Abstract
The continuous use of synthetic hormones as contraceptive pill or hormonal replacement therapy among women is increasing day by day. The widespread use of different formulations as oral contraceptives by women throughout their reproductive cycle has given rise to a serious concern for studying the effects of oral contraceptives on enzymatic profile and DNA damage in peripheral blood lymphocytes among users. The present study was carried out on women taking oral contraceptives. The study was based on the questionnaire having the information of reproductive history, fasting, age, health, nature of menstrual cycle, bleeding and other disease. The profile of the blood serum enzymes i.e. alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), lactate dehydrogenase (LDH), aminotransferases (SGOT and SGPT), serum proteins (albumin and globulin) and DNA damage in lymphocytes was studied among users and non-users. The results of the present study suggest that OCs not only effects enzymatic activity but also results in DNA damage that may vary with the duration of using oral contraceptives. A significant increase in LDH, GGT, SGPT, SGOT, globulin and decrease in ALP as well as albumin was found among users as compared to non-users. The observed DNA damage was more in users as compared to non-users. Hormonal contraceptives seem to exert DNA damage and also have significant effects on blood serum enzymes.
Keywords: Blood serum enzyme, Comet assay, DNA damage, Oral contraceptive pills
Background
Women use exogenous female hormones as oral contraceptives (OCs). Over several decades the side effects of OCs are of major public health interest. Their association with ovarian cancers in particular has drawn an attention of many epidemiologists [1]. Soon after the introduction of OC pills a number of side effects have been reported among users [2]. The contraceptive containing either a combination of an estrogen and progesterone or progesterone only is being used by millions of women [3]. The most effective contraceptive works by suppressing the levels of follicle-stimulating hormone and luteinizing hormone i.e. by reducing metabolic activity of ovary, including the suppression of ovulation [4]. Currently there are several hormonal methods available other than oral pills such as transdermal patches, vaginal rings and intrauterine systems [3]. Although OCs may be useful in preventing of pregnancies but the results from research studies suggest their negative effects on health such as coronary atherosclerosis and myocardial infarction, risk of breast cancer and hepatocellular carcinoma [5–14]. In the present study blood serum enzymes such as alkaline phosphatase (ALP), gamma-glutamyltranseferase (GGT), lactate dehydrogenase (LDH), amino transeferases (SGOT and SGPT), serum proteins (albumin and globulin) and DNA damage in the peripheral blood lymphocytes were studied among women of different age groups using OCs for various durations.
Materials and Methods
Sample Size
A total of 600 subjects were included in the study from Jawahar Lal Nehru Medical College of Aligarh Muslim University, Aligarh, who were 16–above 40 years of age. Among them 300 women were taking OCs preferring continuous months and 300 were non-users healthy women with regular menstrual cycle who were not taking OCs. The detailed questionnaire includes some issues such as age, health history, type of pill, nature of menstrual cycle, any other disease, smoking habit, alcoholism, bleeding etc. About 64 % of women were taking OCs as birth control, 15 % for irregular bleeding, 11 % for dysmenorrhea/pelvic pain, 6 % for premenstrual syndrome and 4 % for menorrhagia. A written consent was taken from each subject taking participation in the study.
Blood Sampling and Processing
After overnight fasting about 2 mL of blood was collected in a vacutainer tube with a clot activator from women in the study sample. The serum was obtained by centrifugation at 3000 rpm for 15 min and analyzed for various enzyme activities.
Blood Serum Analysis
The blood serum was analyzed for enzymes: ALP, GGT, LDH, aminotransferases (SGPT and SGOT) and serum proteins (albumin and globulin) among users and non-users by using commercially available diagnostic test kits (Crest Biosystems kits, India).
Blood Sampling for Comet Assay
After overnight fasting about 2 mL of blood was collected in a heparinised vacutainer tube from each woman (user and non-user). The blood lymphocytes were isolated by Ficoll–Histopaque density gradient centrifugation and washed in PBS [15, 16].
Slide Preparation
The alkaline Single Cell Gel Electrophoresis technique of Singh et al. [17] was followed. The half frosted slides were covered with 1 % normal melting agarose (NMA) at about 45 °C in PBS. The slides were immediately covered with a coverslip and kept at room temperature for about 5 min to allow the agarose to solidify. This layer was followed by the second layer of 0.5 % LMA. Five to 10 µL of cell suspension was mixed with 75 µL of 0.5 % LMA for embedding on slides. After gently removing the coverslip the cell suspension was laid onto the first agarose layer, spread out with a coverslip, and maintained on an ice-cold flat tray for 5 min to solidify. After removal of the coverslip the slides were immersed in cold lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma base, 0.2 mM NaOH, pH 10), 1 % Triton X-100 and 10 % DMSO for overnight at 4 °C. The slides were removed from the lysing solution, drained and placed in a horizontal gel electrophoresis tank near the anode. The tank was filled with fresh electrophoresis buffer (1 mM Na2EDTA and 300 mM NaOH, pH 13) to a level approximately 0.25 cm above the slides. Before electrophoresis, the slides were left in the buffer for 20 min to allow the unwinding of the DNA. Electrophoresis was conducted at 1.6 V/cm for 20 min (300 mA) at room temperature. All steps were conducted under dimmed light (tank was covered with a black cloth) to prevent the occurrence of additional DNA damage by UV light. After electrophoresis, the slides were taken out of the tank. Tris buffer (0.4 M Tris, pH 7.5) was gently added drop wise to neutralise the excess alkali. The neutralising procedure was repeated three times.
