMAM dendrimers has primary amine groups on the surface (pKa = 6.85) and tertiary amine groups within the core (pKa = 3.86).21 The availability of high-density functional groups on the surface of PAMAM dendrimers provides a unique advantage of surface modification of these polymers with various drugs, nucleic acids, and imaging agents.20,22
PAMAM dendrimers were shown to interact with microvasculature and extravasate through endothelium because of their intrinsic charge.23 The net surface charge of dendrimers is based on the nature of surface groups and the generation number of the dendrimers. The net surface charge of PAMAM dendrimers characterized by measuring their zeta potential was: 64.8 ±3.2 mV for G7-NH2 (amine surface), −42.0±1.2 mV for G6.5-COOH (carboxyl dendrimers) and 27.7±1 .1 mV for G7-OH (hydroxyl dendrimers).24 Due to their charge-based ability to interact with biological membranes, PAMAM dendrimers were shown to permeate across epithelial barriers of the gut, therefore, making them potential polymeric carriers for the delivery of drugs and macromolecules across the GI barrier.20 PAMAM dendrimers were also utilized as non-viral gene delivery vectors due to their nanoscale size and their ability to condense DNA thereby protecting DNA from in vivo environment.22,25 PAMAM dendrimers were also shown to be useful in other fields of nanomedicine such as electrochemical bio(sensing),26 and photodynamic therapy.27 However, PAMAM dendrimers suffer from shortcomings such as toxicity, which have limited their clinical and commercial acceptance. Careful consideration in overcoming the toxicity using surface modification could potentially transform PAMAM dendrimers into specific, selective, and biocompatible nanocarriers for delivery of therapeutics across various biological barriers.
This review focuses mainly on the use of PAMAM dendrimers as polymers for oral delivery with emphasis on their mechanism of transport, the influence of surface chemistry and toxicity after oral administration.
Mechanism of transepithelial transport
PAMAM dendrimer interaction with tight junctions
Initial studies of PAMAM dendrimer transport across Caco-2 cell monolayer and in situ perfusion models demonstrated that PAMAM dendrimers are transported across GI epithelium by both para- and transcellular routes.28-32 The detailed mechanistic studies were then performed to elucidate the interaction of PAMAM dendrimers with the tight junction of the epithelium. Tight junctions are protein complexes that include occludin, claudins, junctional-associated membrane protein (JAM), and zonula occludens proteins (ZO-1, ZO-2).33 Claudins are major structural components of tight junction and form a barrier with highly regulated charge-selective pores and play an important role in determining the permeability properties of epithelial and endothelial cells.34,35 Occludin associates with claudins and contributes to the assembly of tight junctions and the establishment of the paracellular barrier.36,37 A thick perijunctional F-actin support the integrity and barrier properties of differentiated epithelia.35
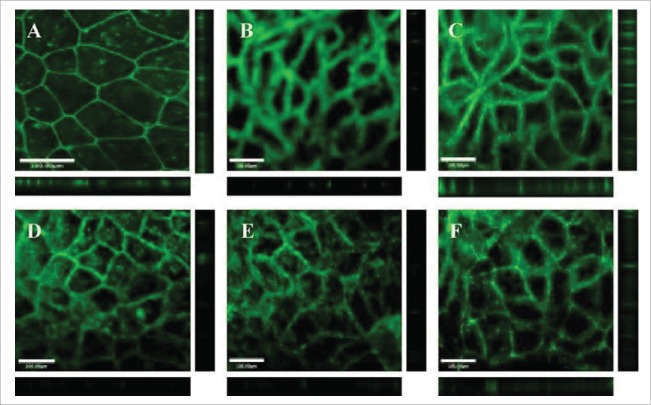
Kitchens et al, investigated whether treatment of Caco-2 cell monolayers with amine-terminated (G2.0-NH2), hydroxyl-terminated (G2.0-OH), and carboxyl-terminated (G1.5-COOH and G3.5-COOH) PAMAM dendrimers resulted in modulation of tight junctions.36 All dendrimers were investigated at 1.0 mM concentration. Changes in the barrier functions of tight junctions were evaluated by visualizing occludin and actin in Caco-2 cell monolayers after treatment with dendrimers using immunofluorescence microscopy. In untreated Caco-2 cells, occludin appears as a sharp honeycomb pattern at the cell apex indicating the intact, tight junction, whereas exposure of PAMAM dendrimers altered this localization causing either occludin diffusion along the lateral plasma membrane or its translocation in the cytosol (Fig. 1).36 Similarly, incubation of Caco-2 cell monolayers with rhodamine phalloidin, a probe specific for filamentous actin, revealed a clear, continuous staining pattern. After incubation with cationic and anionic PAMAM dendrimers, the cells show a clear disruption of actin staining indicating that dendrimers most likely resulted in disruption of perijunctional F-actin belt causing tight junction disassembly.36
Figure 1.
Dendrimers disrupt the integrity of tight junctions in cultured intestinal epithelial cells (A) Caco-2 cells with no polymer treatment. Caco-2 cells incubated for 120 min with 1.0 mM: (B) G2NH2; (C) G2OH; (D) G1.5COOH; (F) G3.5COOH. Main panels illustrate the XY plane; horizontal bars illustrate the Z plane; vertical bars illustrate the Z plane. Scale bars equal 100.00 µm. Figure obtained with permission from reference.36
In another study, Avaritt, et al, 2014, assessed tight junction proteins caludin-1, occludin, and ZO-1 in Caco-2 cell monolayers after treatment with G4-NH2 (0.01 mM) and G3.5-COOH (0.01 and 0.1 mM) dendrimers. Interestingly, for G4-NH2 no difference in staining occurred for actin, claudin-1, or occludin compared to the control. In contrast, G3.5-COOH at higher concentration (0.1 mM) significantly increased staining of all proteins investigated.38 Furthermore, Lin Y et al., 2011, quantitatively evaluated the effect of PAMAM dendrimers on the paracellular permeability of epithelial monolayers. The increase in concentration, incubation time and generation number of cationic and anionic dendrimers resulted in increased paracellular permeability of epithelial cell monolayers.39 Therefore, it was confirmed that PAMAM dendrimers modulate tight junction proteins and increased permeability partly due to the opening of tight junctions, which can be reversible depending on the concentration, generation and surface charge of the dendrimers.
