Abstract
Apolipoprotein A-I (apo A-I) an indispensable component and a major structural protein of high-density lipoprotein (HDL), plays a vital role in reverse cholesterol transport and cellular cholesterol homeostasis since its identification. Its multifunctional role in immunity, inflammation, apoptosis, viral, bacterial infection etc. has crossed its boundary of its potential of protecting cardiovascular system and lowering cardiovascular disease risk, attributing HDL to be known as a protective fat removal particle. Its structural homology with prostacyclin stabilization factor has contributed to its anti-clotting and anti-aggregatory effect on platelet which has potentiated its cardio-protective role as well as its therapeutic efficacy against Alzheimer’s disease. The binding affinity and neutralising action against endotoxin lipopolysaccharide, reduces the toxic manifestations of septic shock. As a negative acute phase protein, it blocks T-cell signalling of macrophages. However the recently identified anti-tumor activity of apo A-I has been highlighted in various models of melanoma, lung cancer, ovarian cancer, lymphoblastic leukaemia, gastric as well as pancreatic cancers. These cancer fighting effects are directed towards regression of tumor size and distant metastasis by its immuno modulatory activity as well as its clearing effect on serum lysophospholipids. This lowering effect on lysophospholipid concentration is utilized by apo A-I mimetic peptides to be used in retarding tumor cell proliferation and as a potential cancer therapeutic agent. Not only that, it inhibits the tumor associated neo-angiogenesis as well as brings down the matrix degrading enzymes associated with tumor metastasis. However this efficient therapeutic potential of apo A-I as an anti tumor agent awaits further future experimental studies in humans.
Keywords: Apolipoprotein A-I, High-density lipoprotein (HDL), Lysophospholipids, Reverse cholesterol transport
Introduction
Apolipoprotein A-I (apo A-I) is the major structural and functional protein component of high density lipoprotein (HDL), or good cholesterol in plasma. It constitutes approximately 70 % of HDL. Apo A-I content of chylomicrons secreted from the intestinal epithelial cells is also immediately handed over to HDL while in the bloodstream [1]. This apo A-I is the primary protein constituent of HDL defining its size and shape, solubilizing its lipid component and helping in reverse cholesterol transport. Being a cofactor for lecithin cholesterol acyl transferase (LCAT), for the formation of most plasma cholesteryl esters, it promotes cholesterol efflux from tissues to the liver for excretion [2].
Apo A-I possesses structural homology with prostacyclin (PGI2) stabilizing factor, and thus may have an anti-clotting effect. Because of its alpha-helix structure it can bind to PGI2 which is originally an unstable substance and causes PGI2 stabilization thereby imparting an important protective anti-aggregatory effect against platelet thrombi formation at sites of vascular damage. Thus it contributes towards its novel anti atherogenic function of prevention of coronary artery disease [3].