Staining
To each slide, 65 µL ethidium bromide (EtBr-20 µg/mL) was added. The slides were covered with a cover-slip, placed in a humidified air-tight container to prevent drying of the gel and analysed within 3–4 h.
Slide Scoring
About 50 cells were scored per slide (three slides/subjects) were scored randomly and analyzed with Cometscore™ software (version 1.5, TriTek Corporation, Sumerduck, VA, USA). DNA tail length was measured and expressed as arbitrary unit.
Statistical Analysis
The obtained data was analyzed by using a software statistical package SPSS version 16.0. Student’s t test was applied for the significant difference among users and non users. One-way analysis of variance (ANOVA) using post hoc Tukey test was applied to compare the differences among the users of different age groups.
Results
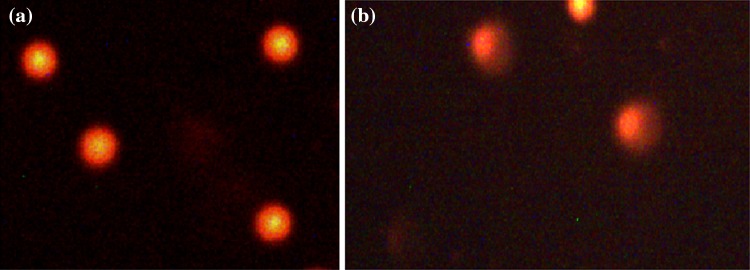
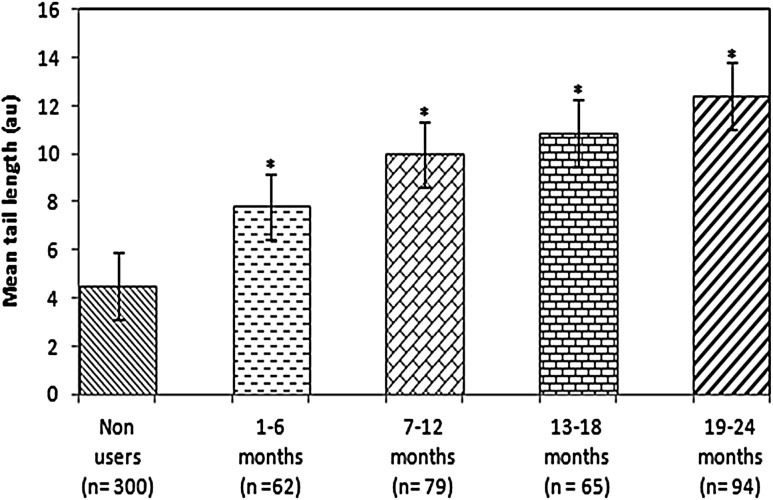
Table 1 shows the age distribution of OCs users and non-users. Table 2 shows the distribution of users according to the duration of using OCs. Most of the users were of 26–30 years of age group. Table 3 describes the composition of OCs used by the women. The highest concentration of estrogens in the combined OCs was 30 µg. Table 4 shows the distribution of various blood serum enzymes in women of different age groups. As is evident from the Table 4, a significant decrease in the ALP (U/L) was observed among the users of all age groups as compared to their respective age group non-users (p < 0.05). Concerning the other enzymes such as LDH (U/L), SGOT (U/L), SGPT (U/L), and GGT (U/L), a significant increase in their levels were found among users of all age groups as compared to non-users to their respective age groups (p < 0.05). The level of serum albumin was also found to be decreased significantly among users of all age groups compared to non users of their respective age group (p < 0.05) (Table 4). The level of serum globulin was found to be significantly higher among users of all age group compared to non users of their respective age group (p < 0.05) (Table 4). Table 5 shows the enzyme profile according to the duration of using the OCs. A duration dependent significant decrease (r = −0.99) in serum ALP was found as compared to the non users (p < 0.05) (Table 5). In other serum enzymes LDH (r = 0.97), SGOT (r = 0.98) and SGPT (r = 0.99) and GGT (r = 0.99) a duration dependent significant increase was found as compared to the non-users (p < 0.05) (Table 5). The serum albumin level (r = 0.99) was also found to be decrease among users in accordance with increase in the duration of use of OCs (p < 0.05) (Table 5). The serum globulin level was found to be increase among users (r = 0.98) with increase in duration of the use of OCs. The results obtained for comet assay performed on the human peripheral blood lymphocytes of users and non-users is shown in Fig. 1a, b. Among women using OCs for 1–6 months showed 1.73-fold (p < 0.05), increase in the mean tail length as compared to control (Fig. 2). The women using OCs for more than 6, 13 and 19 months showed 2.20-fold, 2.40-fold and 2.74-fold significant increase respectively, in the mean tail length as compared to non users (p < 0.05).