Intracellular fate of PAMAM dendrimers
The most common pathway for intracellular uptake of macromolecules is the endocytic pathway. The cellular internalization and subcellular trafficking of FITC-conjugated PAMAM dendrimers have been investigated extensively.39-42 FITC-conjugated PAMAM dendrimers, when visualized by confocal microscopy, revealed that they are internalized within 20 mins, and differentially colocalized with endocytosis markers clathrin, EEA-1 (endosome antigen-1), and LAMP-1 (lysosomal-associated membrane protein 1).40 To further investigate the endocytosis mechanisms of PAMAM transport across Caco-2 cell monolayers, the influence of endocytosis inhibitors such as brefeldin, colchicine, filipin, and sucrose on uptake and transport of G4.0-NH2 across Caco-2 cells was studied.41 In the presence of endocytosis inhibitors, a significant reduction in G4.0-NH2 uptake and permeability was observed. Brefeldin A and colchicine reduced G4.0-NH2 uptake 2-fold and 3-fold respectively.41 Furthermore, co-incubation with filipin and sucrose reduced the rate of G4.0-NH2 uptake almost 3-fold.41 Therefore, it was concluded that in addition to paracellular transport, cationic dendrimers are also endocytosed.
To further investigate whether the surface charge has any influence on cellular uptake mechanism, PAMAM G3.5-COOH dendrimer cellular uptake, intracellular trafficking, transepithelial transport and tight junction modulation in Caco-2 cell monolayers was evaluated.42 Oregon green-conjugated PAMAM G3.5-COOH dendrimers' transport across Caco-2 cells was evaluated in the presence of chemical inhibitors blocking clathrin-, caveolin-, and dynamin-dependent endocytosis pathways. Phenylarsine oxide and mono dansyl cadaverine were clathrin inhibitors, filipin and genistein were caveolin inhibitors, and dynasore was dynamin inhibitor. In the presence of all inhibitors tested, G3.5-COOH showed a reduction in cellular uptake suggesting the involvement of both clathrin- and caveolin-mediated endocytosis pathways in cellular uptake.42 Overall, these studies suggest that PAMAM dendrimers are transported across Caco-2 cell monolayers by a combination of paracellular pathway and endocytosis.
Translocation of PAMAM dendrimers across isolated intestinal tissue and in vivo models
In addition to transepithelial transport studies of PAMAM dendrimers across Caco-2 monolayers, techniques such as in situ perfusion, isolated tissue models using the Ussing chamber set up, and in vivo models were also employed.28,43-46 In vivo models in comparison to Caco-2 cell monolayers comprise mucus layers, supportive mixed cell populations, and basement membrane. Furthermore, these models allow the comparison of differences in the segmental transport throughout different regions of the GI tract.45,47 125I and fluorescein isothiocyanate (FITC)-labeled PAMAM dendrimers were utilized in the majority of instances for evaluation of their uptake and transport across in vivo models.
In an initial study, transport of 125I-labeled anionic PAMAM dendrimers was studied using the everted intestinal sac system in the rat.28 The results have shown that cationic PAMAM dendrimers exhibited greater tissue uptake than their serosal transfer rates across everted rat intestinal sacs at each time point. Conversely, anionic dendrimers showed greater serosal transfer rates than their tissue uptake in the same model.28 This study confirmed the results from Caco-2 monolayer studies that PAMAM dendrimer's generation and surface charge influence their transepithelial transport.
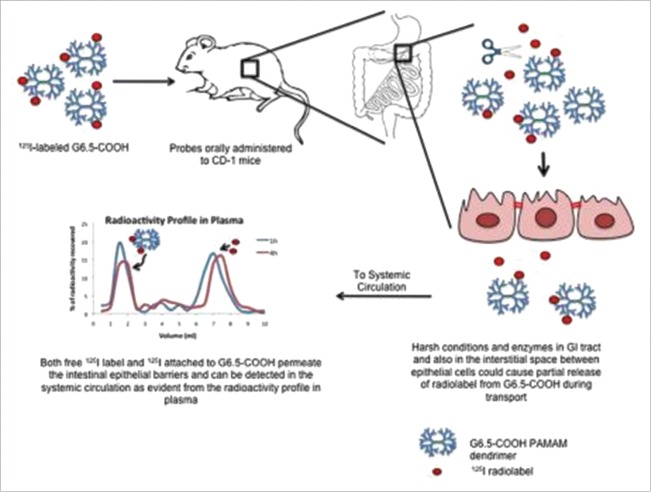
The evidence of oral translocation of 125I-labeled anionic G6.5-COOH dendrimers across GI epithelium was reported after oral administration in CD-1 mice.46 The schematic showing the fate of radiolabeled G6.5 dendrimers in the gastrointestinal tract and evidence of its systemic absorption is provided as Figure 2.46 In this study, the stability of the radiolabeled dendrimers was first evaluated in simulated gastric and intestinal conditions and in an in situ loop perfusion model of mouse GI tissue. These experiments were performed to investigate the possibility that enzymes and other molecules present in those scenarios could potentially cleave radiolabel off dendrimers. Results indicated that radiolabeled G6.5-COOH was relatively intact under simulated and in situ loop perfusion conditions.46 The result revealed that the oral bioavailability of 125I-labeled G6.5-COOH dendrimers plateaued off at about 4 hours and was found to be 9.4 % for 4 h.46
Figure 2.