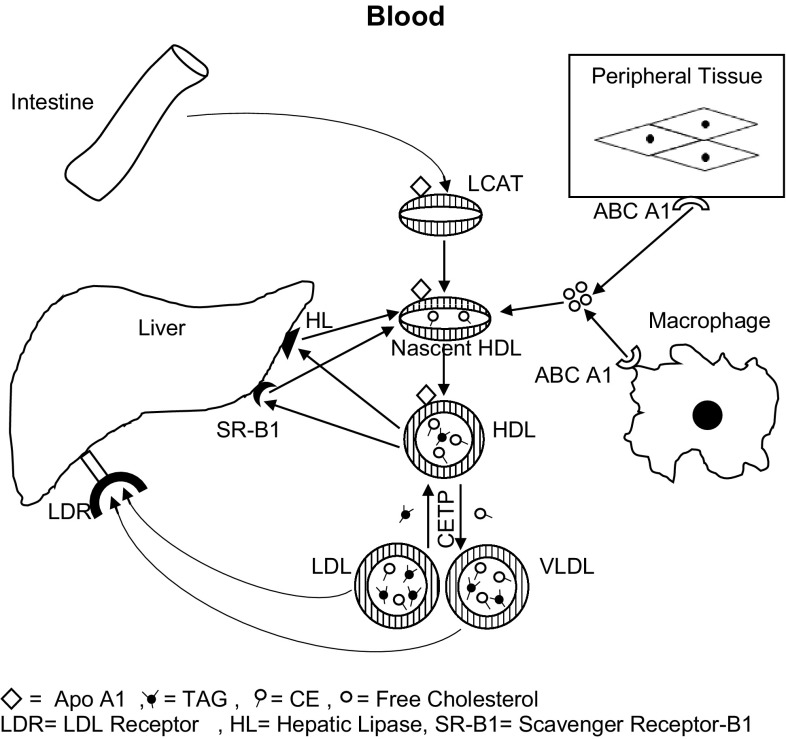
Apo A-I is a key mediator of plasma cholesterol transport and cellular cholesterol homeostasis. During reverse cholesterol transport by HDL, it interacts with different receptors and transporters like ABCAI (ATP Binding Cassette AI), ABCGI (ATP Binding Cassette GI) and Scavenger Receptor BI with structural changes in HDL, mostly discoidal HDL (Fig. 1). Because of its content of amphipathic helices and non helical residues, it possesses flexibility and plasticity with tendency towards self association with other components of HDL for imparting its receptor binding ability and catalytic activity [4].
Fig. 1.
Reverse cholesterol transport
This mechanism of cholesterol efflux from various cells through discoidal HDL depends upon the reconstitution process, stating that apo A-I structure is vital for the role of HDL in reverse cholesterol transport. The same was also seen in macrophage culture [5]. The specific property of apo A-I to remove cholesterol from cells, to interact with lipids, to enhance LCAT activity making HDL responsive to specific receptors and proteins, results in efficient reverse cholesterol transport. However its tertiary structure contributing towards specific lipid binding was explained by various models consisting of phospholipids and apo A-I, like picket fence model, belt model etc. [6].
Adachi et al., have shown the amyloidogenic tendency of N-terminal residues of apo A-I, where an extreme N- terminal small fragment peptide transforms a structure rich with β sheet, that favours amyloid strand formation [7].
In humans Apo AI gene encodes apo A-I [8]. Systemic non-neuropathic amyloidosis and Tangier disease, i.e., a mutations in the ‘ATP-Binding Cassette transporter A1 (ABCA1) gene encoding the membrane transporter ABCA1, which blocks the first step of reverse cholesterol transport, have the manifestations similar to the defects of this gene [9].
The first known molecular abnormality of apolipoproteins was apo A-I Milano, a naturally occurring mutant of apo A-I, first identified by Dr. Cesare Sirtori in Milan, whose presence caused a reduction in HDL cholesterol content and an increase in triglyceride amount in HDL [10]. People with a mutation (E164X) were observed to develop premature coronary artery disease at an early age [11].
As an important constituent of the high-density lipoprotein, a protective “fat removal” particle, apo A-I helps in removal of cholesterol, from white blood cells within artery walls. Thereby it helps in preventing further progression of atherosclerosis by inhibiting fat accumulation within white blood cells and retarding their conversion into foam cells with further degeneration. Recent studies by Huang et al. [12], have stated that both HDLs and its structural protein, apo A-I, obtained from human atheroma are dysfunctional and are grossly oxidized by myeloperoxidase (MPO). In vitro oxidized apo A-I as well as HDL particles, lose their ability of cholesterol acceptance and are poor in their lipid content. They behave as pro inflammatory molecules and initiates the process of atherogenesis increasing the cardiovascular disease risk [12].
Apo A-I in Myeloproliferative Disorder
Apo A-I has been observed to be a prognostic marker in myeloproliferative disorder like polycythemia vera (PV), which is characterized by an acquired gain of function mutation of the JAK2 protein (JAK2V 617F). The maximum serum apo A-I expression for these PV patients is observed in patients with more than 75 % mutated allele. The immunoassay of this Apo A-I in these patients of mutated alleles will help to recognize the PV patients with differentiation of levels of mutated alleles at their diagnosis [13].
