ABSTRACT
Intestinal permeation enhancers (PEs) are key components in ∼12 oral peptide formulations in clinical trials for a range of molecules, primarily insulin and glucagon-like-peptide 1 (GLP-1) analogs. The main PEs comprise medium chain fatty acid-based systems (sodium caprate, sodium caprylate, and N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC)), bile salts, acyl carnitines, and EDTA. Their mechanism of action is complex with subtle differences between the different molecules. With the exception of SNAC and EDTA, most PEs fluidize the plasma membrane causing plasma membrane perturbation, as well as enzymatic and intracellular mediator changes that lead to alteration of intestinal epithelial tight junction protein expression. The question arises as to whether PEs can cause irreversible epithelial damage and tight junction openings sufficient to permit co-absorption of payloads with bystander pathogens, lipopolysaccharides and its fragment, or exo- and endotoxins that may be associated with sepsis, inflammation and autoimmune conditions. Most PEs seem to cause membrane perturbation to varying extents that is rapidly reversible, and overall evidence of pathogen co-absorption is generally lacking. It is unknown however, whether the intestinal epithelial damage-repair cycle is sustained during repeat-dosing regimens for chronic therapy.
KEYWORDS: epithelial toxicity, intestinal permeation enhancers, medium chain fatty acids, oral peptide delivery, sodium caprate, SNAC
Introduction
Pharmaceutical companies have renewed their interest in oral peptides technologies and two biotech companies have formulations that reach primary endpoints in recently-completed Phase III clinical trials for oral salmon calcitonin (sCT) and octreotide.1 Many formulations in trials contain intestinal permeation enhancers (PEs) to assist the poorly-permeable payloads including insulin and glucagon-like Peptide 1 (GLP-1) analogs in overcoming the intestinal epithelial barrier. The main safety concerns regarding the use of PEs are due to associated intestinal epithelial damage that may cause intestinal inflammation, as well as co-absorption of pathogenic “bystander” agents and toxins. This review will focus on PEs that are in formulations which are currently in clinical trials. We discuss whether they truly cause irreversible epithelial damage of clinical relevance and also as to whether there is any evidence to support the widely-held assertion that they permit entry of xenobiotics. For more information about the current status of oral formulations in clinical trials, see a number of recent reviews.1-5
Intestinal PEs in Clinical Trials for Oral Peptides
The mechanism of action is only partly known for PEs in clinical trials and, in many cases, there is consensus that most work in a multitude of ways involving modulation of tight junctions (TJs) arising from initial fluidization of small intestinal epithelial plasma membranes. The main candidates in oral peptide trials are the medium chain fatty acid (MCFA)-based sodium caprate (C10), sodium caprylate (C8), and the C8 derivative (SNAC), as well as acyl carnitines, EDTA, and selected bile salts.6,7 EDTA is different from the other PEs in that it acts by chelating calcium and thus affecting the TJs. In their seminal 1994 review on the toxicology of PEs, Curatolo and Ochoa remind us of the fact that many PEs are surfactants with a history of use in man in drug products and in the food processing industry, and that extensive safety testing was carried out on many of these PEs in the 1950s.8
Fatty Acids
Sodium caprate (C10) is the sodium salt of capric acid and is one of the most widely researched PEs.9-13 Capric acid can be found in dairy products such as milk, at levels as high as 0.2mM and in oils such as coconut. It is an approved food additive with no daily limit set by the World Health Organization and the US Food and Drugs Administration.10,14
C10 is the main PE found in Merrion Pharmaceutical's (Dublin, Ireland) Gastro-Intestinal Permeating Enhancement Technology (GIPET®) technology, a solid dose enteric coated matrix tablet with very high concentrations of the MCFA and the payload; this system was licensed in full to Novo-Nordisk. C10 along with sodium caprylate (C8) is also present in NOD Pharma's (Shanghai, China) oral peptide formulation.2 GIPET® I consists of an enteric coated matric tablet, whereas GIPET® II is a microemulsion in an enteric coated soft gel capsule containing C8 and C10.15 These fatty acids are mild surfactants and act as detergents by fluidising the plasma membrane, leading to an increase in intracellular calcium and alteration of claudin-5 and tricellulin expression in TJs.10,12,16 Emesis, weight loss and a reduction in food consumption were observed in studies involving dogs administered GIPET® I containing a dose of 0.9g/kg/day, but this was likely due to the fact that the dogs ingested 18 tablets a day. No morphological changes were showed upon histological examination of canine intestinal tissue,17 and further studies involving dogs given daily doses of GIPET® I and II were well tolerated.
Chiasma's (Jerusalem, Israel) Transient Permeability Enhancer (TPE®) consists of a combination of pharmaceutical excipients that result in an oily suspension (OS) of hydrophilic particles in a hydrophobic matrix. TPE® has successfully completed pharmacokinetic and pharmacological studies in rats and monkeys and subsequently a multicentre 13 month Phase III trial in 155 acromegaly patients with the somatostatin analog, oral octreotide (Mycappsa™).18,19 It consists of octreotide solubilized with C8 and other common excipients in the hydrophilic component. As with other MCFAs, C8 in the OS temporarily rearranged zonula occludens-1 (ZO-1) in the tight junction.20 Furthermore, to establish safety of long term use, daily doses of oral OS were administered in high doses to Cynomolgus monkeys for 9 months.20 There were no significant changes in bodyweight, electrocardiogram, ophthalmic parameters, haematological scoring or clinical pathology. Adverse effects such as nausea were noted in the Phase III trial, most of which occurred in the first 3 months, but these were comparable to the injectable peptide counterpart, suggesting these were not due to sodium caprylate.
SNAC
The Eligen™ technology (Emisphere, NJ, USA) comprises primarily the 3 low molecular weight molecules, 5–CNAC 8–(N-2-hydroxy-5-chloro-benzyol)-amino-caprylate), SNAC (N-[8-(2-hydroxybenzoyl)amino] caprylate and 4-CNAB (N-(4-chlorosalicyylol)-4-aminobutyrate) for oral co-administration with peptides including GLP-1, insulin, human parathyroid hormone (PTH), and calcitonin.4,21,22 The Eligen® molecules are thought to work by non-covalently complexing with the peptide to form a hydrophobic ion-pair, without reducing peptide bioactivity. SNAC also reduced transepithelial electrical resistance (TEER) and increased membrane fluidity in Caco-2 monolayers and increased the transepithelial transport of heparin in vitro and in vivo.23-25 That TEER was reduced in Caco-2 monolayers more reflected damage caused by the high concentrations of SNAC required to induce permeability increases in vitro rather than any specific effect on tight junction openings. Riley et al., carried out a sub-chronic oral toxicity test of SNAC in rats and found a no observed adverse effect (NOAEL) level of 1 g/kg/day in rats, with a massive dose of 2 g/kg/day eventually causing significant mortality.26 Gastrointestinal (GI) effects such as emesis and diarrhea were observed in studies involving monkeys at a huge dose of ≥ 1.8 g/kg/day. A number of clinical studies have been published involving SNAC and also the related enhancer, 5-CNAC.21,27–29 One study was carried out in which sCT was administered orally with 200mg 5-CNAC twice daily over 2 weeks to 36 men and 37 postmenopausal women.21 Forty-four mild adverse events were observed, some of which were in the placebo group. Analysis of blood, faeces and urine showed no effects on organs. Adverse events relating to the GI tract were reported across all study groups. GI disturbances such as nausea, diarrhea, abdominal pain, vomiting, loose stools and constipation were also shown in a study with 5-CNAC involving 277 postmenopausal women with 32% reporting at least one event.28 Similar effects were seen in another 5-CNAC study which included over 2000 participants and concluded that there were more adverse GI reports in the patients receiving 0.8mg sCT formulated in the 5-CNAC carrier orally compared to the matched placebo (26-30% compared to 46%).29 It was thought that these adverse events were related to sCT as they were comparable to those observed with subcutaneous injection of sCT.
