Abstract
Sepsis is associated with various metabolic derangements as a consequence of inflammatory response, ischemia and oxidative stress. Four parameters of relevance are procalcitonin (PCT), ischemia modified albumin (IMA) pH and lactate. The study was carried out to highlight the concomitant occurrence of sepsis, ischemia and lactic acidosis, all of which could have deleterious effects on organ function. 26 critically ill patients with a provisional diagnosis of sepsis were the test subjects. The control group had 25 apparently healthy volunteers. PCT, lactate and IMA were assayed. PCT was estimated on an automated analyser using electro-chemiluminescence. Lactate and pH were estimated on a blood gas analyzer. Serum IMA was estimated spectrophotometrically by Albumin Cobalt Binding Test. Statistical tools like students ‘t’ test and Venn diagram were employed to depict the outcome of the study. All critically ill patients had significantly higher IMA levels (0.96746 ± 0.73407) as compared to the control group (0.00728 ± 0.00895) with a p value of <0.0001. The Venn diagram was used to depict the finding that all 26 test subjects had elevated levels of IMA, of which PCT was elevated in 22 and lactate in 20. Both PCT and lactate were abnormal in 17 patients. The most significant observation was that all critically ill patients, irrespective of the presence of sepsis or lactic acidosis had elevated levels of IMA which is clearly indicative of the ubiquitous presence of oxidative stress. The Venn diagram is an elegant representation of the concurrent multiple pathophysiological processes which occur in critically ill patients.
Keywords: Venn diagram, Ischemia modified albumin, Lactate, Procalcitonin, Oxidative stress
Introduction
Sepsis is a frequently encountered condition amongst critically ill patients and a common cause of death in intensive care units of hospitals. The death toll has been reported to be comparable to that seen in patients with myocardial infarction. Sepsis has been referred to as a public health disaster [1]. The accurate and timely detection of sepsis still remains challenging as there is no specific biomarker or constellation of tests which could help in diagnosing this condition. In recent times, procalcitonin has been widely used to diagnose sepsis [2]. However, it has been reported that non-specific elevations of PCT can occur in patients under severe stress, especially following surgery or trauma and also in patients after cardiac shock [3]. Moreover the onset of sepsis and the response of counter-regulatory mechanisms that it triggers, leads to the release of several biomolecules such as cytokines, chemokines and vaso-active peptides. These in turn directly impact metabolism at the level of cells tissues and organs. Mitochondrial dysfunction and inhibition of utilization of oxygen is one such effect that has been found to occur in sepsis [4]. It is therefore imperative to analyse and interpret the results not of single tests, but groups of tests in order to understand and elucidate the pathophysiologic mechanisms which occur in patients with sepsis. It is possible that some or all of these might be of relevance in particular cases depending upon the severity of sepsis and the retaliatory response that it provokes. Ischemia modified albumin (IMA) is a novel biomarker, which has been shown to be useful in detecting ischemic oxidative stress [5]. Levels of IMA have to been demonstrated to be increased in various states associated with tissue hypo-perfusion, including sepsis [6, 7]. It is well known that sepsis is associated with metabolic acidosis as a consequence of accumulation of lactate [8]. This study seeks to highlight the importance of simultaneously quantifying and analyzing the following tests namely, PCT, IMA, the acid–base status of the patient represented by the pH of blood and plasma lactate levels.
Materials and Methods
Ethical approval for carrying out the study was obtained from the Institutional Human Ethics Committee of our institution. Informed consent was obtained from patients who were admitted into the critical care unit of the hospital. Samples that were used for the study, were those sent to the clinical laboratory for regular investigations. No separate samples were drawn in order to conduct this study. They were collected soon after the patients were admitted into the critical care unit.