Schematic depicting the fate of radiolabeled G6.5 dendrimers in the gastrointestinal tract and evidence of its subsequent systemic absorption. Figure obtained with permission from reference[46].
Studies involving transport of PAMAM dendrimers across isolated rat and human intestinal tissue using Ussing Chambers have been reported. In an initial study transepithelial transport of FITC-labeled PAMAM dendrimers across isolated rat jejunal mucosae was reported. Results demonstrated that the apparent permeability (Papp) values of FITC-G3.5 PAMAM dendrimers significantly increased over that of free FITC. However, the Papp of FITC-G4.0 dendrimers did not significantly increase over that of free FITC. Moreover, histological evaluation of isolated jejunal tissue after 120 min incubation in Ussing chambers with both anionic and cationic FITC-labeled dendrimers showed that there was no significant membrane disruption due to dendrimer treatments. All tissue samples showed an intact barrier, consistent with the retention of secretory ion transport capacity.45
In a follow-up study, the Papp of PAMAM dendrimers across isolated rat intestinal regional mucosae was evaluated using FITC-labeled dendrimers. Transport of dendrimers across jejunal and colonic regions of isolated rat intestinal mucosae was compared. Results showed that jejunal transport of dendrimers was higher than transport in colonic epithelium. Results from isolated tissue model were compared to Caco-2 monolayer model, and it was reported that monolayer Papp values of dendrimers were comparable to those of jejunal mucosae.43 The results of this study indicate that the transport in jejunal mucosae appears to be the greatest, and small intestine may be the most likely region to target for oral drug delivery using PAMAM dendrimers.
The ability to predict the human intestinal permeability of PAMAM dendrimers based on results obtained from isolated human intestinal tissue was first reported by Dallin et al.44 Human jejunal and colonic tissues received from colectomy, pancreatic duodenectomy, and Roux-en-Y gastric bypass surgery patients. The permeability of PAMAM dendrimers was evaluated along with the penetration enhancing effects on the small paracellular marker mannitol. Results indicated that at 1.0 mM concentration both G4-NH2 and G3.5-COOH dendrimers did not have a statistically significant higher permeability compared to free FITC controls in isolated human jejunum and colonic tissues.44 Therefore, this first in human tissue study suggests that PAMAM dendrimer oral drug delivery may be possible, but it may be limited to highly potent drugs.44
Toxicity of PAMAM dendrimers in the context of oral delivery
It is well reported that PAMAM dendrimers demonstrate a generation-, surface charge-, concentration-and incubation time-dependent cytotoxicity profile in Caco-2 cell monolayers.29 Based on other in vitro cytotoxicity studies, the rank order of cytotoxicity of PAMAM dendrimers is hydroxyl-terminated<carboxyl-terminated<amine-terminated systems.20,31 Cytotoxicity evaluation performed on Caco-2 cells using the lactate dehydrogenase (LDH) assay showed that carboxyl-terminated dendrimers of generations 3.5 and 4.5 were shown to be toxic only at a higher donor concentration of 10.0 mM compared to amine-terminated dendrimers of generations 3.0 and 4.0 which are toxic to 1.0 mM.29,30 The influence of cationic PAMAM G4-NH2 on Madin-Darby canine kidney (MDCK) cell monolayer integrity was performed by determining the permeability of mannitol across MDCK cells.32 Results indicated that at 100 µg/ml concentration of G4-NH2 dendrimer, mannitol permeability increased by 9-fold, suggesting that cell integrity is compromised in the presence of G4.32
Further evaluation of toxicity of PAMAM dendrimers was performed in other in vitro models such as fresh rat blood cells48 and 3-D kidney organoid culture model.49 The release of hemoglobin after addition of PAMAM dendrimers G1.5-G9.5 to fresh rat blood cells (RBCs) and incubation at 37°C for 1 h was spectrophotometrically determined. Results indicate that amine-terminated PAMAM dendrimers displayed concentration and generation-dependent hemolysis, and changes in red cell morphology. All cationic dendrimers except G 1.0 showed hemolytic activity above 1.0 mg/mL. Anionic dendrimers did not show any morphological changes to RBCs up to 2.0 mg/mL.48 In another in vitro study, 3-D kidney organoid proximal tubule cultures were created using isolated murine proximal tubule fractions suspended in a biomedical grade hyaluronic acid based hydrogel. The toxicity of G5-OH dendrimers was assessed using a clinical biomarker generation. Results indicate that G5-OH PAMAM dendrimers elicited in vivo-relevant kidney biomarkers and cell viability that closely reflect toxicity markers reported in vivo in rodent nephrotoxicity models exposed to same G5-OH dendrimers.49
Several in vivo studies were reported that were focused on the toxicity of PAMAM dendrimers of different generations and surface charge, after oral administration.24,46,50 PAMAM dendrimers of generations 3.5, 4.0, 6.5, and 7.0 corresponding to 3 different surface functionalities: carboxyl-, amine, and hydroxyl-terminated were administered orally and intravenously. The results have shown that orally administered doses of PAMAM dendrimers were tolerated at a higher dose when compared to intravenously administered doses. PAMAM G4-NH2 was tolerated up to 100 mg/kg after oral administration while anionic and hydroxyl-terminated PAMAM dendrimers of the corresponding generation were tolerated at 300 mg/kg. Furthermore, PAMAM G7.0-NH2 was found to be toxic at 50 mg/kg while anionic and hydroxyl-terminated PAMAM dendrimers of same generations were tolerated at 300 mg/kg.24,50 To eliminate the local toxicity of dendrimers as the cause of increased permeation resulting in enhanced oral bioavailability, an acute toxicity study after oral administration of G6.5-COOH PAMAM dendrimer was conducted using CD-1 mice. Results indicate that G6.5-COOH did not show any signs of toxicity after oral administration at a dose (1 mg/kg) employed in bioavailability studies.46
Surface modification of PAMAM dendrimers to reduce toxicity and enhance transepithelial transport
Because of their potential to transport across epithelial barriers, it would be desirable to enhance their transport without eliciting toxicity. This enhanced transport can be achieved by surface modification of PAMAM dendrimers using various small molecule ligands.20,22 The initial attempts in this direction involved conjugation of lauric acid to cationic PAMAM dendrimers, generation 2.0 to 4.0 and evaluation of their cytotoxicity and permeability across Caco-2 cell monolayers.51,52 Results have shown that conjugation of PAMAM dendrimers with 6 and 9 lauroyl chains per molecule of PAMAM reduced toxicity and improved permeability. The study involving investigation of the mechanism of transport of lauroyl conjugated dendrimers suggested that lauroyl moieties enhance penetration of dendrimers across Caco-2 cells.52 In an attempt to reduce toxicity, PAMAM dendrimers were partially- or fully- acetylated, and their transport across Caco-2 cells was evaluated.53 The results of this study suggest that the cytotoxicity of the cationic PAMAM G4-NH2 can be reduced by surface acetylation while maintaining permeability.