It is worth noting that the amount of 5-CNAC administered in clinical trials to humans (200mg twice daily) would equate to 0.005g/kg/day for a 80kg man which is 200 times lower than the NOEL of 1g/kg/day found in rats and doses found to be toxic in monkeys (1.8g/kg/day).29 This suggests that the concentration needed to be efficacious in humans is below that which has been found to be toxic in animals.
SNAC is already on the market in an oral product containing large doses of the agent combined in tablets with vitamin B12.30 It has also recently been tested in Phase II trials for the long-acting GLP-1 analog, semaglutide, by Novo Nordisk1 and has now entered Phase III.31
Acyl Carnitines
Carnitines, such as lauroyl carnitine chloride (LCC) and palmitoyl carnitine chloride (PCC), have been shown to act as PEs since the 1980s and have been included in formulations by Enteris Technologies (NJ, USA) for parathyroid hormone (a PTH 1-34 analog) and the analgesic, CR845. The Peptelligence™ technology consists of an enteric coated tablet containing citric acid, the peptide, excipients and acyl carnitines. rhPTH (1–34)OH, an anabolic peptide for the treatment of osteoporosis in postmenopausal women was formulated with either LCC or PCC and tested in a Phase II trial. LCC and PCC have been found to reduce TEER and act by fluidising the plasma membrane and altering the expression of tight junction proteins such as ZO-1, Claudins (1,3 and 5) in vitro.32,33 An oral sCT (Tbria™) completed Phase III in a study carried out by Tarsa Therapeutics in 2015, but citric acid and not carnitines were included in the formulation in this first application of this technology to the FDA, although some literature remains somewhat confused over this point.34 A study of a formulation containing [rhPTH(1-31)NH2] with an acylcarnitine in postmenopausal women with osteoporosis had adverse events such as abdominal pain, but they were attributed to PTH rather than the acylcarnitine.35
Bile Salts
Bile salts including sodium taurodeoxycholate, ursodeoxycholate, taurocholate and chenodeoxycholate have been used to enhance the intestinal permeability of insulin.36 Bile salts are naturally occurring in the small intestine, at concentrations up to 8mM in the fasted state and 18mM in the fed state, and can form mixed micelles with lecithin, fatty acids and cholesterol.37,38 The NOD formulation is an enteric coated nanoparticle consisting of insulin and other components, one of which is a bile salt. Oramed's (Jerusalem, Israel) POD™ technology consists of PEs including EDTA and bile salts combined with a peptide in an oily suspension with omega-3 fatty acids.1 Oramed has completed 2 Phase IIa trials for insulin and recently enrolled patients for a Phase IIb trial.1
Due to the fact that a lot of the formulations in clinical trials are proprietary, the accurate determination and concentrations of PE(s) included may be undisclosed and most trials are not published in peer-reviewed literature. This makes it difficult to comment on the effects they may have on epithelial damage and repair and, therefore, apart from summaries of trial safety, the specific evidence to assess is primarily based on preclinical studies. In clinical trials the PE is administered in a formulation containing both the active pharmacological ingredient (API) and it is usually not possible to discriminate the adverse events.
Damage and Repair
It has been hypothesized that the high concentrations of PEs required to be efficacious in man are more consistent with an initial event of perturbation of the plasma membrane rather than selective paracellular flux enhancement.39 The peptides may then traverse either paracellularly, and/or transcellularly in mixed micelles with bile salts, although evidence for the latter pathway is yet to be fully deciphered.40 It is known that the concentration of PE and the time it is in contact with the epithelia plays a role in the extent of the perturbation caused.
Normal repair mechanism
In order for a PE to perturb the intestine it must first overcome physiological defense mechanisms. Extrinsic barriers including mucus, bicarbonate and prostaglandins (PGE) as well as gastrointestinal (GI) motility are involved in preventing damage to small intestinal tissue.8,41,42 The small intestine can be exposed to and can normally cope with a range of challenges: e.g. alcohol, food (spices and fatty meals) and drug molecules (e.g., non-steroidal anti-inflammatory drugs, laxatives and chenodeoxycholate, a bile salt used to dissolve gall stones).42-44 Every 3 days the small intestinal epithelia is entirely renewed, and each day, 1011 epithelial cells are shed.45,46 There is a high rate of cellular turnover and the intestine has a high capacity to replace cells, due to migrating stem cells from the intestinal crypt (Fig 1). There are also a reserve of stem cells that are dormant until epithelial injury occurs, at which point they are recruited to assist with restoration.46,47 Intestinal repair is a complex process which involves many factors that are still not completely understood. For a compressive review of repair and restoration of the intestinal barrier see Blikslager et al.48
Figure 1.