Apo A-I in Alzheimer’s Disease
Apo A-I as a component of good cholesterol HDL, not only protects against heart disease, but also has profound effect on CNS with preserving brain health. It defends the brain against the cognitive deficits of Alzheimer’s disease (AD). In vitro studies have highlighted that apo A-I prevents the deposition of Aβ in cerebral blood vessels, and directly inhibits the aggregation of Aβ to lessen plaque formation [13]. This apo A-I is protective in nature to lessen neuroinflammation in brain including less microglial and astrocyte activation and preserves the memory (Fig. 2). Though enough apo A-I is not made in brain, yet enough apo A-I crosses blood brain barrier to give its presence in brain. Because of its role in reverse cholesterol transport, alteration of cholesterol homeostasis is associated with AD. Absence of apo A-I is associated with cerebral amyloid angiopathy and disturbed brain health. Interventions that raise apo A-I level may act as potential therapeutics for AD [14].
Fig. 2.
Apo A1 in brain
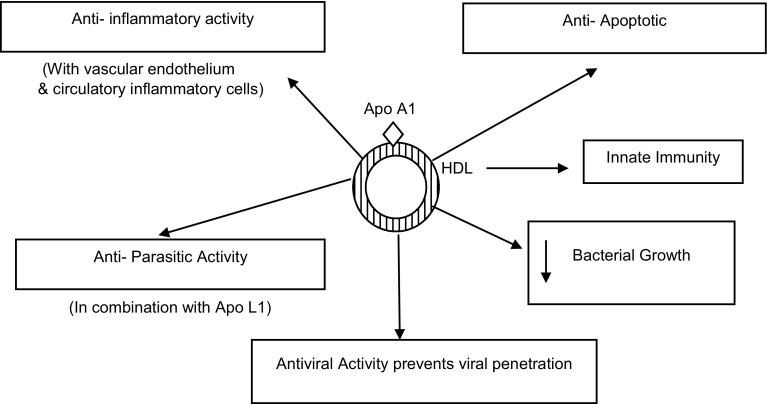
Antimicrobial and Antiviral Activity
Apo A1/HDL is situated at the nexus of a number of physiologically significant immune, anti inflammatory and anti-apoptotic functions [15]. Antimicrobial activity was also observed with apo A-I containing lipoprotein particle like HDL, which directly affects bacterial growth and promotes the self defence mechanism of normal and immune compromised individuals [15]. Interestingly, a vast number of studies of HDL have suggested a list of expanded activities for the lipoprotein beyond simply contributing as a cholesterol carrier from tissues to liver. This includes anti-inflammatory, anti-parasitic, anti-apoptotic, and innate immune activities [16–18]. As an anti-inflammatory molecule it mediates its action with the vascular endothelium and circulating inflammatory cells [16–18]. Apo A-I exhibits its anti-parasitic effect function by forming a complex with apolipoprotein L1, a minor apolipoprotein component of HDL, a parasite-specific lysosomal pore forming protein, a trypanosome lytic factor (Fig. 3) [19].
Fig. 3.
Antimicrobial and antiviral role of apo A-I
Antibacterial activities of HDL are attributed primarily to apo A-I, which conjugates with and neutralizes both bacterial endotoxin and lipoteichoic acid [20, 21]. Apo A-I of lipoprotein HDL can act to protect against endotoxin lipopolysachharide (LPS) and this protection is augmented by lipoprotein free plasma fraction (LFF). In human and experimental animals LPS produces multiple pathophysiological changes like fever, diarrhea, hypotensive shock, disseminated intravascular coagulation and multiple organ failure as lethal effects of LPS. LPS binds to LPS binding protein (LBP) in plasma to form LPS-LBP complex that attaches to membrane associated CD14 receptor on monocyte/macrophage, activating the process of cytokine release and pathologic consequences. With the help of two binding sites, apo A-I binds to lipopolysaccharides both directly and indirectly and neutralizes its toxicity causing its inactivation, while LFF only enhances this ability of apo A-I [22].