Amino acids ornithine and arginine were conjugated to PAMAM dendrimers and their permeability across Caco-2 and intestinal pig epithelial cell (IPEC-J2) monolayer were investigated.54,55 In both cell lines, there was no significant difference in cytotoxicity between polyamine-conjugated dendrimer and native dendrimers. Furthermore, a concentration and time-dependent enhancement in permeability was observed from apical to the basolateral side for the polyamine conjugated PAMAM dendrimers.54,55 Another proven strategy to improve biocompatibility and pharmacokinetics of polymeric drug carriers in vivo is PEGylation. PEGylation of anionic dendrimers G3.5-COOH and G4.5-COOH with PEG 750 kDa did not significantly alter cytotoxicity up to a concentration of 0.1 mM.56 Overall, the above studies indicate that surface modification of PAMAM dendrimers can result in a reduction of cytotoxicity while either maintaining or increasing transepithelial permeability.
PAMAM-dendrimer-drug conjugates and complexes for oral delivery
Due to the availability of functional groups and presence of void volume inside dendrimers, therapeutics can be covalently attached to- or complexed by ion-ion and van der Waals interactions or entrapped in void spaces of PAMAM dendrimers. During the past decade, there have been many reports of such PAMAM dendrimer-drug conjugates and complexes. The investigations have included both in vitro and in vivo evaluation of transepithelial transport of PAMAM drug conjugates and complexes. Discussion about all PAMAM drug conjugates and complexes is out of the scope of this review. However, a few examples will be discussed below, and a list is provided in Table 1.
Table 1.
List of PAMAM dendrimer-drug conjugates investigated for oral delivery.
| PAMAM-Drug | Year | Conjugation or Complexation | Model | Ref |
|---|---|---|---|---|
| PAMAM G0,G1,G2-quercetin | 2016 | Complexation | In vivo-rat | 63 |
| PEG-PAMAM-probucol in nanostructured lipid carriers | 2015 | Co-delivery | In vivo-mouse | 64 |
| PAMAM-resveratrol | 2015 | Complexation | In vitro-stability | 65 |
| PAMAM G0,G1,G2-puerarin | 2013 | Complexation | In vivo-rat | 66 |
| PAMAM G4.0, G3.5-camptothecin | 2013 | Co-delivery | In vivo-rat | 61 |
| PEG-PAMAM-Simvastatin | 2013 | Complexation | In vivo-rat | 67 |
| PAMAM G1.5,G2,G2.5,G3-silybin | 2011 | Complexation | In vivo-rat | 68 |
| PAMAM-SN38 | 2011 | Covalent conjugation | In vitro-Caco-2 | 60 |
| PAMAM-doxorubicin | 2008 | Complexation | In vivo-rat | 58 |
| PAMAM-naproxen | 2007 | Covalent conjugation | In vitro-Caco-2 | 69 |
| PAMAM-propranolol | 2003 | Covalent conjugation | In vitro-Caco-2 | 57 |
An initial study reported conjugation of propranolol, a poorly soluble drug, and a p-glycoprotein (P-up) substrate, with PAMAM G3.0-NH2 or lauroyl-modified PAMAM G3.0-NH2 using a chloroacetyl spacer. The results of this study showed that conjugation of propranolol with dendrimers enhanced solubilization overcame P-gp efflux and increased apical to basolateral transport of the dendrimers across Caco-2 monolayers.57 In another study, oral absorption of PAMAM G3.0-NH2-doxorubicin conjugate was investigated in rats. The absorption kinetics was evaluated using a simple non-compartmental model using plasma concentration vs. time data after oral gavage of PAMAM-doxorubicin conjugates. The results reported a 300 fold increase in bioavailability of the doxorubicin when delivered as PAMAM-doxorubicin conjugate as compared to the free drug following a single oral dose in rats.58
Studies in our lab involved covalent conjugation as well as complexation of 7-ethyl-10-hydroxy-camptothecin (SN-38), a potent topoisomerase inhibitor and a biologically active metabolite of irinotecan hydrochloride, with PAMAM dendrimers. Initially, SN-38 was complexed to PAMAM G4.0-NH2, and its permeability was assessed across Caco-2 monolayers.59 The results indicated that PAMAM G4.0-NH2 – SN-38 conjugate showed up to 10-fold higher permeability and 100-fold higher uptake than free SN-38. However, the complex was not stable under acidic conditions.59 In a follow-up study, SN-38 was covalently conjugated to PAMAM G3.5-COOH with glycine and β-alanine spacers and their transepithelial transport was evaluated on Caco-2 cell monolayers.60 G3.5-Gly-SN38 at 100 µM and G3.5- βala-SN38 at 10 µM and 100 µM showed a statistically significant increase in apical to basolateral SN-38 flux relative to free SN-38 (p < 0 .001).60 However, the SN-38 flux was concentration dependent for G3.5-gly-SN38 while it was unchanged for G3.5-βala-SN38 between treatment with 10 and 100 µM concentrations. The data, therefore, suggests that conjugate with glycine linker may be transported primarily by a concentration-driven process, such as paracellular diffusion and conjugate with β-alanine linker may follow a saturable process such as transcellular transport.60