Table 1.
Age distribution of oral contraceptives non-users and users
| Age groups (years) | Non-users (N = 300) | Users (N = 300) |
|---|---|---|
| 16–20 | 30 | 31 |
| 21–25 | 55 | 60 |
| 26–30 | 89 | 95 |
| 31–35 | 34 | 42 |
| 36–40 | 48 | 30 |
| ≥41 | 44 | 42 |
Table 2.
Distribution of users on the basis of using oral contraceptives
| Duration of contraceptive use (months) | OC users |
|---|---|
| 1–6 | 62 |
| 7–12 | 79 |
| 13–18 | 65 |
| 19–24 | 94 |
Table 3.
Description of oral contraceptive formulations used by women
| Progestin (mg) | Estrogens (ethinyl estradiol) (µg) | |
|---|---|---|
| Combined oral contraceptives | ||
| Desogestrel | 0.5 | 10 |
| Levonorgestrol | 0.10 | 30 |
| Medroxyprogesterone | 0.15 | 20 |
| Drospirenone | 0.10 | 10 |
| Gestodene | 0.15 | 30 |
| Norgestimate | 0.75 | 10 |
| Norethindrone acetate | 0.5 | 10 |
| Desogene | 0.10 | 30 |
| Norgestrel | 0.15 | 10 |
| Progestin only | 20 | |
| Ethynodiol diacetate | 0.5 | – |
| Desogestrel plus | 0.35 | – |
| Levonorgestrel | 0.3 | – |
Table 4.
Enzyme profile in the blood serum of oral contraceptive users and non users in different age groups
| Age group (years) | Sample size (N) | Alkaline phosphatase (U/L) Mean ± SD | Lactate dehydrogenase (U/L) Mean ± SD | Aminotransferases | γ Glutamyltransferase (U/L) Mean ± SD | Serum proteins | ||
|---|---|---|---|---|---|---|---|---|
| SGOT (U/L) Mean ± SD | SGPT (U/mL) Mean ± SD | Albumin (g/dL) Mean ± SD | Globulin (g/dL) Mean ± SD | |||||
| 16–20 | ||||||||
| U | 30 | 24.45 ± 8.46*,a | 413.37 ± 103.44*,a | 31.74 ± 6.03*,a | 10.79 ± 1.21*,a | 28.90 ± 5.11*,a | 3.09 ± 0.47*,a | 3.46 ± 0.30*,a |
| NU | 31 | 91.03 ± 21.93 | 261.14 ± 78.47 | 21.24 ± 6.60 | 6.89 ± 2.13 | 23.3 ± 5.39 | 3.91 ± 0.42 | 3.21 ± 0.31 |
| 21–25 | ||||||||
| U | 55 | 28.11 ± 9.92*,a | 411.41 ± 105.31*,a | 29.23 ± 5.69*,a | 12.30 ± 0.95*,a,b | 30.78 ± 5.84*,a | 2.95 ± 0.33*,a | 3.45 ± 0.29*,a |
| NU | 60 | 96.07 ± 19.39 | 259.13 ± 75.41 | 20.31 ± 6.23 | 9.51 ± 1.23 | 24.98 ± 5.56 | 3.91 ± 0.39 | 3.19 ± 0.33 |
| 26–30 | ||||||||
| U | 89 | 31.66 ± 12.98*,a | 410.52 ± 101.11*,a | 28.34 ± 5.75*,a | 13.95 ± 1.29*,b | 29.82 ± 5.08*,a | 2.91 ± 0.33*,a | 3.43 ± 0.27*,a |
| NU | 95 | 88.97 ± 19.99 | 261.11 ± 77.38 | 20.71 ± 6.41 | 8.09 ± 1.90 | 25.35 ± 5.65 | 3.89 ± 0.43 | 3.21 ± 0.29 |
| 31–35 | ||||||||
| U | 34 | 25.07 ± 9.00*,a | 415.49 ± 108.94*,a | 27.49 ± 5.41*,a | 11.45 ± 1.13*,a,b | 31.89 ± 5.91*,a | 2.89 ± 0.32*,a | 3.44 ± 0.29*,a |
| NU | 42 | 86.17 ± 21.77 | 261.21 ± 78.41 | 16.60 ± 5.43 | 7.41 ± 1.94 | 23.38 ± 5.41 | 3.88 ± 0.42 | 3.18 ± 0.30 |
| 36–40 | ||||||||