The selection criteria for the study subjects were based on the physicians’ provisional diagnosis at the time of admission. Critically ill patients admitted into the ICU with a requisition for PCT analysis and arterial blood gas analysis formed the test group known as the ‘Critically Ill Group’ or the ‘Test Group’. This group consisted of 26 subjects, 17 males and 9 females with an age range of 34–82 years. The ‘control group’ comprised of 25 apparently healthy human adult volunteers with 19 males and 6 females in the age range of 44–75 years. These samples were obtained from voluntary blood donors.
Methods
Five milliliter of random venous blood sample was collected using Becton–Dickinson ‘vacutainers’ and were transported to the clinical biochemistry laboratory within 15 min. The samples were allowed to clot, centrifuged thereafter and the serum obtained used for analysis of procalcitonin and ischemia modified albumin.
Heparinized arterial blood samples transported on ice were used for arterial blood gases including pH and lactate. These tests were done as soon as the sample was received on an arterial blood gas analyser Cobas b 221.
Estimation of Serum PCT
Procalcitonin was estimated by a method based upon electro-chemiluminescence using the Sandwich principle. The PCT in the sample reacted with a biotinylated monoclonal specific antibody and also with a monoclonal PCT specific labelled antibody with a ruthenium complex to form a sandwich complex. A second reagent, streptavidin-coated micro particles, interacted with the complex and was bound to the solid phase via interaction between biotin and streptavidin. The reaction mixture was aspirated into the measuring cell where micro particles were magnetically captured onto the surface of the electrode. On application of a voltage to the electrode, a chemiluminescent emission was induced and measured by a photomultiplier. Results were determined by a calibration curve which was instrument- specifically generated by a two point calibration. The instrument used was ELECSYS 2010 a fully automated analyzer. A value <0.5 ng/mL represents a low risk of severe sepsis and/or septic shock [9].
The Albumin Cobalt Binding Test (ACB Test)
The ACB test was used the estimate the serum levels of IMA. The principle of this test is based on the measurement of unbound cobalt after adding a known amount in a serum sample with IMA, as it is known that IMA has impaired metal binding as opposed to structurally normal albumin. The test involved the addition of 200 µL of the patients’ serum to 50 µL of cobalt chloride (1 g/L) followed by vigorous mixing. It was incubated for 10 min. This is followed by addition of 50 µL of dithiothretol (DTT) (1.5 g/L) and 2 min incubation at room temperature. DTT binds to unbound cobalt and serves as a selective indicator for the same. Finally 1 mL of physiological saline was added. A Gilford spectrophotometer at 470 nm was to read the IMA levels in absorbance units (ABSU). The blank was prepared using the same steps but with exclusion of DTT [7, 10].
Lactate and pH Estimation
L lactate and pH were estimated using arterial blood, amperometrically on Cobas b 221 system. Lactate oxidase that was immobilized in the lactate biosensor converted lactate to pyruvate and hydrogen peroxide. The hydrogen peroxide that was liberated was oxidised in a platinum electrode to produce a current. The current is proportional to the concentration of lactate in the test sample. A value >2.2 mmol/L was taken to be abnormal [11]. The biological reference interval for pH of blood was considered to be 7.35–7.45 [12].
Statistical Analysis
The Venn diagram, Students ‘t’ test and Pearson product-moment correlation coefficient were used for data analysis.
A Venn diagram is a diagrammatic representation consisting of intersecting circles. It portrays similarities, differences, and relationships between groups. Similarities between groups are segregated within intersecting portions of the circles, while differences are represented in the non-intersecting portions of the circles.