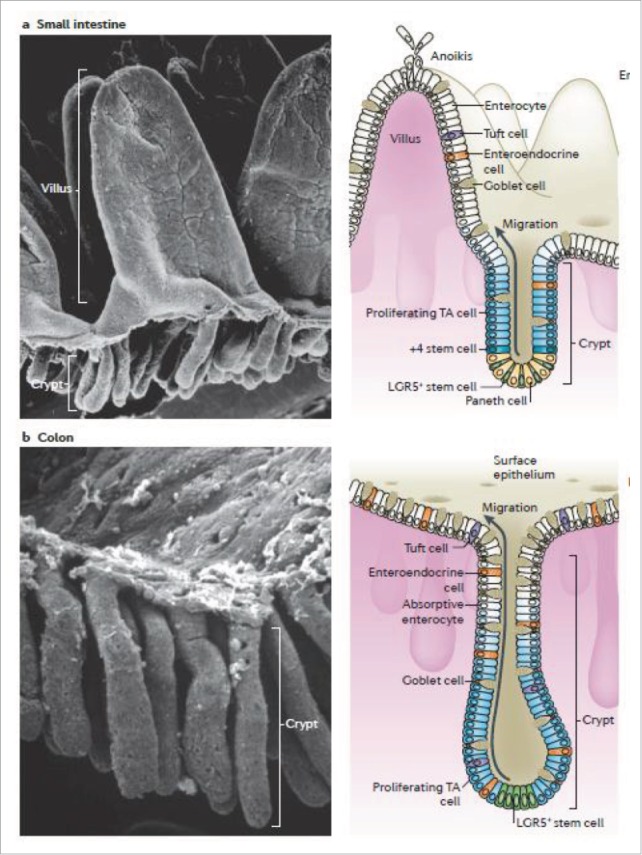
Intestinal epithelium: Scanning electron micrograph (SEM) (left) of (a) small intestine, villus can be observed, (b) colon which has no villi but multiple crypts. Diagram of the intestine (right) showing the crypt which contain stem cells which divide into proliferating transint-amplyfing (TA) cells which differentiate into cells such as enterocytes, goblet cells (which secrete mucus) and tuft cells. +4 stem cells are believed to act as reserve stem cells to replace LGR5+ stem cells during injury thus restoring the normal cell renewal process. During cell renewal, which takes 3-5 days, cells migrate from the crypt up toward the villi. At the top of the villi, anoikis or programmed cell death occurs. This is also the site of cell sloughing during intestinal injury. Reproduced with permission.46
The main outcome from repair is to restore the integrity of the barrier. Stage 1 involves contraction of the villi to reduce the surface area that requires repair.48 Synthesis of cyto-protective prostaglandin E analogs and prostacyclin also increase after injury.49 Stage 2 is restitution or migration, where adjacent healthy epithelia begin crawling using lamellipodia in order to patch exposed areas between cells.43 A number of cofactors including polyamines and trefoil peptides are also involved. Stage 3 is restoration of the barrier involving repair and closure of paracellular spaces. For the repair to be complete, the TJs must become fully functional again; ZO-1 and ZO-2 are particularly important as they are integral to assembly and maintenance of TJs as they have physical contact with most of the other TJ proteins.50 Within 18-24 h cell proliferation from the crypt cells is activated. Inflammation also plays a role in damage repair: neutrophils in the lamina propria and sub-epithelium are sources of inflammatory prostaglandins and IL-1β; they also affect tight junctions and increase paracellular permeability when migrating across the tissue by releasing TJ-damaging proteases.48 Studies have been carried out on the mechanism by which damage caused by ischemia is repaired 48 Damage includes cell sloughing at the tip of the villi and in the crypts, depending on the extent of the damage, and this is commonly seen with PEs in rodent studies. This indicates that the intestinal epithelia is highly robust and would suggest that any damage caused by PEs should be equally capable of being rapidly repaired, and this has been confirmed in a range of in vitro, ex vivo and in vivo preclinical intestinal models, as well as in a human study with C10.17
PE-induced intestinal epithelial damage
At effective concentrations, sodium caprate (C10), has been shown to reversibly reduce TEER in Caco-2 monolayers.9,12,51 The tight junctional integrity can be monitored in real time by quantifying TEER, many PEs fluidise the epithelial plasma membrane and ultimately rearrange tight junctions, so TEER decreases are seen as a secondary effect. C10 reversibly reduced TEER at 8.5 mM in Caco-2 monolayers, which then recovered within 4 h of exposure.13 This study also investigated the effect of pre-treatment with the prostaglandin analog, misoprostol, which is a cytoprotectant and prevents damage induced by repeat dosing of non-steroidal anti-inflammatory drugs (NSAIDs). PGE regulates a number of intestinal processes such as blood flow and motility and in part prevented both the C10-induced permeability increases and epithelial damage in monolayers and in rat in situ intestinal loop instillations.13
High content analysis (HCA) is a cell-based high throughput assay which analyses sub-lethal cytotoxicity parameters including plasma membrane permeability (PMP) and intracellular calcium. PMP after exposure to 8.5mM C10 was transiently increased and recovered to control levels after 8 h.13 Damage and recovery of the intestinal barrier histology to exposure to 8.5 mM C10 was further confirmed in isolated rat intestinal mucosae in Ussing chambers and also in situ instillations of intact rat intestinal loops when exposed to 100mM C10. In the in situ intestinal instillation rat model, repair occurred within 30-60 min; due to an intact mesenteric blood supply, this was much faster than in the in vitro models.39 In the in situ study, fluorescein isothiocyanate-labeled dextran (4 kDa) (FD4) was instilled either at the same time or 10, 30 or 60 min following addition of C10. The highest absolute bioavailability (33%) and membrane perturbation was seen when C10 and FD4 were co-administered together, whereas FD4 instilled 60min after C10 had a bioavailability of just 4% . The lack of FD4 bioavailability from the protocols where C10 was administered ahead of it was likely due to the intestinal barrier recovering from C10 in advance of FD4 exposure, although it is also possible that luminal concentrations of C10 dropped off as it permeated per se (Fig 2). Studies were also carried out in 24 human subjects investigating the ratio of mannitol and lactulose (LMER) in urine to see the effects of C10 on intestinal paracellular permeability in vivo. The two sugars were given orally 20, 40 and 60 mins following intra-jejunal administration of 0.5g C10. Similarly to the results obtained by Wang et al, only when the sugar was administered 20 min after the C10 was an increase in LMER seen, whereas no differences were detected at 40 and 60 min gaps. This human study also suggests that the epithelium had repaired from the mild challenge before the sugars were ingested.17
Figure 2.
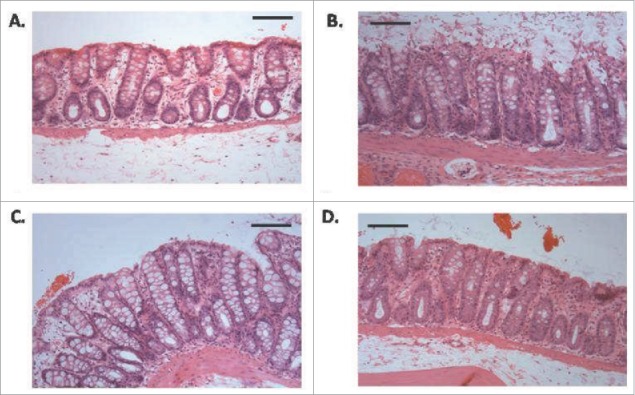
Histology of rat colonic tissue (haemotoxylin and eosin stained) following instillations (A) saline control, (B) C10 (100 mM) after 10 min (C) C10 (100 mM) after 30 min, (D) and C10 (100mM) after 60 min. Horizontal bars = 250 μm. Cell sloughing, one of the first signs of mucosal damage can be observed in B. Note that these high concentrations are the minimum required to induce increased permeability in preclinical rat models in vivo. Reproduced with permission.39
Chiasma also tested their Transient Permeability Enhancer (TPE®) formulation in rats using a similar study design.20 FD4 was administered into the jejunum via a cannula 0, 30 and 60 min after their formulation and it was found that the area under the curve (AUC) was reduced when the FD4 was administered 60 min (8 ± 1 AU) after the C8-containing formulation compared to 10min (64 ± 15 AU). These results suggest that, as with C10, the effects on intestinal permeability are temporary and reversible for TPE®.