| U | 48 | 27.03 ± 13.20*,a | 411.39 ± 101.44*,a | 28.49 ± 5.89*,a | 13.34 ± 0.98*,a,b | 30.23 ± 5.43*,a | 3.20 ± 0.53*,a | 3.50 ± 0.39*,a |
| NU | 30 | 82.35 ± 18.60 | 261.15 ± 78.39 | 19.41 ± 6.14 | 8.15 ± 1.44 | 24.34 ± 5.14 | 3.89 ± 0.44 | 3.19 ± 0.31 |
| ≥41 | ||||||||
| U | 44 | 19.47 ± 9.76*,a | 413.12 ± 109.45*,a | 29.41 ± 5.91*,a | 12.25 ± 0.97*,a,b | 32.25 ± 5.01*,a | 3.08 ± 0.45*,a | 3.49 ± 0.35*,a |
| NU | 42 | 83.11 ± 26.86 | 261.10 ± 78.35 | 20.14 ± 6.28 | 7.42 ± 1.89 | 22.65 ± 5.13 | 3.88 ± 0.41 | 3.20 ± 0.32 |
U user, NU non users
* Significant difference between oral contraceptive users and non-users (p < 0.05). Superscript of different alphabets denotes significant difference between different age groups among oral contraceptive users (ANOVA) post hoc Tukey test
Table 5.
Enzyme profile in blood serum on the basis of duration (months) of using oral contraceptives
| Duration (months) | N | Alkaline phosphatase (U/L) | Lactate dehydrogenase (U/L) | Aminotransferases | |||||
|---|---|---|---|---|---|---|---|---|---|
| SGOT (U/L) | SGPT (U/mL) | ||||||||
| 1–6 | 62 | 60.53 ± 5.74*,a | Y = 68.455 − 1.553X; r = −0.99; p < 0.0010 | 365.35 ± 33.52*,a | Y = 344.05 + 2.7780X; r = 0.971; p < 0.0005 | 24.83 ± 5.78*,a | Y = 21.680 + 0.55900X; r = 0.985; p < 0.0027 | 29.54 ± 2.35*,a | Y = 26.70 + 0.51850X; r = 0.996; p < 0.0004 |
| 7–12 | 79 | 48.07 ± 8.11*,b | 372.69 ± 41.29*,a,b | 28.15 ± 4.79*,b | 33.11 ± 2.47*,b | ||||
| 13–18 | 65 | 39.81 ± 6.54*,c | 389.55 ± 48.94*,b | 32.83 ± 5.67*,c | 36.47 ± 2.41*,c | ||||
| 19–24 | 94 | 32.22 ± 4.22d | 415.29 ± 49.62c | 34.45 ± 6.63*,d | 38.79 ± 2.05*,d | ||||
| Non users | 300 | 87.16 ± 14.76 | 363.11 ± 26.84 | 27.93 ± 3.86 | 26.52 ± 3.28 | ||||
| Duration (months) | N | γ-Glutamyltransferase (U/L) | Serum proteins | ||||
|---|---|---|---|---|---|---|---|
| Albumin (g/dL) | Globulin (g/dL) | ||||||
| 1–6 | 62 | 26.51 ± 4.76*,a | Y = 25.34 + 0.23183X; r = 0.97; p < 0.0007 | 3.80 ± 0.34*,a | Y = 4.0750 − 0.0355X; r = −0.97; p < 0.0007 | 3.51 ± 0.48*,a | Y = 3.385 + 0.02483X; r = 0.986; p < 0.0002 |
| 7–12 | 79 | 28.69 ± 5.40*,a,b,c | 3.71 ± 0.30*,a | 3.73 ± 0.44*,b | |||
| 13–18 | 65 | 29.04 ± 4.6*,b,c | 3.50 ± 0.28*,b | 3.81 ± 0.51*,b,c | |||
| 19–24 | 94 | 31.03 ± 5.49*,c | 3.16 ± 0.37*,c | 3.98 ± 0.54*,d | |||
| Non users | 300 | 22.84 ± 5.67 | 4.24 ± 0.36 | 3.38 ± 0.46 | |||
U user, NU non users
* Significant difference between oral contraceptive users and non-users (p < 0.05). Superscript of different alphabets denotes significant difference between different age groups among oral contraceptive users (ANOVA) post hoc Tukey test
Fig. 1.
Comet assay performed in human peripheral blood lymphocytes: a non users and b users
Fig. 2.