Results
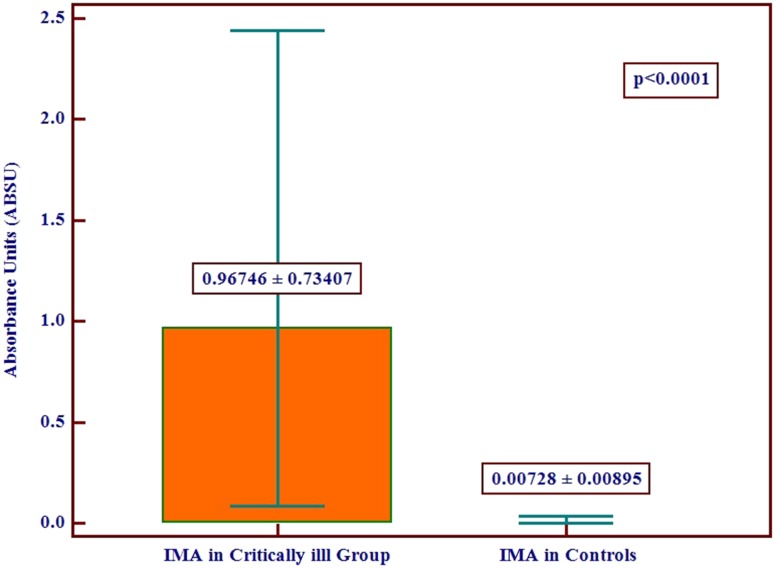
The IMA levels in the two groups have been illustrated (Fig. 1). There was a statistically highly significant difference in serum IMA levels between the critically ill group and the controls with a p value <0.0001. The IMA in the critically ill group was 0.96746 ± 0.73407 ABSU, while in the control group it was 0.00728 ± 0.00895 ABSU. All critically ill patients had IMA levels considerably higher than those in the control group. The mean IMA level in the patient group was 13 times higher than in the control group. The level of IMA in the control group was negligibly small although it was measured and expressed in numbers.
Fig. 1.
Graph depicting mean IMA levels with standard deviation of the test and control groups
The ‘critically ill group’ also had elevated levels of PCT with values ranging from 0.111 to 500 ng/mL. Any value >0.5 ng/mL was taken as abnormal [9]. 22 out of the 26 critically ill patients were diagnosed as having sepsis.
The lactate levels in the ‘critically ill group’ was 4.2 ± 3.0156 mmol/L while the pH was 7.2877 ± 0.1183. Twenty critically ill patients had lactic acidosis with lactate and pH levels higher than the reference range.
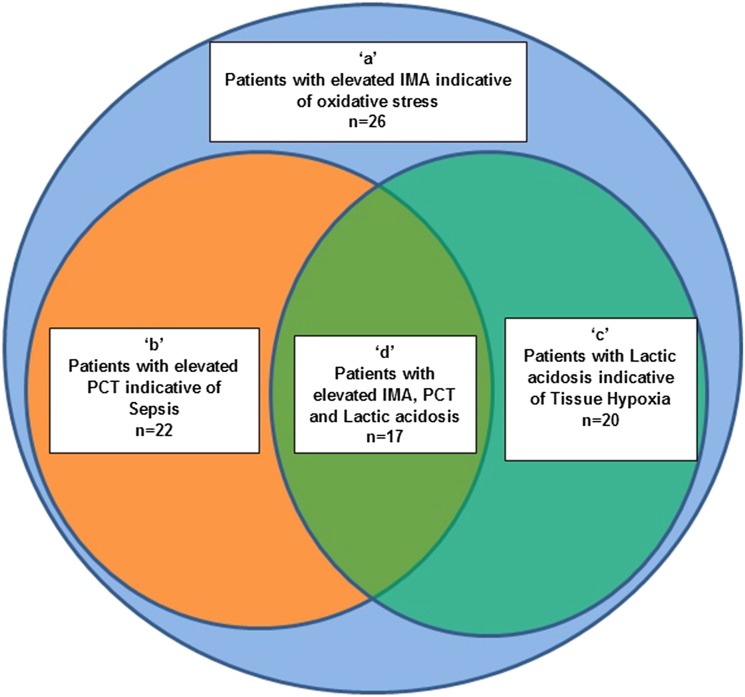
The Venn diagram (Fig. 2) represents the number of patients with biochemical evidence of sepsis, lactic acidosis and both in the ‘critically ill group’. The outermost circle ‘a’ represents the fact that the entire group consisting of 26 critically ill patients had elevated IMA levels. Enclosed within are two intersecting circles ‘b’ and ‘c.’ Circle ‘b’ represents patients with elevated PCT while circle ‘c’ represents those with elevated lactate alone. The intersection area marked ‘d’ highlights patients with maximum criticality as they present with all three pathological phenomena oxidative stress, sepsis as well as lactic acidosis.