Swenson et al. also investigated toxicity of a number of surfactants including the bile salts, sodium taurocholate (TC) and sodium taurodeoxycholate (TDC) in rats.45 Using an intestinal single pass perfusion model, they used biochemical read-outs (e.g., lactase dehydrogenase (LDH)) and morphological changes (e.g. shortening of the villi) to assess PE-induced damage associated with absorption of the marker, phenol red. Their results suggest that damage was reversible in this model within 1-3 h after removal of surfactant, which would likely occur during normal gut transit. Using pig ileal tissue in Ussing chambers, Gookin et al. found that the bile salt sodium deoxycholate (6 mM, 15 min) reduced TEER, but that recovery was also observed within 3 h.49 Epithelial villous tip damage was observed post-treatment, but it had begun to be restored at 210 min (Fig 3). Overall, intestinal epithelia structural and functional recovery seems to occur within 1-3 h in many different in vitro and in vivo bioassays with most of the PEs that are currently present in oral peptide formulations in clinical trials.
Figure 3.
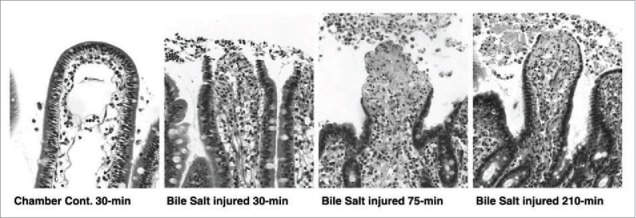
Histologic appearance of porcine tissue after removal from the Ussing chamber. Tissue was treated with the bile salt, sodium deoxycholate. After 30min, epithelial loss is visible from the tips of villi. Epithelial losses continued for the first 45-min of recovery culminating in peak injury at time = 75-min. Between 75 and 210-min there was partial restitution of the injured villi by flattened to cuboidal migrating enterocytes. Uninjured control tissue maintained epithelial continuity throughout the study period. Magnification, 314X. Reproduced with permission.49
PE-induced intestinal permeability of LPS and xenobiotics
One of concerns about PEs is that the compromised barrier may allow translocation of potentially dangerous bystanders such as bacteria, viruses, lipopolysaccharide (LPS), LPS fragments, toxins and allergens. LPS refers to the cell wall endotoxin components of Gram-negative bacteria and it consists of a hydrophobic lipid (Lipid A) and a hydrophilic carbohydrate core with polysaccharide O-antigen, which can promote an inflammatory response.52 LPS in the gut lumen does not normally permeate intestinal epithelia, whereas, when the intestinal barrier is compromised in certain diseased states, it is thought to have some capacity to translocate.53,54 A recurring concern over the safety of intestinal PEs is therefore co-absorption of LPS or other xenobiotics.10 This concern is controversial, as theoretically the maximum diameter of an open small intestinal TJ is ∼10nm when co-administered with PEs working in part by that mechanism, whereas the TJ diameter is ∼1nm in the normal state.55 LPS itself induces an increase in intestinal permeability via an intracellular mechanism involving TLR-4 receptors, however, this was only observed in vitro when LPS was present in the basolateral chamber of Caco-2 monolayers or in vivo when injected via intra-peritoneal route.56,57 It has also been shown that gut ischemia increases the permeability of LPS by passive transcellular diffusion58 and in addition, Shigella-derived LPS was trafficked across epithelial cells by endosomal compartments.59 This suggests that the presence of LPS in the gut lumen does not affect intestinal permeability per se, but there is debate over whether its passage can be promoted in vivo by selected PEs, and if so, to what extent.
The following analysis may help clarify matters. The relationship between the molecular weight (MW) and paracellular permeability across intestinal epithelia has been well established, with limited permeability detected for polyethylene glycol (PEG) >700 Da across human jejunal tissue.60 C8-induced permeation enhancement of FITC-dextrans across the rat small intestinal epithelium in vivo was inversely correlated with dextran MW and insignificant permeation was detected at 70 kDa.20 As the MW of enterobacterial toxins range from 70-900 kDa and LPS is >100 kDa, it would suggest that PEs are unlikely to open TJs sufficiently to allow ’by-stander' LPS or xenobiotics to permeate.20 Furthermore, E. coli (pHTK3) was not able to translocate across Caco-2 monolayers in the presence of C10, but it was translocated in the presence of the strong detergent, Triton®-X-100 (16mM),10 easily accounted for by the irreversible monolayer damage and compromised barrier. S. typhimurium was not able to translocate across isolated rat ileal mucosa upon co-exposure to C10 (1-30mM).61 The lack of facilitation by C10 may in part be due to known anti-bacterial effects of MCFA, which may pose questions on its possible unbalancing effects on gut microbiota, although this would be unlikely to occur due to the rapid absorption of MCFAs in the gut. In relation to other oral peptide technologies based on nanotechnology, when LPS was administered to mice orally for 7 consecutive days with or without co-administration of a chitosan-based insulin-entrapped nanoparticle (Fig 4), there was no increase in LPS serum levels nor damage to hepatic tissue.62 Furthermore, the cell-penetrating peptide (CPP) penetratin, acts as a transient transcellular absorption enhancer based on non-covalent electrostatic interaction with the insulin payload, not unlike the mechanism of the Eligen® technology. When penetratin was also orally co-administered with LPS daily for 7 days in mice, there was no significant increase levels in liver enzymes.63 Although, one particular study demonstrated an increase in serum LPS after prolonged exposure to food- and pharmaceutical grade emulsifiers, carboxymethylcellulose (1% w/v) or polysorbate-80 (1% w/v) in wild type and IL-10 knock-out mice.64
Figure 4.
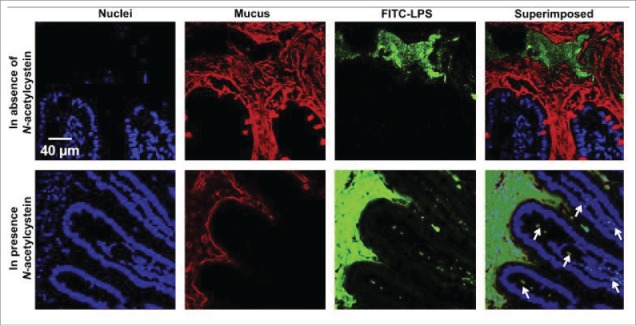
Confocal images showing the intestinal absorption of FITC-LPS (green) after its oral administration in the absence/presence of a mucolytic agent (N-acetylcysteine). In the presence of the mucolytic agent, the mucus layer (red) became thinner, and FITC-LPS was observed underneath the epithelium (indicated in the superimposed image by the white arrows), an indication of the intestinal absorption of LPS. Reproduced with permission.62
However, the foregoing is predicated on the risk for bystander entry solely according to a MW and molecular diameter-based argument. This can lead to other more subtle aspects being overlooked, such as the potential of PE-induced permeability of LPS fragments,65 permeability of food allergens,66,67 damage due to repeat dosing of PEs, and exacerbation of damage to people with impaired intestinal function due to disease state or dietary intake.68-70
Models for Testing Safety and Toxicity of PEs
Transwell® filter models of cultured human intestinal epithelial cell lines are often used as an initial in vitro screening of PEs, and typically involves culture of a Caco-2 cell monolayer on a porous semi-permeable membranes.51 The monolayer integrity can monitored by quantifying TEER. However, cell based assays are well-known to overestimate the damage that occurs upon exposure to PEs, as the repair mechanism may not be fully intact due to a lack of mucus, co-factors, and supporting cell types.45 As the filter model lacks a blood supply, signaling events and cytokines may not be present at the level of in vivo, resulting in a reduced capacity for cell division and repair. This can also be seen with in vitro toxicity testing of PEs using Caco-2 cells with the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) MTS and lactate dehydrogenase (LDH) assays, which show high levels of damage compared to in vivo studies for the same agents.13
Mucus can buffer the effects of a PE, however the Caco-2 model fails to account for this unless an alternative mucus-producing epithelial co-culture or a sub-clone is used (e.g., HT29-MTX-E12).71 Similarly, 3-dimensional (3D) dynamic in vitro Caco-2 and human microvascular endothelial cells (hMECs) showed morphology similar to microvilli in vivo.72 Attempts are being made to create more physiologically relevant cell models such as human gut-on-a-chip constructs (Fig 5), in which Caco-2 can be cultured to mimic the biomechanical, structural and pathophysiological properties of the human gut, including spontaneous villi formation.73
Figure 5.