Apo A-I, when either expressed or injected in vivo, spontaneously lipidates forming HDL, and demonstrates a number of innate immune activities including antiviral effects for both enveloped and non-enveloped DNA and RNA viruses by causing direct viral inactivation [23]. Anti viral activity of Apo A1 against human immuno deficiency virus (HIV) and Herpes virus has been come out showing HDL to have broad anti viral activity by preventing virus penetration into the cell as it prevents virus induced cell fusion at normal concentration in blood (Fig. 3) [24, 25].
Role in Immune System
Apo A-I content of HDL have been suggested to be important in preventing autoimmunity and loss of self tolerance. Patients with auto immune diseases like systemic lupus erythematosus and rheumatoid arthritis have been found to be associated with decreased level of HDL, pointing to their relation with regulatory T cells, i.e., by altering the cholesterol content of lipid rafts in immune cells. Treatment with apo A-I reduces inflammation by preventing lymphnode enlargement, cholesterol accumulation in lymphocytes, as well as their activation and proliferation, thereby augmenting the effectiveness of the lymph node T regulatory response [26, 27].
Anti-inflammatory Role of apo A-I
Like a “negative” acute-phase reactant, apo A-I functions as an anti-inflammatory molecule and exerts its action by blocking the interplay between T cells and monocytes. It interferes with T cell signaling of monocytes and thus inhibits TNF-a and IL-1b production. It also inhibits LPS activated monocyte by the transfer of LPS from LPS- binding protein to HDL, that inactivates it contributing to the anti inflammatory role of HDL both in acute and chronic inflammatory diseases. Furthermore, apo A-I inhibits monocyte inflammatory functions in peripheral blood mononuclear cells activated by either specific antigens or lectins without affecting cell proliferation. These results demonstrate a new anti-inflammatory activity of HDL-associated apo A-I that might have modulating functions in nonseptic conditions [28]. The inhibition of TNF-α and IL-1 has proved to be successful in the treatment of human diseases like rheumatoid arthritis, Crohns disease and other immunoinflammatory diseases [29–31]. Such inflammatory response is also seen in atherosclerosis, even in its earliest form—the fatty streak. Therefore, T lymphocyte-signaling of monocytes may occur in atherosclerosis. Gene transfer of apo A-I has shown to reduce the size of previously existing atherosclerotic lesion consisting of macrophages and foam cells in mouse models and their susceptibility to atherosclerosis [32, 33]. Expression of apo A-I and/or apo A-IV helps in protecting against atherosclerosis even in hyperlipidemia cases [34].
Anti Tumor Activity of apo A-I
Recently the anti tumor activity of apo A-I has been highlighted. It had revealed itself as a therapeutic molecule against cancer, because of its cancer fighting effects in mouse models of melanoma and lung cancers. In mice, injected apo A-I expressed reduction in already developed palpable tumors and metastases by modulating the immune system of the host to create an environment not suitable for the tumor [35]. In another study conducted in mouse models with ovarian cancer, it was demonstrated that ovarian cancers are associated with lowered apo A-I concentration. Overexpression of human apo A-I in transgenic mice have shown inhibition of tumour growth and improved survival rate. Decreased apo A-I levels have been reported in the serum of patients with pancreatic cancer, gastric cancer, and ovarian cancer [36–38]. Apo A-I along with apo A-11, transthyretin are found decreased by more than two fold in pancreatic cancers [36]. Chong et al. in their study of gastric cancers got varied expression of apo A-I in plasma in different size tumors. The expression is more reduced in large size tumors than the small size tumors [37]. Amongst others like transthyretin, transferrin, beta-haemoglobin, Apo A-I is one of the biomarker for ovarian cancer, as suggested by Kozak et al. [38], these together with CA-125 may help in detecting early stage ovarian cancer [38]. A lowered apo A-I, along with low HDL-C and elevated triglyceride were observed in acute lymphoblastic leukemia (ALL) [39]. Scribano et al. [40], showed that patients with ALL who recovered after getting suitable chemotherapy documented a significant increase in plasma apo A-I levels along with total cholesterol, HDL2, HDL3, etc. [40].