A more recent study involved investigation of PAMAM dendrimers as absorption enhancers for oral delivery of camptothecin, an anti-cancer agent with low oral bioavailability and gastrointestinal toxicity.61 In this study, camptothecin (5mg/kg) was formulated and co-delivered with cationic G4.0-NH2, anionic G3.5-COOH PAMAM dendrimers in CD-1 mice.61 Both cationic and anionic dendrimers resulted in an approximate 2 to 3-fold oral absorption enhancement of camptothecin in vivo at 2 h. However, PAMAM dendrimers alone did not cause an increase in blood levels of the paracellular uptake marker 14C-mannitol at 2 h suggesting that, at the doses tested, tight junction modulation was not observed in vivo and that the increase in absorption of camptothecin was not due to the opening of tight junctions.61
Future outlook
The oral administration remains the most patient compliant route for therapeutics. Many of the major drawbacks associated with oral delivery can be overcome using polymer based delivery systems. PAMAM dendrimers are one such polymer that have shown promise in enhancing oral delivery of drugs with poor bioavailability. Research in various in vitro and in vivo models has shown that PAMAM dendrimers have the ability to enhance transepithelial transport of therapeutics. The major challenges that involve clinical translation of PAMAM dendrimers as efficient oral delivery carriers are their toxicity and biocompatibility. There is a need for strategies to mitigate the toxicity of dendrimers while maintaining their permeability across the transepithelial barrier. Surface charge is determined to be the most dominant reason for toxicity of dendrimers. Therefore, strategies to alter the surface of PAMAM dendrimers using novel chemistries would be of utmost importance for the success of these carriers as oral delivery carriers in a clinical setting.
Permeability experiments on isolated human tissues have been reported to have a strong correlation to fraction absorbed in humans.62 Evaluation of PAMAM dendrimer permeability across isolated human tissue revealed important aspects of their transepithelial transport that influence their translation to the clinic. Based on correlation curves, an overall predicted fraction absorbed for 13-14 kDa dendrimers was approximately 30% in humans. However, this value may be an overestimation because the correlation curves are prepared from Papp values of small molecular weight pharmaceuticals and markers across intestinal epithelial barriers and have inherent assumptions that these probes do not influence the transport properties of epithelial barriers.
Another major area involves appropriate analytical techniques for quantification of intact and degraded PAMAM dendrimers in various biological fluids and tissues after oral administration. Current methods that are based on either radiolabeling or conjugation with a fluorophore are cumbersome, lack specificity, and often suffer from stability issues. The design and development of linker chemistries for PAMAM dendrimer-drug conjugates play a vital role, as the linker must be stable in the GI tract and the blood stream, but susceptible to cleavage at the target site of action.
Based on published literature and experimental evidence from our lab, PAMAM dendrimers can be beneficial for a) moderately enhancing the intestinal permeability of highly potent drugs with poor oral bioavailability, b) oral delivery of targetable systems intended for site-specific release of drugs, and c) for delivery of polymer-therapeutics along with penetration enhancement.
In conclusion, PAMAM dendrimers can provide a platform for oral drug delivery, provided their drawbacks can be overcome by strategies such as optimizing the dose of the carrier and drug, surface modification to overcome toxicity, reliable analytical techniques for detection, appropriate design of site-specific linkers, and employment of penetration enhancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Support was provided in part from a National Institutes of Health grant R01ES024681.