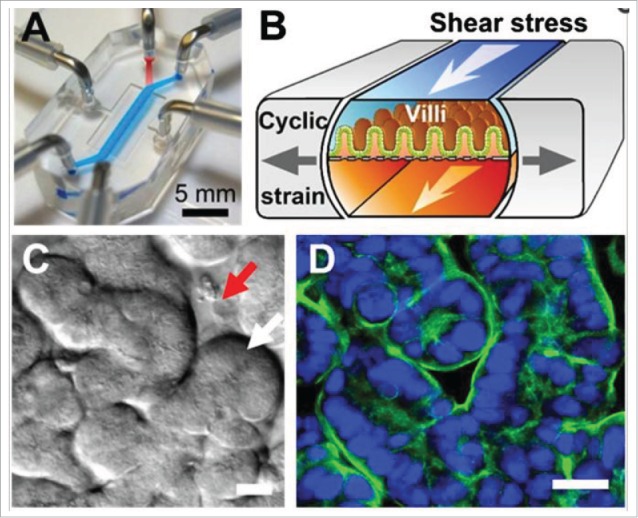
Human gut-on-a-chip in vitro model of Caco-2 cells leads to spontaneous formation of villi (C and D) and also incorporates more physiological relevant factors such as mimicking peristaltic biomechanics. Adapted with permission.79
Isolated ex vivo intestinal tissue from rat and human has been tested with PEs in Ussing chambers,74-76 as well as in everted rat gut sacs.77 Although these systems account for villi, increased transporter expression and mucus secretion, they lack blood supply which assists complete mechanistic repair of damaged tissue. Barthe et al., recommend that the intestinal sac model cannot be relied upon for accurate flux data beyond 60 min, even when maintained in culture medium.78 Isolated intestinal tissue mucosae mounted in Ussing chambers is also sensitive to oxygen tension and nutrient supply, while edge damage can occur during chamber mounting. The most relevant systems therefore to test intestinal damage are oral gavage and intact intra-intestinal loop instillations/perfusions as these use intact tissue with a mesenteric blood supply.43 Instillations/perfusions reveal local damage to the intestinal segment, but repair occurs more readily than in in vitro. These studies cannot however, examine repeat-dosing regimens in which damage may be accumulated and not have sufficient time to fully recover before the next challenge. Such models provide information on mucosal inflammatory responses and on serum biomarkers of normally excluded molecules present in the GI tract. Finally, instillation/perfusion models can permit a histological scoring system to be established, such as the one used by Swenson et al.45 For example, changes in villi height could be a useful tool in assessing damage induced in such models by PE-containing formulations and may allow for rank ordering the toxicity potential of families of PEs.49,79
Conclusion
It has well known that small intestinal epithelial damage is caused by many of the PEs in current oral peptide clinical trials and the extent of the damage seems to vary between them. For the majority, histological damage is temporary and repairable and it is not unlike the stress the intestine undergoes on a day-to-day basis from food, alcohol, and a range of therapeutics including aspirin. It is still unknown however if chronic repeat dosing of such PEs in man could overcome the body's natural ability to repair or create conditions for allergies or autoimmune conditions. Since some of these PEs including SNAC, C8, C10 and acyl carnitines are currently in advanced clinical trials, it is conceivable that several enhancer-based formulations will soon be on pharmacy shelves for selected highly potent oral peptides with a high therapeutic index. Post-marketing surveillance will decipher the true toxicological effects of repeat dosing of selected PEs in such high doses. A conservative approach would suggest that PE-containing formulations should not be prescribed for patients with inflammatory bowel disease, irritable bowel syndrome or celiac disease.
Supplementary Material
Disclosure of potential conflicts of interest
David Brayden has consulted for one or more companies mentioned within. No other potential conflicts of interest were disclosed.
Funding
This work was supported by the Science Foundation Ireland Center for Medical Devices (CURAM), 13/RC/2073, by the Irish Department of Agriculture FIRM grant (NUTRADEL), 11/F/042, and by the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement 281035 ('TRANS-INT').
References
- [1]. Aguirre T, Teijeiro-Osorio D, Rosa M, Coulter I, Alonso MJ, Brayden DJ. Current status of selected oral peptide technologies in advanced preclinical development and in clinical trails. Adv Drug Deliv Rev 2016; In Press; http://dx.doi.org/ 10.1016/j.addr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- [2]. Maher S, Duffy B, Ryan A, Brayden DJ. Formulation strategies to improve oral peptide delivery. Pharm Pat Anal 2014; 3:313–36; PMID:24998290; http://dx.doi.org/ 10.4155/ppa.14.15 [DOI] [PubMed] [Google Scholar]
- [3]. Moroz E, Matoori S, Leroux JC. Oral delivery of macromolecular drugs: Where we are after almost 100 years of attempts. Adv Drug Deliv Rev 2016; In Press; http://dx.doi.org/ 10.1016/j.addr.2016.01.010 [DOI] [PubMed] [Google Scholar]
- [4]. Karsdal MA, Riis BJ, Mehta N, Stern W, Arbit E, Christiansen C, Henriksen K. Lessons learned from the clinical development of oral peptides. Br J Clin Pharmacol 2015; 79:720–32; PMID:25408230; http://dx.doi.org/ 10.1111/bcp.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Zijlstra E, Heinemann L, Plum-Morschel L. Oral insulin reloaded: a structured approach. J Diabetes Sci Technol 2014; 8:458–65; PMID:24876606; http://dx.doi.org/ 10.1177/1932296814529988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta 2009; 1788:892–910; PMID:18983815; http://dx.doi.org/ 10.1016/j.bbamem.2008.09.016 [DOI] [PubMed] [Google Scholar]
- [7]. Aungst BJ. Absorption enhancers: applications and advances. AAPS J 2012; 14:10–8; PMID:22105442; http://dx.doi.org/ 10.1208/s12248-011-9307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Curatolo WJ, Ochoa R. Safety assessment of intestinal permeability enhancers in drug absorption trends. In: de Boer AG, Ed. Drug absorption enhancement - concepts, possibilities, limitations and trends: Hanswood Academy Publishers, 1994:367–89 [Google Scholar]
- [9]. Shima M, Yohdoh K, Yamaguchi M, Kimura Y, Adachi S, Matsuno R. Effects of medium-chain fatty acids and their acylglycerols on the transport of penicillin V across Caco-2 cell monolayers. Biosci Biotechnol Biochem 1997; 61:1150–5; PMID:9255979; http://dx.doi.org/ 10.1271/bbb.61.1150 [DOI] [PubMed] [Google Scholar]
- [10]. Maher S, Leonard TW, Jacobsen J, Brayden DJ. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv Drug Deliv Rev 2009; 61:1427–49; PMID:19800376; http://dx.doi.org/ 10.1016/j.addr.2009.09.006 [DOI] [PubMed] [Google Scholar]