Pro-inflammatory and pro-angiogenic lysophospholipids such as lysophosphatidic acid (LPA) have been repeatedly shown to be associated with tumor progression and poor prognosis, and are normally cleared from the serum by apo A-I, which is downregulated in ovarian, gastric and pancreatic cancer patients. Both apo A-I as well as apo A-I mimetic peptides have the ability to bind pro-inflammatory phospholipids like LPA [41], but the apo A-I mimetic peptides bind LPA with a greater affinity than apo A-I, causing their removal from serum and lowering their serum levels. Thus apo A-I controls the rate of cell proliferation in a tumor and the tumor growth [42].
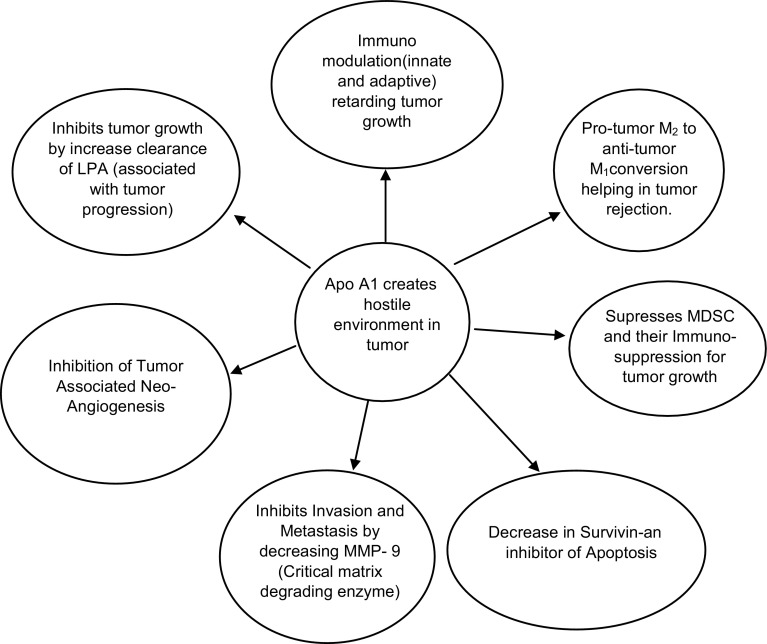
The tumor growth associated with evasion of immune system of tumor cell, its associated angiogenesis, its migration, distant metastasis, all are dependent upon disordered immune system. The efficacy of apo A-I to modulate the immunological environment of the tumor, to retard the tumor growth has been explained by, Zamanian-Daryoush et al. [15]. Accordingly the anti cancer effect of apo A-I is not due to direct inhibition of tumor cell replication or cell death, rather indirect by affecting both arms, (innate and adaptive)of immune system, as well as by inhibiting neo- angiogenesis. Tumor-associated macrophages, that form a constant part of the tumor microenvironment in many distinct types of malignancy polarize towards an anti-inflammatory M2 phenotype with expressed scavenger receptor A (SR A), hence promoting tumor progression. However, a change in phenotype expression takes place by apo A-I in the tumor-associated macrophages, from a pro-tumor M2 to an anti-tumor M1 phenotype responsible for tumor rejection, thus helping to establish the therapeutic efficacy of apo A-I against cancers [15, 43]. These SR A on macrophages can be therapeutically targeted in vivo with the small peptide inhibitor 4F, a D- amino acid peptide, an apo A-I mimetic, by blocking SRA-mediated macrophage adhesion, and help to prevent tumor progression and distant metastasis. Both 4F and SRA exhibit their action using the same signaling pathway. Besides other anti neoplastic effects like antiangiogenesis, anti-inflammatory, antioxidant activity with lowering of serum LPA, 4F helps in retardation of tumor growth [44].