Comet tail lengths in non-users and in women taking oral contraceptives for various duration. Asterisk represents significant difference between oral contraceptive users and non-users (p < 0.05)
Discussion
Hormonal contraceptives are in clinical practice for more than 70 years for the prevention of ovulation, implantation and sperm penetration into ovum. Estrogen or progesterone or both are also used in hormonal replacement therapy and are given to women after menopause [18]. However the hormonal replacement therapy has been reported to be associated with increased risk of cancer [19, 20]. There are several reports on the genotoxic potential of estrogens and synthetic progestins in vivo and in vitro [21–28]. Most of the oral formulations have a definite proportion of estrogen and synthetic progesterone [29]. However, the composition of the OCs can vary from country to country so as the response toward the drugs forms the basis of pharmacogenomics [30]. The reports on the genotoxic potential in women’s using steroid hormones as an OCs or HRT are available [31, 32]. In the present study the serum enzymes studied are liver enzymes that are present normally inside the hepatocytes and are released into the blood when there is hepatocellular damage. The elevated level of ALP is associated with disorders of liver and bone. The elevated serum ALP levels represent the presence of osteoblastic activity. Gamma glutamyl transferase comes from cell lining of biliary tract and is found in the blood. The elevated levels of GGT signify obstructive diseases of the biliary tract and liver cancers. One of the most important diagnostic uses of LDH test is the diagnosis of myocardial infarction or heart attack. Glutamic-oxaloacetic transaminase (SGOT) occurs in large concentrations in the heart and liver with moderate amounts in skeletal muscle, kidneys, and pancreas. GOT levels can be used to diagnose myocardial infarction and severe angina of the heart, and liver damage. Glutamic-pyruvic transaminase (SGPT) is found in significant quantities in liver, kidney, and skeletal muscle. When liver cells are damaged, GOT and GPT levels rise especially early in the disease [33–35].
In early 80s it was well documented that the use of OCs was associated with the benign hepatic adenoma. The association was further confirmed by Palmer et al. [36]. The use of OCs have been reported to be associated with the higher plasma retinol, 25-hydroxy vitamin D, total iron binding capacity, total chlolesterol, LDL-cholesterol and triacyglycerol [37, 38]. Concerning the serum enzymes levels in our present study an increase in LDH, SGPT, SGOT and GGT was found among users. This increase was found to be in correlation with the duration of using OCs. The use of OCs has been reported to alter lipids and enzymes profile among OCs users [38, 39]. As most of the drugs are metabolized by liver hence it is becomes difficult to correlate whether larger doses of drugs can cause adaptative changes in the form of increasing the detoxifying enzymes, or increase in the metabolic functions of liver. DNA damage and increase in the frequency of sister chromatid exchanges in peripheral blood lymphocytes have been reported in OCs users [40]. In one of the study the extent of DNA damage and SCEs were due to the altered hormonal profile of the users and the DNA damage reported was found to be non-significant compared to the pregnant women [41]. The increased in the mean tail length in our study may also be correlated to the altered hormonal profile. The increased in the DNA damage has been correlated with the induction of various cancers. There are reports on the increase in lipid profile among OCs users and increased risk of atherogenesis and coronary arterial disease [42]. The mechanism by which progestins induce tumour development is not very clear. It has been suggested that induction of homo oxygenases leads to the conversion of procarcinogenes–carcinogens [43] or by stimulation of the genetically altered pre-neoplastic cells [44]. The relationship between the carcinogens and sex hormones is not very clear, however a series of or combination of genotoxic and epigenetic changes have been reported to be associated with OC users [45]. In our earlier in vitro studies synthetic progestins have been reported to generate reactive oxygen species (ROS) [27]. Estrogens have been reported to generate free radicals by metabolic redox cycling between quinone and hydroquinone forms of estrogens [46]. β-Estradiol was reported to be mutagenic and genotoxic effects on the blood cells of Oreochromis niloticus [47]. In cultured cells northindrone has been reported to induce double stranded breaks (DSBs) [48]. In our present study the DNA damage was found to correlate with the duration of using the OCs (Y = 6.6100 + 0.24433X; r = 0.987; p < 0.0047). Steroid hormones have been reported to increase aminotransferases among OC users [48, 49]. In our earlier study with triglycerides, HDL and LDL, the level of serum markers were found to be high among users [50]. Thus, it is concluded that the intake of OC affects the level of various blood serum enzymes and it may depends on the estrogens and progestin dosage or the androgenic activity of the progestin [51]. Concerning the DNA damage in blood lymphocytes, the damage was found to increase with increase in the duration of using OCs. Earlier studies have revealed association between DNA damage and malignancies [52, 53]. Nearly all OCs have some amount of estrogens that is converted into catecholestrogenes and produces oxygen radicals that leads to DNA damage [54].
Conclusion
Although estrogens are present normally in women, but the presence of it in the form of OCs may lead to high concentration in human body. Hence, it is suggested that while prescribing OCs to the women various life style factors (cigarette smoking, alcohol consumption etc.) that may possibly enhance DNA damage should be taken in consideration. The biochemical parameters (enzymatic profile of various blood serum enzymes) should continuously be monitored as estrogens may also act as tumour promoting agent due to their estrogens-receptor mediated mitogenic activity.