- [11]. Fan D, Wu X, Dong W, Sun W, Li J, Tang X. Enhancement by sodium caprate and sodium deoxycholate of the gastrointestinal absorption of berberine chloride in rats. Drug Dev Ind Pharm 2013; 39:1447–56; PMID:23020091; http://dx.doi.org/ 10.3109/03639045.2012.723219 [DOI] [PubMed] [Google Scholar]
- [12]. Brayden DJ, Gleeson JP, Walsh EG. A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells. Eur J Pharm Biopharm 2014; 88:830–39; PMID:25460147; http://dx.doi.org/ 10.1016/j.ejpb.2014.10.008 [DOI] [PubMed] [Google Scholar]
- [13]. Brayden DJ, Maher S, Bahar B, Walsh E. Sodium caprate-induced increases in intestinal permeability and epithelial damage are prevented by misoprostol. Eur J Pharm Biopharm 2015; 94:194–206; PMID:26026287; http://dx.doi.org/ 10.1016/j.ejpb.2015.05.013 [DOI] [PubMed] [Google Scholar]
- [14]. Administration UFaD. Salts of fatty acids, In: Food additives permitted for direct addition to food for human consumption. CFR, 2008 [Google Scholar]
- [15]. Walsh EG, Adamczyk BE, Chalasani KB, Maher S, O'Toole EB, Fox JS, Leonard TW, Brayden DJ. Oral delivery of macromolecules: rationale underpinning Gastrointestinal Permeation Enhancement Technology (GIPET). Ther Deliv 2011; 2:1595–610; PMID:22833984; http://dx.doi.org/ 10.4155/tde.11.132 [DOI] [PubMed] [Google Scholar]
- [16]. Krug SM, Amasheh M, Dittmann I, I C, Fromm M, Amasheh S. Sodium caprate as an enhancer of macromolecule permeation across tricullular tight junctions of intestinal cells. Biomaterials 2013; 34:275–82; PMID:23069717; http://dx.doi.org/ 10.1016/j.biomaterials.2012.09.051 [DOI] [PubMed] [Google Scholar]
- [17]. Leonard TW, Lynch J, McKenna MJ, Brayden DJ. Promoting absorption of drugs in humans using medium-chain fatty acid-based solid dosage forms: GIPET. Expert Opin Drug Deliv 2006; 3:685–92; PMID:16948563; http://dx.doi.org/ 10.1517/17425247.3.5.685 [DOI] [PubMed] [Google Scholar]
- [18]. Melmed S, Popovic V, Bidlingmaier M, Mercado M, Lely AJvd, Biermasz N, Bolanowski M, Coculescu M, Schopohl J, Racz K, et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter Phase III trial. J Clin Endocrinol Metab 2015; 100:1699–708; PMID:25664604; http://dx.doi.org/ 10.1210/jc.2014-4113 [DOI] [PubMed] [Google Scholar]
- [19]. Tuvia S, Atsmon J, Teichman SL, Katz S, Salama P, Pelled D, Landau I, Karmeli I, Bidlingmaier M, Strasburger CJ, et al. Oral octreotide absorption in human subjects: comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression. J Clin Endocrinol Metab 2012; 97:2362–9; PMID:22539587; http://dx.doi.org/ 10.1210/jc.2012-1179 [DOI] [PubMed] [Google Scholar]
- [20]. Tuvia S, Pelled D, Marom K, Salama P, Levin-Arama M, Karmeli I, Idelson G, Landau I, Mamluk R. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm Res 2014; 31:2010–21; PMID:24558008; http://dx.doi.org/ 10.1007/s11095-014-1303-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Karsdal MA, Byrjalsen I, Henriksen K, Riis BJ, Lau EM, Arnold M, Christiansen C. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthritis Cartilage 2010; 18:150–9; PMID:19747581; http://dx.doi.org/ 10.1016/j.joca.2009.08.004 [DOI] [PubMed] [Google Scholar]
- [22]. Gschwind HP, Glaenzel U, Waldmeier F, Wirz B, Sabia HD, Picard F, Weiss HM, Choi L, Swart PJ, Vasudevan A, et al. Metabolism and disposition of the oral absorption enhancer 14C-radiolabeled 8-(N-2-hydroxy-5-chlorobenzoyl)-amino-caprylic acid (5-CNAC) in healthy postmenopausal women and supplementary investigations in vitro. European J Pharm Sci 2012; 47:44–55; http://dx.doi.org/ 10.1016/j.ejps.2012.04.023 [DOI] [PubMed] [Google Scholar]
- [23]. Hess S, Rotshild V, Hoffman A. Investigation of the enhancing mechanism of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate effect on the intestinal permeability of polar molecules utilizing a voltage clamp method. European J Pharm Sci 2005; 25:307–12; http://dx.doi.org/ 10.1016/j.ejps.2005.03.003 [DOI] [PubMed] [Google Scholar]
- [24]. Brayden D, Creed E, O'Connell A, Leipold H, Agarwal R, Leone-Bay A. Heparin absorption across the intestine: effects of sodium N-[8-(2-hydroxybenzoyl)amino]caprylate in rat in situ intestinal instillations and in Caco-2 monolayers. Pharm Res 1997; 14:1772–9; PMID:9453067; http://dx.doi.org/ 10.1023/A:1012192115828 [DOI] [PubMed] [Google Scholar]
- [25]. Alani AW, Robinson JR. Mechanistic understanding of oral drug absorption enhancement of cromolyn sodium by an amino acid derivative. Pharm Res 2008; 25:48–54; PMID:17846867; http://dx.doi.org/ 10.1007/s11095-007-9438-6 [DOI] [PubMed] [Google Scholar]
- [26]. Riley MG, Castelli MC, Paehler EA. Subchronic oral toxicity of salcaprozate sodium (SNAC) in Sprague-Dawley and Wistar rats. Int J Toxicol 2009; 28:278–93; PMID:19636071; http://dx.doi.org/ 10.1177/1091581809337737 [DOI] [PubMed] [Google Scholar]
- [27]. Bittner B, McIntyre C, Tian H, Tang K, Shah N, Phuapradit W, Ahmed H, Chokshi H, Infeld M, Fotaki N, et al. Phase I clinical study to select a novel oral formulation for ibandronate containing the excipient sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC). Die Pharmazie 2012; 67:233–41; PMID:22530305 [PubMed] [Google Scholar]
- [28]. Tanko LB, Bagger YZ, Alexandersen P, Devogelaer JP, Reginster JY, Chick R, Olson M, Benmammar H, Mindeholm L, Azria M, et al. Safety and efficacy of a novel salmon calcitonin (sCT) technology-based oral formulation in healthy postmenopausal women: acute and 3-month effects on biomarkers of bone turnover. J Bone Miner Res 2004; 19:1531–8; PMID:15312255; http://dx.doi.org/ 10.1359/JBMR.040715 [DOI] [PubMed] [Google Scholar]
- [29]. Karsdal MA, Byrjalsen I, Alexandersen P, Bihlet A, Andersen JR, Riis BJ, Bay-Jensen AC, Christiansen C. Treatment of symptomatic knee osteoarthritis with oral salmon calcitonin: results from two phase 3 trials. Osteoarthritis Cartilage 2015; 23:532–43; PMID:25582279; http://dx.doi.org/ 10.1016/j.joca.2014.12.019 [DOI] [PubMed] [Google Scholar]