Fig. 2.
Venn diagram showing the interacting pathophysiological mechanisms in the test group
Amongst the four parameters that were analyzed in the critically ill group, there was no correlation between IMA and PCT, IMA and lactate as well as IMA and pH. There was a statistically significant positive correlation between lactate and PCT with a ‘p’ value of 0.0094 and an ‘r’ value of 0.4995. Also there was a negative correlation between lactate and pH with a ‘p’ value of 0.0143 and an ‘r’ value of −0.4747.
Discussion
The most significant observation of this study was that, all critically ill patients irrespective of the presence of lactic acidosis or sepsis had elevated levels of IMA. It is a well established fact that following an episode of ischemia and oxidative stress the N terminal end of albumin undergoes a change in conformation which results in a decreased ability of the biomolecule to bind cobalt. The product formed is referred to as IMA which could serve as a ubiquitous indicator of ischemic damage [13]. This signifies that in critically ill patients, regardless of the underlying pathophysiological changes, damage at the bimolecular level is an inevitable event and may be a prelude to the development of organ dysfunction. It has been reported that IMA levels are higher in patients with myocardial ischemia against those without it [14]. A prospective study done in a large number of patients had shown that serum malondialdehyde levels are higher in patients with sepsis [15]. Malondialdehyde is considered to be the stable end-product of lipid peroxidation a by-product of free radical damage to the cellular membranes which are rich in poly-unsaturated fatty acids [16]. The results of our study is in keeping with this report, as elevated IMA levels help to detect and quantify oxidative damage at the molecular level.
High levels of IMA can signal a perpetuation of peroxidative damage and can clinically identify patients at a risk of myocardial ischemia. It is not unreasonable to surmise that, at least some of these patients might have had co-existing undiagnosed coronary artery disease and in such a scenario, the risk of fatal outcome becomes enormous. The Venn diagram has given an elegant representation of the concurrent multiple pathophysiological processes which occur in critically ill patients. It has been used to highlight the simultaneous existence of sepsis, ischemia and lactic acidosis, all of which could have deleterious effects on organ function. Sepsis by itself produces several deleterious effects on the heart. It leads to the release of cytokines, nitric oxide, endothelins and prostanoids, all of which are known to have inhibitory effects on the myocardium [17].
The third factor that interacts in a sub-group of patients is the metabolic acidosis as evidenced by low pH and elevated lactate in a number of these patients. The significant correlation that is seen between lactate and PCT could be taken evidence of the cause and effect relationship between these two. Determination of lactate levels help to recognize the occult state of hypo-perfusion that occurs in patients with septic shock. The shift in the metabolism from an aerobic to an anaerobic mode can severely limit the production of ATP with profound functional effects on vital organ systems culminating in a fatal multi-organ failure [18, 19]. Measuring and monitoring lactate levels is therefore of utmost importance if mitigating interventions need to be instituted.
The study has brought out very elegantly the multiple biochemical phenomena which operate in critically ill patients. The use of Venn diagram serves to illustrate this in a simple but comprehensive manner. The limitation of the study was that the analysis was restricted to single samples. It was not carried forward to see the effects of good or bad prognosis, as well as the effects of therapeutic interventions, on these parameters.
To sum up, this study has sought to highlight the coexistence of oxidative stress, sepsis and lactic acidosis in several critically ill patients. These could be diagnosed and monitored by estimating four parameters that are easily measurable namely, IMA, PCT, pH and lactate. The Venn diagram gives an excellent diagrammatic representation of the interacting pathological mechanisms in critically ill patients, some or all of which might be operative sub-sets of patients.
Compliance with Ethical Standards
Conflict of interest
None.