References
- [1].Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev 2016; PMID:26772138 [DOI] [PubMed] [Google Scholar]
- [2].GBI Research Oral drug delivery market report: Patients to see innovations. Contract Pharma. 2012 May 30 [cited 2016 Jan 7]. Available from http://www.contractpharma.com/issues/2012-06/view_features/oral-drug-delivery-market-report
- [3].Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol 2008; 22:391-409; PMID:18492562; http://dx.doi.org/ 10.1016/j.bpg.2007.11.002 [DOI] [PubMed] [Google Scholar]
- [4].Al-Hilal TA, Alam F, Byun Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliv Rev 2013; 65:845-64; PMID:23220326; http://dx.doi.org/ 10.1016/j.addr.2012.11.002 [DOI] [PubMed] [Google Scholar]
- [5].Balimane PV, Chong S. Cell culture-based models for intestinal permeability: a critique. Drug Discov Today 2005; 10:335-43; PMID:15749282; http://dx.doi.org/ 10.1016/S1359-6446(04)03354-9 [DOI] [PubMed] [Google Scholar]
- [6].Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14:141-53; PMID:24566914; http://dx.doi.org/ 10.1038/nri3608 [DOI] [PubMed] [Google Scholar]
- [7].Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev 2012; 64:557-70; PMID:22212900; http://dx.doi.org/ 10.1016/j.addr.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 1998; 60:143-59; PMID:9558458; http://dx.doi.org/ 10.1146/annurev.physiol.60.1.143 [DOI] [PubMed] [Google Scholar]
- [9].Yun Y, Cho YW, Park K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Deliv Rev 2013; 65:822-32; PMID:23123292; http://dx.doi.org/ 10.1016/j.addr.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Russell-Jones GJ. The potential use of receptor-mediated endocytosis for oral drug delivery. Adv Drug Deliv Rev 2001; 46:59-73; PMID:11259833; http://dx.doi.org/ 10.1016/S0169-409X(00)00127-7 [DOI] [PubMed] [Google Scholar]
- [11].Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2001; 2:149-59; PMID:11260520; http://dx.doi.org/ 10.1034/j.1600-0854.2001.020301.x [DOI] [PubMed] [Google Scholar]
- [12].Zaro JL. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J 2015; 17:83-92; PMID:25269430; http://dx.doi.org/ 10.1208/s12248-014-9670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle technologies for oral drug delivery. Clin Gastroenterol Hepatol 2014; 12:1605-10; PMID:24981782; http://dx.doi.org/ 10.1016/j.cgh.2014.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duncan R. Polymer conjugates for drug targeting. From inspired to inspiration! J Drug Target 2006; 14:333-5; PMID:17092833; http://dx.doi.org/ 10.1080/10611860600833880 [DOI] [PubMed] [Google Scholar]
- [15].Duncan R, Ringsdorf H, Satchi-Fainaro R. Polymer therapeutics–polymers as drugs, drug and protein conjugates and gene delivery systems: past, present and future opportunities. J Drug Target 2006; 14:337-41; PMID:17092834; http://dx.doi.org/ 10.1080/10611860600833856 [DOI] [PubMed] [Google Scholar]
- [16].Andrianov AK, Payne LG. Polymeric carriers for oral uptake of microparticulates. Adv Drug Deliv Rev 1998; 34:155-70; PMID:10837676; http://dx.doi.org/ 10.1016/S0169-409X(98)00038-6 [DOI] [PubMed] [Google Scholar]
- [17].Bakhru SH, Furtado S, Morello AP, Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev 2013; 65:811-21; PMID:23608641; http://dx.doi.org/ 10.1016/j.addr.2013.04.006 [DOI] [PubMed] [Google Scholar]
- [18].Truong-Le V, Lovalenti PM, Abdul-Fattah AM. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Adv Drug Deliv Rev 2015; 93:95-108; PMID:26277263; http://dx.doi.org/ 10.1016/j.addr.2015.08.001 [DOI] [PubMed] [Google Scholar]
- [19].Tomalia DA. A new class of polymers: starburst-dendritic macromolecules. Polymer 1985; 17:117-32; http://dx.doi.org/ 10.1295/polymj.17.117 [DOI] [Google Scholar]
- [20].Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev 2012; 64:571-88; PMID:21983078; http://dx.doi.org/ 10.1016/j.addr.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tomalia DA, Naylor AM, Goddard WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed 1990; 29:138-75; http://dx.doi.org/ 10.1002/anie.199001381 [DOI] [Google Scholar]
- [22].Yellepeddi VK, Kumar A, Palakurthi S. Surface modified poly(amido)amine dendrimers as diverse nanomolecules for biomedical applications. Expert Opin Drug Deliv 2009; 6:835-50; PMID:19637972; http://dx.doi.org/ 10.1517/17425240903061251 [DOI] [PubMed] [Google Scholar]
- [23].El-Sayed M, Kiani MF, Naimark MD, Hikal AH, Ghandehari H. Extravasation of poly(amidoamine) (PAMAM) dendrimers across microvascular network endothelium. Pharm Res 2001; 18:23-8; PMID:11336349; http://dx.doi.org/ 10.1023/A:1011066408283 [DOI] [PubMed] [Google Scholar]
- [24].Thiagarajan G, Greish K, Ghandehari H. Charge affects the oral toxicity of poly(amidoamine) dendrimers. Eur J Pharm Biopharm 2013; 84:330-4; PMID:23419816; http://dx.doi.org/ 10.1016/j.ejpb.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kumar A, Yellepeddi VK, Davies GE, Strychar KB, Palakurthi S. Enhanced gene transfection efficiency by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. Int J Pharm 2010; 392:294-303; PMID:20363307; http://dx.doi.org/ 10.1016/j.ijpharm.2010.03.054 [DOI] [PubMed] [Google Scholar]
- [26].Bahadir EB, Sezginturk MK. Poly(amidoamine) (PAMAM): An emerging material for electrochemical bio(sensing) applications. Talanta 2016; 148:427-38; PMID:26653469; http://dx.doi.org/ 10.1016/j.talanta.2015.11.022 [DOI] [PubMed] [Google Scholar]