- [30]. Castelli MC, Wong DF, Friedman K, Riley MG. Pharmacokinetics of oral cyanocobalamin formulated with sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): an open-label, randomized, single-dose, parallel-group study in healthy male subjects. Clin Ther 2011; 33:934–45; PMID:21722960; http://dx.doi.org/ 10.1016/j.clinthera.2011.05.088 [DOI] [PubMed] [Google Scholar]
- [31]. Nordisk N. Novo Nordisk to initiate phase 3a development of oral semaglutide , a once-daily oral GLP-1 analogue. http://www.novonordisk.com/bin/getPDF.1947638.pdf 2015 [Google Scholar]
- [32]. Duizer E, van der Wulp C, Versantvoort CH, Groten JP. Absorption enhancement, structural changes in tight junctions and cytotoxicity caused by palmitoyl carnitine in Caco-2 and IEC-18 cells. J Pharmacol Exp Ther 1998; 287:395–402; PMID:9765361 [PubMed] [Google Scholar]
- [33]. Hochman JH, Fix JA, LeCluyse EL. In vitro and in vivo analysis of the mechanism of absorption enhancement by palmitoylcarnitine. J Pharmacol Exp Ther 1994; 269:813–22; PMID:8182550 [PubMed] [Google Scholar]
- [34]. Lewis AL, Richard J. Challenges in the delivery of peptide drugs: an industry perspective. Ther Deliv 2015; 6:149–63; PMID:25690084; http://dx.doi.org/ 10.4155/tde.14.111 [DOI] [PubMed] [Google Scholar]
- [35]. Henriksen K, Andersen JR, Riis BJ, Mehta N, Tavakkol R, Alexandersen P, Byrjalsen I, Valter I, Nedergaard BS, Teglbjaerg CS, et al. Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1-31)NH(2)] in postmenopausal women with osteoporosis. Bone 2013; 53:160–6; PMID:23234813; http://dx.doi.org/ 10.1016/j.bone.2012.11.045 [DOI] [PubMed] [Google Scholar]
- [36]. Eldor R, Kidron M, Arbit E. Open-label study to assess the safety and pharmacodynamics of five oral insulin formulations in healthy subjects. Diabetes Obes Metab 2010; 12:219–23; http://dx.doi.org/ 10.1111/j.1463-1326.2009.01153.x [DOI] [PubMed] [Google Scholar]
- [37]. Porter CJ, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 2007; 6:231–48; PMID:17330072; http://dx.doi.org/ 10.1038/nrd2197 [DOI] [PubMed] [Google Scholar]
- [38]. Aungst BJ. Intestinal permeation enhancers. J Pharm Sci 2000; 89:429–42; PMID:10737905; http://dx.doi.org/ 10.1002/(SICI)1520-6017(200004)89:4%3c429::AID-JPS1%3e3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- [39]. Wang X, Maher S, Brayden DJ. Restoration of rat colonic epithelium after in situ intestinal instillations of the absorption promoter, sodium caprate. Ther Deliv 2010; 1:75–82; PMID:22816121; http://dx.doi.org/ 10.4155/tde.10.5 [DOI] [PubMed] [Google Scholar]
- [40]. Gradauer K, Nishiumi A, Unrinin K, Higashino H, Kataoka M, Pedersen BL, Buckley ST, Yamashita S. Interaction with mixed micelles in the intestine attenuates the permeation enhancing potential of alky maltosides. Mol Pharm 2015; 12:2245–53; PMID:25874852; http://dx.doi.org/ 10.1021/mp500776a [DOI] [PubMed] [Google Scholar]
- [41]. Smale S, Bjarnason I. Determining small bowel integrity following drug treatment. Br J Clin Pharmacol 2003; 56:284–91; PMID:12919176; http://dx.doi.org/ 10.1046/j.1365-2125.2003.01942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 2008; 135:41–60; PMID:18549814; http://dx.doi.org/ 10.1053/j.gastro.2008.05.030 [DOI] [PubMed] [Google Scholar]
- [43]. Narkar Y, Burnette R, Bleher R, Albrecht R, Kandela A, Robinson JR. Evaluation of mucosal damage and recovery in the gastrointestinal tract of rats by a penetration enhancer. Pharm Res 2008; 25:25–38; PMID:18161013; http://dx.doi.org/ 10.1007/s11095-007-9509-8 [DOI] [PubMed] [Google Scholar]
- [44]. Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev 2015; 14:479–89; PMID:25676324; http://dx.doi.org/ 10.1016/j.autrev.2015.01.009 [DOI] [PubMed] [Google Scholar]
- [45]. Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res 1994; 11:1132–42; PMID:7971714; http://dx.doi.org/ 10.1023/A:1018984731584 [DOI] [PubMed] [Google Scholar]
- [46]. Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014; 15:19–33; PMID:24326621; http://dx.doi.org/ 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- [47]. Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013; 495:65–9; PMID:23446353; http://dx.doi.org/ 10.1038/nature11965 [DOI] [PubMed] [Google Scholar]
- [48]. Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 2007; 87:545–64; PMID:17429041; http://dx.doi.org/ 10.1152/physrev.00012.2006 [DOI] [PubMed] [Google Scholar]
- [49]. Gookin JL, Galanko JA, Blikslager AT, Argenzio RA. PG-mediated closure of paracellular pathway and not restitution is the primary determinant of barrier recovery in acutely injured porcine ileum. Am J Physiol 2003; 285:G967–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36:204–12; PMID:25263012; http://dx.doi.org/ 10.1016/j.semcdb.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2007; 2:2111–9; PMID:17853866; http://dx.doi.org/ 10.1038/nprot.2007.303 [DOI] [PubMed] [Google Scholar]
- [52]. Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem 2008; 15:1697–705; PMID:18673219; http://dx.doi.org/ 10.2174/092986708784872393 [DOI] [PubMed] [Google Scholar]
- [53]. Benoit R, Rowe S, Watkins SC, Boyle P, Garrett M, Alber S, Wiener J, Rowe MI, Ford HR. Pure endotoxin does not pass across the intestinal epithelium in vitro. Shock 1998; 10:43–8; PMID:9688090; http://dx.doi.org/ 10.1097/00024382-199807000-00008 [DOI] [PubMed] [Google Scholar]
- [54]. Ge Y, Ezzell RM, Warren HS. Localization of endotoxin in the rat intestinal epithelium. J Infect Dis 2000; 182:873–81; PMID:10950783; http://dx.doi.org/ 10.1086/315784 [DOI] [PubMed] [Google Scholar]