Acknowledgments
The authors are thankful to University Grant Commission (UGC), New Delhi for the award of project entitled: “Biochemical and Cytogenetic effects of Oral Contraceptives among women of different reproductive histories” [F. No. 39-582/2010(SR)] to Dr. Yasir Hasan Siddique, Department of Zoology, Aligarh Muslim University, Aligarh.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no competing interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Siskind V, Green A, Bain C, Purdie D. Beyond ovulation: oral contraceptives and epithelial ovarian. Epidemiology. 2000;11:106–110. doi: 10.1097/00001648-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:277–284. doi: 10.1023/A:1008945721786. [DOI] [PubMed] [Google Scholar]
- 3.Palan PR, Strube F, Letko J, Sadikovic A, Mikhail MS. Effects of Oral, vaginal, and transdermal hormonal contraception on serum levels of coenzyme Q10, vitamin E, and total antioxidant activity. Obstet Gynecol Int. 2010;2010:1–4. doi: 10.1155/2010/925635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petitti DB. Combination estrogen–progestin oral contraceptives. N Engl J Med. 2003;349:1443–1450. doi: 10.1056/NEJMcp030751. [DOI] [PubMed] [Google Scholar]
- 5.Engel HJ, Engel E, Lichtlen PR. Coronary atherosclerosis and myocardial infarction in young women role of oral contraceptives. Eur Heart J. 1983;4:1–8. doi: 10.1093/oxfordjournals.eurheartj.a061365. [DOI] [PubMed] [Google Scholar]
- 6.Jick H, Jick S, Myers MW, Vasilakis C. Risk of acute myocardial infarction and low-dose combined oral contraceptives. Lancet. 1996;347:627–628. doi: 10.1016/S0140-6736(96)91334-3. [DOI] [PubMed] [Google Scholar]
- 7.Lewis MA, Heinemann LA, Spitzer WO, MacRae KD, Bruppacher R. The use of oral contraceptives and the occurrence of acute myocardial infarction in young women. Results from the Transnational Study on Oral Contraceptives and the Health of Young Women. Contraception. 1997;56:129–140. doi: 10.1016/S0010-7824(97)00118-2. [DOI] [PubMed] [Google Scholar]
- 8.Dunn N, Thorogood M, Faragher B, de Caestecker L, MacDonald TM, Mann R. Oral contraceptives and myocardial infarction: results of the MICA case–control study. BMJ. 1999;318:1579–1583. doi: 10.1136/bmj.318.7198.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanis BC, van den Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, Rosendaal FR. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787–1793. doi: 10.1056/NEJMoa003216. [DOI] [PubMed] [Google Scholar]
- 10.Sidney S, Siscovick DS, Petitti DB, Schwartz SM, Quesenberry CP, Psaty BM, Koepsell TD. Myocardial infarction and use of low-dose oral contraceptives: a pooled analysis of 2 US studies. Circulation. 1998;98(11):1058–1063. doi: 10.1161/01.CIR.98.11.1058. [DOI] [PubMed] [Google Scholar]
- 11.Grabrick DM, Hartmann LC, Cerhan JR, Vierkant RA, Therneau TM, Vachon CM, Sellers TA. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. J Am Med Assoc. 2000;284:1791–1798. doi: 10.1001/jama.284.14.1791. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg L, Zhang Y, Coogan PF, Strom BL, Palmer JR. A case–control study of oral contraceptive use and incident breast cancer. Am J Epidemiol. 2009;169:473–479. doi: 10.1093/aje/kwn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanford JL, Brinton LA, Hoover RN. Oral contraceptives and breast cancer: results from an expanded case–control study. Br J Cancer. 1989;60:375–381. doi: 10.1038/bjc.1989.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuberger J, Forman D, Doll R, Williams R. Oral contraceptives and hepatocellular carcinoma. BMJ. 1986;92:1355–1357. doi: 10.1136/bmj.292.6532.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajpayee M, Pandey AK, Parmar D, Mathur N, Seth PK, Dhawan A. Comet assay responses in human lymphocytes are not influenced by the menstrual cycle: a study in healthy Indian females. Mutat Res. 2005;565:163–172. doi: 10.1016/j.mrgentox.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Kapiszewska M, Kalemba M, Grzesiak A, Kocemba K. The level of endogenous DNA damage in lymphocytes isolated from blood is associated with the fluctuation of 17β estradiol concentration in the follicular phase of healthy young women. Acta Biochim Pol. 2005;52:535–539. [PubMed] [Google Scholar]