- [27].Narsireddy A, Vijayashree K, Adimoolam MG, Manorama SV, Rao NM. Photosensitizer and peptide-conjugated PAMAM dendrimer for targeted in vivo photodynamic therapy. Int J Nanomedicine 2015; 10:6865-78; PMID:26604753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wiwattanapatapee R, Carreno-Gomez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm Res 2000; 17:991-8; PMID:11028947; http://dx.doi.org/ 10.1023/A:1007587523543 [DOI] [PubMed] [Google Scholar]
- [29].El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Release 2002; 81:355-65; PMID:12044574; http://dx.doi.org/ 10.1016/S0168-3659(02)00087-1 [DOI] [PubMed] [Google Scholar]
- [30].El-Sayed M, Rhodes CA, Ginski M, Ghandehari H. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int J Pharm 2003; 265:151-7; PMID:14522128; http://dx.doi.org/ 10.1016/S0378-5173(03)00391-0 [DOI] [PubMed] [Google Scholar]
- [31].Kitchens KM, El-Sayed ME, Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv Drug Deliv Rev 2005; 57:2163-76; PMID:16289433; http://dx.doi.org/ 10.1016/j.addr.2005.09.013 [DOI] [PubMed] [Google Scholar]
- [32].Tajarobi F, El-Sayed M, Rege BD, Polli JE, Ghandehari H. Transport of poly amidoamine dendrimers across Madin-Darby canine kidney cells. Int J Pharm 2001; 215:263-7; PMID:11250111; http://dx.doi.org/ 10.1016/S0378-5173(00)00679-7 [DOI] [PubMed] [Google Scholar]
- [33].Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 1995; 269:G467-75; PMID:7485497 [DOI] [PubMed] [Google Scholar]
- [34].Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525-69; PMID:23589827; http://dx.doi.org/ 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1:a002584; PMID:20066090; http://dx.doi.org/ 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm Res 2006; 23:2818-26; PMID:17094034; http://dx.doi.org/ 10.1007/s11095-006-9122-2 [DOI] [PubMed] [Google Scholar]
- [37].Knutson L, Knutson F, Knutson T. Permeability in the gsatrointestinal tract In: Lennernas JBDH, ed. Oral drug asbsorption: prediction and assessment. New York: Marcel Dekker, 2000:11-6. [Google Scholar]
- [38].Avaritt BR, Swaan PW. Intracellular Ca2+ release mediates cationic but not anionic poly(amidoamine) (PAMAM) dendrimer-induced tight junction modulation. Pharm Res 2014; 31:2429-38; PMID:24648136; http://dx.doi.org/ 10.1007/s11095-014-1338-y [DOI] [PubMed] [Google Scholar]
- [39].Lin YL, Khanafer K, El-Sayed ME. Quantitative evaluation of the effect of poly(amidoamine) dendrimers on the porosity of epithelial monolayers. Nanoscale 2010; 2:755-62; PMID:20648321; http://dx.doi.org/ 10.1039/b9nr00407f [DOI] [PubMed] [Google Scholar]
- [40].Kitchens KM, Foraker AB, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 cells. Pharm Res 2007; 24:2138-45; PMID:17701324; http://dx.doi.org/ 10.1007/s11095-007-9415-0 [DOI] [PubMed] [Google Scholar]
- [41].Kitchens KM, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol Pharm 2008; 5:364-9; PMID:18173246; http://dx.doi.org/ 10.1021/mp700089s [DOI] [PubMed] [Google Scholar]
- [42].Goldberg DS, Ghandehari H, Swaan PW. Cellular entry of G3.5 poly (amido amine) dendrimers by clathrin- and dynamin-dependent endocytosis promotes tight junctional opening in intestinal epithelia. Pharm Res 2010; 27:1547-57; PMID:20411406; http://dx.doi.org/ 10.1007/s11095-010-0153-3 [DOI] [PubMed] [Google Scholar]
- [43].Hubbard D, Bond T, Ghandehari H. Regional morphology and transport of PAMAM dendrimers across isolated rat intestinal tissue. Macromol Biosci 2015; 15:1735-43; PMID:26332343; http://dx.doi.org/ 10.1002/mabi.201500225 [DOI] [PubMed] [Google Scholar]
- [44].Hubbard D, Enda M, Bond T, Moghaddam SP, Conarton J, Scaife C, Volckmann E, Ghandehari H. Transepithelial transport of PAMAM sendrimers across isolated human intestinal tissue. Mol Pharm 2015; 12:4099-107; PMID:26414679; http://dx.doi.org/ 10.1021/acs.molpharmaceut.5b00541 [DOI] [PubMed] [Google Scholar]
- [45].Hubbard D, Ghandehari H, Brayden DJ. Transepithelial transport of PAMAM dendrimers across isolated rat jejunal mucosae in ussing chambers. Biomacromolecules 2014; 15:2889-95; PMID:24992090; http://dx.doi.org/ 10.1021/bm5004465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Thiagarajan G, Sadekar S, Greish K, Ray A, Ghandehari H. Evidence of oral translocation of anionic G6.5 dendrimers in mice. Mol Pharm 2013; 10:988-98; PMID:23286733; http://dx.doi.org/ 10.1021/mp300436c [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Borchardt SPR, Wilson G. Models for assessing drug absorption and metabolism. Pharmaceutical Biotechnology. New York and London: Plenum Press, 1996. [Google Scholar]
- [48].Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release 2000; 65:133-48; PMID:10699277; http://dx.doi.org/ 10.1016/S0168-3659(99)00246-1 [DOI] [PubMed] [Google Scholar]
- [49].Astashkina AI, Jones CF, Thiagarajan G, Kurtzeborn K, Ghandehari H, Brooks BD, Grainger DW. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials 2014; 35:6323-31; PMID:24814424; http://dx.doi.org/ 10.1016/j.biomaterials.2014.04.060 [DOI] [PubMed] [Google Scholar]
- [50].Greish K, Thiagarajan G, Herd H, Price R, Bauer H, Hubbard D, Burckle A, Sadekar S, Yu T, Anwar A, et al.. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology 2012; 6:713-23; PMID:21793770; http://dx.doi.org/ 10.3109/17435390.2011.604442 [DOI] [PubMed] [Google Scholar]