- [55]. Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525–69; PMID:23589827; http://dx.doi.org/ 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol 2013; 182:375–87; PMID:23201091; http://dx.doi.org/ 10.1016/j.ajpath.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Tomita M, Ohkubo R, Hayashi M. Lipopolysaccharide transport system across colonic epithelial cells in normal and infective rat. Drug Metab Pharmacokinet 2004; 19:33–40; PMID:15499167; http://dx.doi.org/ 10.2133/dmpk.19.33 [DOI] [PubMed] [Google Scholar]
- [58]. Drewe J, Beglinger C, Fricker G. Effect of ischemia on intestinal permeability of lipopolysaccharides. Eur J Clin Invest 2001; 31:138–44; PMID:11168452; http://dx.doi.org/ 10.1046/j.1365-2362.2001.00792.x [DOI] [PubMed] [Google Scholar]
- [59]. Beatty WL, Méresse S, Gounon P, Davoust J, Mounier J, Sansonetti PJ, Gorvel J-P. Trafficking of Shigella lipopolysaccharide in polarized intestinal epithelial cells. J Cell Biol 1999; 145:689–98; PMID:10330399; http://dx.doi.org/ 10.1083/jcb.145.4.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Linnankoski J, Makela J, Palmgren J, Mauriala T, Vedin C, Ungell AL, Lazorova L, Artursson P, Urtti A, Yliperttula M. Paracellular porosity and pore size of the human intestinal epithelium in tissue and cell culture models. J Pharm Sci 2010; 99:2166–75; PMID:19827099; http://dx.doi.org/ 10.1002/jps.21961 [DOI] [PubMed] [Google Scholar]
- [61]. Cox AB, Rawlinson LA, Baird AW, Bzik V, Brayden DJ. In vitro interactions between the oral absorption promoter, sodium caprate (C(10)) and S. typhimurium in rat intestinal ileal mucosae. Pharm Res 2008; 25:114–22; PMID:17546408; http://dx.doi.org/ 10.1007/s11095-007-9354-9 [DOI] [PubMed] [Google Scholar]
- [62]. Sonaje K, Lin KJ, Tseng MT, Wey SP, Su FY, Chuang EY, Hsu CW, Chen CT, Sung HW. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011; 32:8712–21; PMID:21862121; http://dx.doi.org/ 10.1016/j.biomaterials.2011.07.086 [DOI] [PubMed] [Google Scholar]
- [63]. Nielsen EJ, Kamei N, Takeda-Morishita M. Safety of the cell-penetrating peptide penetratin as an oral absorption enhancer. Biol Pharm Bull 2015; 38:144–6; PMID:25744470; http://dx.doi.org/Ref60 [DOI] [PubMed] [Google Scholar]
- [64]. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015; 519:92–6; PMID:25731162; http://dx.doi.org/ 10.1038/nature14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Cani PD, Everard A. Keeping gut lining at bay: impact of emulsifiers. Trends Endocrinol Metab 2015; 26:273–4; PMID:25887492; http://dx.doi.org/ 10.1016/j.tem.2015.03.009 [DOI] [PubMed] [Google Scholar]
- [66]. Mine Y, Zhang JW. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int Arch Allergy Immunol 2003; 130:135–42; PMID:12673067; http://dx.doi.org/ 10.1159/000069009 [DOI] [PubMed] [Google Scholar]
- [67]. Chen T, Liu X, Ma L, He W, Li W, Cao Y, Liu Z. Food allergens affect the intestinal tight junction permeability in inducing intestinal food allergy in rats. Asian Pac J Allergy Immunol 2014; 32:345–53; PMID:25543046 [DOI] [PubMed] [Google Scholar]
- [68]. Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci 2009; 1165:195–205; PMID:19538307; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Perrier C, Corthesy B. Gut permeability and food allergies. Clin Exp Allergy 2011; 41:20–8; PMID:21070397; http://dx.doi.org/ 10.1111/j.1365-2222.2010.03639.x [DOI] [PubMed] [Google Scholar]
- [70]. Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PloS One 2014; 9:e96864; PMID:24828436; http://dx.doi.org/ 10.1371/journal.pone.0096864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Petersen SB, Nolan G, Maher S, Rahbek UL, Guldbrandt M, Brayden DJ. Evaluation of alkylmaltosides as intestinal permeation enhancers: comparison between rat intestinal mucosal sheets and Caco-2 monolayers. European J Pharm Sci 2012; 47:701–12; http://dx.doi.org/ 10.1016/j.ejps.2012.08.010 [DOI] [PubMed] [Google Scholar]
- [72]. Pusch J, Votteler M, Göhler S, Engl J, Hampel M, Walles H, Schenke-Layland K. The physiological performance of a three-dimensional model that mimics the microenvironment of the small intestine. Biomaterials 2011; 32:7469–78; PMID:21764120; http://dx.doi.org/ 10.1016/j.biomaterials.2011.06.035 [DOI] [PubMed] [Google Scholar]
- [73]. Kim HJ, Ingber DE. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol 2013; 5:1130–40; PMID:23817533; http://dx.doi.org/ 10.1039/c3ib40126j [DOI] [PubMed] [Google Scholar]
- [74]. Petersen SB, Nielsen LG, Rahbek UL, Guldbrandt M, Brayden DJ. Colonic absorption of salmon calcitonin using tetradecyl maltoside (TDM) as a permeation enhancer. Eur J Pharm Sci 2013; 48:726–34; PMID:23354154; http://dx.doi.org/ 10.1016/j.ejps.2013.01.009 [DOI] [PubMed] [Google Scholar]
- [75]. Gleeson JP, Heade J, Ryan SM, Brayden DJ. Stability, toxicity and intestinal permeation enhancement of two food-derived antihypertensive tripeptides, Ile-Pro-Pro and Leu-Lys-Pro. Peptides 2015; 71:1–7; PMID:26048090; http://dx.doi.org/ 10.1016/j.peptides.2015.05.009 [DOI] [PubMed] [Google Scholar]
- [76]. Foltz M, Cerstiaens A, van Meensel A, Mols R, van der Pijl PC, Duchateau GS, Augustijns P. The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 2008; 29:1312–20; PMID:18490081; http://dx.doi.org/ 10.1016/j.peptides.2008.03.021 [DOI] [PubMed] [Google Scholar]
- [77]. Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted gut sac model as a tool in pharmaceutical research: limitations and applications. J Pharm Pharmacol 2012; 64:326–36; PMID:22309264; http://dx.doi.org/ 10.1111/j.2042-7158.2011.01391.x [DOI] [PubMed] [Google Scholar]
- [78]. Barthe L, Woodley JF, Kenworthy S, Houin G. An improved everted gut sac as a simple and accurate technique to measure paracellular transport across the small intestine. Eur J Drug Metab Pharmacokinet 1998; 23:313–23; http://dx.doi.org/ 10.1007/BF03189357 [DOI] [PubMed] [Google Scholar]
- [79]. Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 2016; 113:E7–E15; http://dx.doi.org/ 10.1073/pnas.1522193112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