- 17.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 18.Moore JS, Monson JP, Kaltsas G, Putignano P, Wood PJ, Sheppard MC, Stewart PM. Modulation of 11β-hydroxysteroid dehydrogenase isozymes by growth hormone and insulin-like growth factor: in vivo and in vitro studies. J Clin Endocrinol Metab. 1999;84:4172–4177. doi: 10.1210/jcem.84.11.6108. [DOI] [PubMed] [Google Scholar]
- 19.Speroff L, Glass RH, Kase NG. Oral contraception. In: Fritz MA, Speroff L, editors. Clinical gynecologic endocrinology and infertility. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 867–944. [Google Scholar]
- 20.Pike MC, Ross RK. Progestins and menopause: epidemiological studies of risks of endometrial and breast cancer. Steroids. 2000;65:659–664. doi: 10.1016/S0039-128X(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 21.Siddique YH, Afzal M. Evaluation of genotoxic potential of synthetic progestin chlormadinone acetate. Toxicol Lett. 2004;153:221–225. doi: 10.1016/j.toxlet.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Siddique YH, Afzal M. Evaluation of genotoxic potential of ethynodiol diaacetate in human lymphocytes in vitro. Curr Sci. 2004;86:1161–1165. [Google Scholar]
- 23.Dorn SB, Degen GH, Muller T, Bonacker D, Joosten HF, van der Louw J, Bolt HM. Proposed criteria for specific and non-specific chromosomal genotoxicity based on hydrophobic interactions. Mutat Res. 2007;628:67–75. doi: 10.1016/j.mrgentox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Dorn SB, Bolt HM, Thevis M, Diel P, Degen GH. Micronucleus induction in V79 cells by the anabolic doping steroids desoxymethyltestosterone (madol) and 19-norandrostenedione. Toxicol Lett. 2008;183:58–64. doi: 10.1016/j.toxlet.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Seraj MJ, Umemoto A, Tanaka M, Kajikawa A, Hamada K, Monden Y. DNA adduct formation by hormonal steroids in vitro. Mutat Res. 1996;370:49–59. doi: 10.1016/S0165-1218(96)90126-3. [DOI] [PubMed] [Google Scholar]
- 26.Siddique YH, Ara G, Beg T, Afzal M. Genotoxic potential of medroxyprogesterone acetate in cultured human peripheral blood lymphocytes. Life Sci. 2006;80:212–218. doi: 10.1016/j.lfs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Siddique YH, Afzal M. A review on the genotoxic effects of some synthetic progestins. Int J Pharmacol. 2008;4:410–430. doi: 10.3923/ijp.2008.410.430. [DOI] [Google Scholar]
- 28.Yan ZH, Lu GH, Yang XF. Single and combined effects of estrone and 17β-estradiol on male gold fish. Biomed Environ Sci. 2013;26:176–184. doi: 10.3967/0895-3988.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Siddique YH, Beg T, Afzal M. Genotoxic potential of ethinylestradiol in cultured mammalian cells. Chem Biol Interact. 2005;151:133–141. doi: 10.1016/j.cbi.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Franconi F, Campesi I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and women. Br J Pharmacol. 2014;171:580–594. doi: 10.1111/bph.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozcagli E, Sardas S, Biri A. Assessment of DNA damage in postmenopausal women under hormone replacement therapy. Maturitas. 2005;51:280–285. doi: 10.1016/j.maturitas.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Kayikcioglu F, Gunes M, Baltaci V, Koçak M, Alpas I, Haberal A. Sister-chromatid exchange frequencies in postmenopausal hormone replacement patients. Mutat Res. 2000;452:37–39. doi: 10.1016/S0027-5107(00)00033-6. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan MM. Alkaline phosphatase. Gastroenterology. 1972;62:452–468. [PubMed] [Google Scholar]
- 34.Rosalki SB. Gamma-glutamyl transpeptidase. Adv Clin Chem. 1975;17:53–107. doi: 10.1016/S0065-2423(08)60248-6. [DOI] [PubMed] [Google Scholar]
- 35.Vroon DH, Israili Z. Alkaline phosphatase and gamma glutamyltransferase. 3. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 36.Palmer JR, Rosenberg L, Kaufman DW, Warshauer ME, Stolley P, Shapiro S. Oral contraceptive use and liver cancer. Am J Epidemiol. 1989;130:878–881. doi: 10.1093/oxfordjournals.aje.a115420. [DOI] [PubMed] [Google Scholar]
- 37.Thane CW, Bates CJ, Prentice A. Oral contraceptives and nutritional status in adolescent British girls. Nutr Res. 2002;22:449–462. doi: 10.1016/S0271-5317(02)00356-1. [DOI] [Google Scholar]