Research Involving Human Participants and/or Animals
This work was done on samples of blood sent to the clinical biochemistry laboratory for routine investigations from patients. Tests were carried out in the left-over samples. Blood was not drawn for the purpose of the study. No animals were used in this study.
Informed Consent
General consent was obtained from patients for all investigations done. No invasive procedures were carried out in this study. Anonymity was maintained.
Contributor Information
Prashanth Ashok Kumar, Email: wizardakp@ymail.com.
Usha Anand, Email: ushaanandashwin@gmail.com.
References
- 1.Reinhart K, Bauer M, Riedermann CN, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609–634. doi: 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudhir U, Venkatachalaiah RV, Kumar TA, Rao MY, Kempegowda P. Significance of serum procalcitonin in sepsis. Indian J Crit Care Med. 2011;15(1):1–5. doi: 10.4103/0972-5229.78214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorge H, Schondube FA, Dorge P, Seipelt R, Voss M, Messmer BJ. Procalcitonin is a valuable prognostic marker in cardiac surgery but not specific for infection. Thorac Cardiovasc Surg. 2003;51:322–326. doi: 10.1055/s-2003-45425. [DOI] [PubMed] [Google Scholar]
- 4.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–264. doi: 10.1111/j.1476-5381.2009.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24:333–341. doi: 10.2133/dmpk.24.333. [DOI] [PubMed] [Google Scholar]
- 6.Yerlikaya FH, Kurban S, Mehmetoglu I, Annagur A, Altunhan H, Erbay E, et al. Serum ischemia-modified albumin levels at diagnosis and during treatment of late-onset neonatal sepsis. J Matern Fetal Neonatal Med. 2014;27:1723–1727. doi: 10.3109/14767058.2013.876621. [DOI] [PubMed] [Google Scholar]
- 7.Prashanth AK, Anand U. Clinical significance of ischemia modified albumin in critically ill patients with sepsis. Indian J Clin Biochem. 2015;30:194–197. doi: 10.1007/s12291-014-0434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clec’h C, Ferriere F, Karuobi P, Fosse JP, Cupa M, Hoang P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166–1169. doi: 10.1097/01.CCM.0000126263.00551.06. [DOI] [PubMed] [Google Scholar]
- 10.Chawla R, Goyal N, Calton R. Ischemia modified albumin: a novel marker for acute coronary syndromes. Indian J Clin Biochem. 2006;21:77–82. doi: 10.1007/BF02913070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 12.Schwalfenberg GK. The alkaline diet: is there evidence that an alkaline pH diet benefits health? J Environ Public Health. 2012;2012:727630. doi: 10.1155/2012/727630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellidag HY, Eren E, Aydin O, Akgol E, Yalcinkaya S, Sezer C, et al. Ischemia modified albumin levels and oxidative stress in patients with bladder cancer. Asian Pac J Cancer Prev. 2013;14:2759–2763. doi: 10.7314/APJCP.2013.14.5.2759. [DOI] [PubMed] [Google Scholar]
- 14.Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski JC. Role of “ischemia modified albumin”, a new biochemical marker of myocardial ischemia, in the early diagnosis of acute coronary syndromes. Emerg Med J. 2004;21:29–34. doi: 10.1136/emj.2003.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss SL, Deutschman CS. Elevated malondialdehyde levels in sepsis—something to ‘stress’ about. Crit Care. 2014;18:125. doi: 10.1186/cc13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 18.Krishna U, Joshi SP, Modh M. An evaluation of serial blood lactate measurement as an early predictor of shock and its outcome in patients of trauma or sepsis. Indian J Crit Care Med. 2009;13:66–73. doi: 10.4103/0972-5229.56051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phypers B, Tom Pierce JM. Lactate physiology in health and disease. Contin Edu Anaesth Crit Care Pain. 2006;6:128–132. doi: 10.1093/bjaceaccp/mkl018. [DOI] [Google Scholar]