Distant metastasis is associated with degradation of basement membranes and remodeling of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs),that operates with a large number of non-matrix proteins necessary for neo angiogenesis, MMPs concentration are observed to be low in many invasive, metastatic tumors. The potent anti tumorigenic activity of apo A-I is attributed to inhibition of tumor-associated angiogenesis and concomitant reduction in MMP-9 protein levels and activity, a critical matrix degrading enzyme whose action culminates in the availability of pro-angiogenic factors [45].
Myeloid-derived suppressor cells (MDSCs) behave as negative immunomodulators and potent stimulators of tumor growth [46] by aggregating within the tumor and cause T-cell suppression, thus resulting in tumor induced immunosuppression. Their number increases both within and outside the tumor, in response to various molecules present within the tumor itself. However, apo A-I also suppresses this increase of MDSCs, both within and outside the tumor and help in tumor regression [15, 43]. Not only that, apo A-I also causes decline in survivin, an inhibitor of apoptosis and cell cycle promoter, responsible for recurrence and reduced survival in melanoma, as shown by Piras et al. [47] (Fig. 4) [15, 43, 47].
Fig. 4.
Anti tumor role of apo A-I
Conclusion
The potent immunomodulatory effects with resultant anti-tumor biological activity of apo A-I suggests that pharmacological delivery of apo A-I may be exploited for therapeutic gain as an anticancer agent, for regression of tumor and its metastasis. Administration of apo A-I or apo A-I elevating drugs have shown potential therapeutic benefit, for cardiovascular diseases [48–51]. Recent studies regarding, this apolipoprotein has revealed to have anti-tumour activity partly through modulation of immune cell function via regulation of cholesterol content in critical subcellular compartments. Its potential therapeutic benefit in regression of tumor metastases in many animal studies has already been proved. However sufficient experimental studies in humans is lacking except for malignant melanoma and needs further studies.
References
- 1.Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K, Thornton SJ. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nat Rev Drug Discov. 2008;7(1):84–99. doi: 10.1038/nrd2353. [DOI] [PubMed] [Google Scholar]
- 2.Phillips JC, Wrigglers W, Li Z, Schulten K. Predicting the structure of apolipoprotein AI in reconstituted high density lipoprotein disks. Biophys J. 1997;73:2337–2346. doi: 10.1016/S0006-3495(97)78264-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yui Y, Aoyama T, Morishita H, Takahashi M, Takatsu Y, Kawai C. Serum prostacyclin stabilizing factor is identical to apolipoprotein A-I (apo A-I). A novel function of apo A-I. J Clin Invest. 1988;82(3):803–807. doi: 10.1172/JCI113682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oda MN, Budamagunta MS, Borja MS, Petrlova J, Voss JC, Lagerstedt JO. The secondary structure of Apolipoprotein A-1 on 9.6 nm reconstituted high density lipoprotein determined by EPR Spectroscopy. FEBS J. 2013;280(14):3416–3424. doi: 10.1111/febs.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuellar LÁ, Prieto ED, Cabaleiro LV, Garda HA. Apolipoprotein A-I configuration and cell cholesterol efflux activity of discoidal lipoproteins depend on the reconstitution process. Biochim Biophys Acta. 2014;1841(1):180–189. doi: 10.1016/j.bbalip.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Frank PG, Marcel YL. Apolipoprotein A-I: structure-function relationships. J Lipid Res. 2000;41(6):853–872. [PubMed] [Google Scholar]
- 7.Adachi E, Kosaka A, Tsuji K, Mizuguchi C, Kawashima H, Shigenaga A, et al. The extreme N-terminal region of human apolipoprotein A-I has a strong propensity to form amyloid fibrils. FEBS Lett. 2014;588(3):389–394. doi: 10.1016/j.febslet.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Arinami T, Hirano T, Kobayashi K, Yamanouchi Y, Hamaguchi H. Assignment of the apolipoprotein A-I gene to 11q23 based on RFLP in a case with a partial deletion of chromosome 11, del(11)(q23.3→qter) Hum Genet. 1990;85(1):39–40. doi: 10.1007/BF00276323. [DOI] [PubMed] [Google Scholar]