- 38.Naz F, Jyoti S, Akhtar N, Afzal M, Siddique YH. Lipid profile of women using oral contraceptive pills. Pak J Biol Sci. 2012;15:947–950. doi: 10.3923/pjbs.2012.947.950. [DOI] [PubMed] [Google Scholar]
- 39.Machado RB, de Melo NR, Maia H, Cruz AM. Effect of a continuous regimen of contraceptive combination of ethinylestradiol and drospirenone on lipid, carbohydrate and coagulation profiles. Contraception. 2010;81:102–106. doi: 10.1016/j.contraception.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Naz F, Jyoti S, Akhtar N, Afzal M, Siddique YH. Genotoxic damage in cultured human peripheral blood lymphocytes of oral contraceptive users. Egypt J Med Hum Genet. 2012;13:301–305. doi: 10.1016/j.ejmhg.2012.06.002. [DOI] [Google Scholar]
- 41.Biri A, Civelek E, Karahalil B, Şardaş S. Assessment of DNA damage in women using oral contraceptives. Mutat Res. 2002;521:113–119. doi: 10.1016/S1383-5718(02)00217-6. [DOI] [PubMed] [Google Scholar]
- 42.Santos MCS, Rebelo ACS, Zuttin RS, César MC, Catai AM, Silva E. Influence of oral contraceptive use on lipid levels and cardiorespiratory responses among healthy sedentary women. Rev Bras Fisioter. 2008;12:188–194. doi: 10.1590/S1413-35552008000300006. [DOI] [Google Scholar]
- 43.Schulte-Hermann R, Timmermann-Trosiener I, Schuppler J. Promotion of spontaneous preneoplastic cells in rat liver as a possible explanation of tumor production by nonmutagenic compounds. Cancer Res. 1983;43:839–844. [PubMed] [Google Scholar]
- 44.Ochs H, Düsterberg B, Günzel P, Schulte-Hermann R. Effect of tumor promoting contraceptive steroids on growth and drug metabolizing enzymes in rat liver. Cancer Res. 1986;46:1224–1232. [PubMed] [Google Scholar]
- 45.Braz MG, Salvadori DM. Lack of genotoxicity induced by endogenous and synthetic female sex hormones in peripheral blood cells detected by alkaline comet assay. Environ Mol Mutagen. 2007;48:414–420. doi: 10.1002/em.20295. [DOI] [PubMed] [Google Scholar]
- 46.Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424:107–115. doi: 10.1016/S0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 47.Sponchiado G, de Lucena Reynaldo EMF, de Andrade ACB, de Vasconcelos EC, Adam ML, deOliveira CMR. Genotoxic effects in erythrocytes of Oreochromis niloticus exposed to nanograms-per-liter concentration of 17β-estradiol (E2): an assessment using micronucleus test and comet assay. Water Air Soil Pollut. 2011;218:353–360. doi: 10.1007/s11270-010-0649-9. [DOI] [Google Scholar]
- 48.Gallmeier E, Winter JM, Cunningham SC, Kahn SR, Kern SE. Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005;26:1811–1820. doi: 10.1093/carcin/bgi132. [DOI] [PubMed] [Google Scholar]
- 49.Siest G, Visvikis S, Herbeth B, Gueguen R, Vincent-Viry M, Sass C, Chevrier P. Objectives, design and recruitment of a familial and longitudinal cohort for studying gene–environment interactions in the field of cardiovascular risk: the Stanislaus cohort. Clin Chem Lab Med. 1998;36:35–42. doi: 10.1515/CCLM.1998.007. [DOI] [PubMed] [Google Scholar]
- 50.Schiele F, Vincent-Viry M, Fournier B, Starck M, Siest G. Biological effects of eleven combined oral contraceptives on serum triglycerides, gamma-glutamyltransferase, alkaline phosphatase, bilirubin and other biochemical variables. Clin Chem Lab Med. 1998;36:871–878. doi: 10.1515/CCLM.1998.153. [DOI] [PubMed] [Google Scholar]
- 51.Naz F, Jyoti S, Afzal M, Siddique Yh. Biochemical effects of oral contraceptives among users: a review. Int J Pharmacol. 2012;8:314–320. doi: 10.3923/ijp.2012.314.320. [DOI] [Google Scholar]
- 52.Smith TR, Miller MS, Lohman KK, Case LD, Hu JJ. DNA damage and breast cancer risk. Carcinogenesis. 2003;24:883–889. doi: 10.1093/carcin/bgg037. [DOI] [PubMed] [Google Scholar]
- 53.Colleu-Durel S, Guitton N, Nourgalieva K, Leveque J, Danic B. Genomic instability and breast cancer. Oncol Rep. 2001;8:1001–1005. doi: 10.3892/or.8.5.1001. [DOI] [PubMed] [Google Scholar]
- 54.Bolton JL. Quinoids, quinoid radicals, and phenoxyl radicals formed from estrogens and antiestrogens. Toxicology. 2002;2002(1771):55–65. doi: 10.1016/S0300-483X(02)00195-6. [DOI] [PubMed] [Google Scholar]