- [51].Jevprasesphant R, Penny J, Attwood D, McKeown NB, D'Emanuele A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm Res 2003; 20:1543-50; PMID:14620505; http://dx.doi.org/ 10.1023/A:1026166729873 [DOI] [PubMed] [Google Scholar]
- [52].Jevprasesphant R, Penny J, Attwood D, D'Emanuele A. Transport of dendrimer nanocarriers through epithelial cells via the transcellular route. J Control Release 2004; 97:259-67; PMID:15196753; http://dx.doi.org/ 10.1016/j.jconrel.2004.03.022 [DOI] [PubMed] [Google Scholar]
- [53].Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug Chem 2007; 18:2054-60; PMID:17960872; http://dx.doi.org/ 10.1021/bc0603889 [DOI] [PubMed] [Google Scholar]
- [54].Pisal DS, Yellepeddi VK, Kumar A, Kaushik RS, Hildreth MB, Guan X, Palakurthi S. Permeability of surface-modified polyamidoamine (PAMAM) dendrimers across Caco-2 cell monolayers. Int J Pharm 2008; 350:113-21; PMID:17913410; http://dx.doi.org/ 10.1016/j.ijpharm.2007.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pisal DS, Yellepeddi VK, Kumar A, Palakurthi S. Transport of surface engineered polyamidoamine (PAMAM) dendrimers across IPEC-J2 cell monolayers. Drug Deliv 2008; 15:515-22; PMID:18720134; http://dx.doi.org/ 10.1080/10717540802321826 [DOI] [PubMed] [Google Scholar]
- [56].Sweet DM, Kolhatkar RB, Ray A, Swaan P, Ghandehari H. Transepithelial transport of PEGylated anionic poly(amidoamine) dendrimers: implications for oral drug delivery. J Control Release 2009; 138:78-85; PMID:19393702; http://dx.doi.org/ 10.1016/j.jconrel.2009.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].D'Emanuele A, Jevprasesphant R, Penny J, Attwood D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J Control Release 2004; 95:447-53; PMID:15023456; http://dx.doi.org/ 10.1016/j.jconrel.2003.12.006 [DOI] [PubMed] [Google Scholar]
- [58].Ke W, Zhao Y, Huang R, Jiang C, Pei Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci 2008; 97:2208-16; PMID:17879294; http://dx.doi.org/ 10.1002/jps.21155 [DOI] [PubMed] [Google Scholar]
- [59].Kolhatkar RB, Swaan P, Ghandehari H. Potential oral delivery of 7-ethyl-10-hydroxy-camptothecin (SN-38) using poly(amidoamine) dendrimers. Pharm Res 2008; 25:1723-9; PMID:18438703; http://dx.doi.org/ 10.1007/s11095-008-9572-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Goldberg DS, Vijayalakshmi N, Swaan PW, Ghandehari H. G3.5 PAMAM dendrimers enhance transepithelial transport of SN38 while minimizing gastrointestinal toxicity. J Control Release 2011; 150:318-25; PMID:21115079; http://dx.doi.org/ 10.1016/j.jconrel.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sadekar S, Thiagarajan G, Bartlett K, Hubbard D, Ray A, McGill LD, Ghandehari H. Poly(amido amine) dendrimers as absorption enhancers for oral delivery of camptothecin. Int J Pharm 2013; 456:175-85; PMID:23933439; http://dx.doi.org/ 10.1016/j.ijpharm.2013.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sjoberg A, Lutz M, Tannergren C, Wingolf C, Borde A, Ungell AL. Comprehensive study on regional human intestinal permeability and prediction of fraction absorbed of drugs using the Ussing chamber technique. Eur J Pharm Sci 2013; 48:166-80; PMID:23103351; http://dx.doi.org/ 10.1016/j.ejps.2012.10.007 [DOI] [PubMed] [Google Scholar]
- [63].Madaan K, Lather V, Pandita D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv 2016; 23:254-62; PMID:24845475; http://dx.doi.org/ 10.3109/10717544.2014.910564 [DOI] [PubMed] [Google Scholar]
- [64].Qi R, Li YZ, Chen C, Cao YN, Yu MM, Xu L, He B, Jie X, Shen WW, Wang YN, et al.. G5-PEG PAMAM dendrimer incorporating nanostructured lipid carriers enhance oral bioavailability and plasma lipid-lowering effect of probucol. J Control Release 2015; 210:160-8; PMID:26003044; http://dx.doi.org/ 10.1016/j.jconrel.2015.05.281 [DOI] [PubMed] [Google Scholar]
- [65].Chauhan AS. Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann N Y Acad Sci 2015; 1348:134-40; PMID:26173478; http://dx.doi.org/ 10.1111/nyas.12816 [DOI] [PubMed] [Google Scholar]
- [66].Gu L, Wu Z, Qi X, He H, Ma X, Chou X, Wen X, Zhang M, Jiao F. Polyamidomine dendrimers: an excellent drug carrier for improving the solubility and bioavailability of puerarin. Pharm Dev Technol 2013; 18:1051-7; PMID:22303809; http://dx.doi.org/ 10.3109/10837450.2011.653822 [DOI] [PubMed] [Google Scholar]
- [67].Kulhari H, Kulhari DP, Prajapati SK, Chauhan AS. Pharmacokinetic and pharmacodynamic studies of poly(amidoamine) dendrimer based simvastatin oral formulations for the treatment of hypercholesterolemia. Mol Pharm 2013; 10:2528-33; PMID:23692066; http://dx.doi.org/ 10.1021/mp300650y [DOI] [PubMed] [Google Scholar]
- [68].Huang X, Wu Z, Gao W, Chen Q, Yu B. Polyamidoamine dendrimers as potential drug carriers for enhanced aqueous solubility and oral bioavailability of silybin. Drug Dev Ind Pharm 2011; 37:419-27; PMID:20942611; http://dx.doi.org/ 10.3109/03639045.2010.518150 [DOI] [PubMed] [Google Scholar]
- [69].Najlah M, Freeman S, Attwood D, D'Emanuele A. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int J Pharm 2007; 336:183-90; PMID:17188439; http://dx.doi.org/ 10.1016/j.ijpharm.2006.11.047 [DOI] [PubMed] [Google Scholar]