- 9.Tall AR, Wang N. Tangier disease as a test of reverse cholesterol transport hypothesis. J Clin Invest. 2000;106(10):1205–1207. doi: 10.1172/JCI11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschini G, Sirtori M, Gianfranceschi G, Sirtori CR. Relation between the HDL apoproteins and A-I isoproteins in subjects with the AIMilano abnormality. Metab Clin Exp. 1981;30(5):502–509. doi: 10.1016/0026-0495(81)90188-8. [DOI] [PubMed] [Google Scholar]
- 11.Dastani Z, Dangoisse C, Boucher B, Desbiens K, Krimbou L, Dufour R, et al. A novel nonsense apolipoprotein A-I mutation (apoA-I(E136X)) causes low HDL cholesterol in French Canadians. Atherosclerosis. 2006;185(1):127–136. doi: 10.1016/j.atherosclerosis.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, DiDonato JA, Levison BS, Schmitt D, Li L, Wu Y, et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med. 2014;20(2):193–203. doi: 10.1038/nm.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mossuz P, Bouamrani A, Brugière S, Arlotto M, Hermouet S, Lippert E, et al. Apolipoprotein A1: a new serum marker correlated to JAK2 V617F proportion at diagnosis in patients with polycythemia vera. Proteomics Clin Appl. 2007;1(12):1605–1612. doi: 10.1002/prca.200601051. [DOI] [PubMed] [Google Scholar]
- 14.Koldamova RP, Lefterov IM, Lefterova MI, Lazo JS. Apolipoprotein AI directly interacts with amyloid precursor protein and inhibits A beta aggregation and toxicity. Biochemistry. 2001;40(12):3553–3560. doi: 10.1021/bi002186k. [DOI] [PubMed] [Google Scholar]
- 15.Zamanian-Daryoush M, Lindner D, Tallant TC, Wang Z, Buffa J, Klipfell E, et al. The cardioprotective protein apolipoprotein A I promotes potent anti-tumorigenic effects. J Biol Chem. 2013;288(29):21237–21252. doi: 10.1074/jbc.M113.468967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50(Suppl):S195–S200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein:it’s not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, Van Den Abbeele J, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–87. doi: 10.1038/nature01461. [DOI] [PubMed] [Google Scholar]
- 20.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunfeld C, Marshall M, Shisenega JK, Moser AH, Tobias P, Feingold KR. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J Lipid Res. 1999;40:245–254. [PubMed] [Google Scholar]
- 22.Ma J, Liao XL, Lou B, Wu MP. Role of apolipoprotein A-I in protecting against endotoxin toxicity. ActaBiochim Biophys Sin (Shanghai) 2004;36(6):419–424. doi: 10.1093/abbs/36.6.419. [DOI] [PubMed] [Google Scholar]
- 23.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it’s not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22(1):9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh IP, Chopra AK, Coppenhaver DH, Ananatharamaiah GM, Baron S. Lipoproteins account for part of the broad non-specific antiviral activity of human serum. Antiviral Res. 1999;42(3):211–218. doi: 10.1016/S0166-3542(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 25.Srinivas RV, Birkedal B, Owens RJ, Ananatharamaiah GM, Segrest JP, Compans RW. Antiviral effects of Apolipoprotein AI and its synthetic amphipathic peptide analogs. Virology. 1990;176(1):48. doi: 10.1016/0042-6822(90)90229-K. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, et al. Apo lipoprotein A1 Modulates regulatory T cells in auto immune LDL r-/-, Apo A 1-/- mice. J Biol Chem. 2010;285(46):16158. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc Res. 2014;103(3):372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 28.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, III, Roux-Lombard P, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.V97.8.2381. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann M, Taylor P, Paleolog E, Brennan FM, Maini RN. Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn’s disease: analysis of the mechanism of action predicts utility in other diseases. Transpl Proc. 1998;30:4126–4127. doi: 10.1016/S0041-1345(98)01365-7. [DOI] [PubMed] [Google Scholar]
- 30.Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, ATTRACT Study Group et al. Infliximab(chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/S0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- 31.Kalden JR, Breedveld FC, Burkhardt H, Burmester GR. Immunological treatment of autoimmune diseases. Adv Immunol. 1998;68:333–418. doi: 10.1016/S0065-2776(08)60564-7. [DOI] [PubMed] [Google Scholar]
- 32.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoproteinA-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.CIR.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 33.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vergnes L, Baroukh N, Ostos MA, Castro G, Duverger N, Nanjee MN, et al. Expression of human apolipoprotein A-I/C-III/A-IV gene cluster in mice induces hyperlipidemia but reduces atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2266–2274. doi: 10.1161/01.ATV.20.10.2267. [DOI] [PubMed] [Google Scholar]
- 35.Neale, T.: HDL component an enemy to cancer in mice. http://www.medpagetoday.com/Cardiology/Dyslipidemia/40579 (2013). Accessed 22 July 2013.
- 36.Ehmann M, Felix K, Hartmann D, Schnölzer M, Nees M, Vorderwülbecke S, et al. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205–214. doi: 10.1097/01.mpa.0000250128.57026.b2. [DOI] [PubMed] [Google Scholar]
- 37.Chong P-K, Lee H, Zhou J, Liu S-C, Loh MCS, So JBY, et al. Reduced plasma APOA1 level is associated with Gastric Tumor Growth in MKN45 mouse xenograft mode. J Proteomics. 2010;73(8):1632–1640. doi: 10.1016/j.jprot.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Kozak KR, Su F, Whitelegge JP, Faull K, Reddy S, Farias-Eisner R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 39.Halton JM, Nazir DJ, McQueen MJ, Barr RD. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer. 1998;83:379–384. doi: 10.1002/(SICI)1097-0142(19980715)83:2<379::AID-CNCR24>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Scribano D, Baroni S, Pagano L, Zuppi C, Lione G, Giardina B, et al. Return to normal values of lipid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica. 1996;81:343–345. [PubMed] [Google Scholar]
- 41.Li H, Wang D, Zhang H, Kirmani K, Zhao Z, Steinmetz R, et al. Lysophosphatidic acid stimulates cell migration, invasion, and colony formation as well as tumorigenesis/metastasis of mouse ovarian cancer in immunocompetent mice. Mol Cancer Ther. 2009;8:1692–1701. doi: 10.1158/1535-7163.MCT-08-1106. [DOI] [PubMed] [Google Scholar]
- 42.Su F, Kozak KR, Imaizumi S, Gao F, Amneus MW, Grijalva V, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc Natl Acad Sci USA. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neyen C, Pluddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. J Immunol. 2013;190:000–000. [DOI] [PMC free article] [PubMed]
- 44.Neyen C, Mukhopadhyay S, Gordon S, Hagemann T. An apolipoprotein A-I mimetic targets scavenger receptor A on tumor-associated macrophages A prospective anticancer treatment. Oncoimmunology. 2013;2(6):e24461. doi: 10.4161/onci.24461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hofmann UB, Houben R, Bröcker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–314. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piras F, Murtas D, Minerba L, Ugalde J, Floris C, Maxia C, et al. Nuclear survivin is associated with disease recurrence and poor survival in patients with cutaneous malignant melanoma. Histopathology. 2007;50:835–842. doi: 10.1111/j.1365-2559.2007.02695.x. [DOI] [PubMed] [Google Scholar]
- 48.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ, et al. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.ATV.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 49.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 50.Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 51.Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J Lipid Res. 2009;50:894–907. doi: 10.1194/jlr.M800622-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]