Abstract
Recent interest in two-pore channels (TPCs) has resulted in a variety of studies dealing with the functional role and mechanism of action of these endo-lysosomal proteins in diverse physiological processes. With the availability of mouse lines harbouring mutant alleles for Tpcnl and/or Tpcn2 genes, several studies have made use of them to validate, consolidate and discover new roles for these channels not only at the cellular level but, importantly, also at the level of the whole organism. The different mutant mouse lines that have been used were derived from distinct genetic manipulation strategies, with the aim of knocking out expression of TPC proteins. However, the expression of different residual TPC sequences predicted to occur in these mutant mouse lines, together with the varied degree to which the effects on Tpcn expression have been studied, makes it important to assess the true knockout status of some of the lines. In this review we summarize these Tpcn mutant mouse lines with regard to their predicted effect on Tpcn expression and the extent to which they have been characterized. Additionally, we discuss how results derived from studies using these Tpcn mutant mouse lines have consolidated previously proposed roles for TPCs, such as mediators of NAADP signalling, endo-lysosomal functions, and pancreatic β cell physiology. We will also review how they have been instrumental in the assignment of new physiological roles for these cation channels in processes such as membrane electrical excitability, neoangiogenesis, viral infection and brown adipose tissue and heart function, revealing, in some cases, a specific contribution of a particular TPC isoform.
Keywords: Acrosome; Arrhythmia; Autophagy; Cardiac Hypertrophy; Endo-Lysosomes; Knockout; Metabolism; Myocardial Infarction; Neoangiogenesis; Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP); PI(3,5)P2; Reporter Gene; Trafficking; Two-Pore Channels (TPCN, TPC)
Introduction
The endo-lysosomal two-pore channels (TPCs) have recently been the focus of intense scrutiny regarding their proposed role in the Ca2+-signalling pathway regulated by the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP) (Jentsch et al., 2015; Morgan and Galione, 2014).
Originally cloned in 2000 by way of their homology with voltage-gated Ca2+ and Na+-channels (Ishibashi et al., 2000), it took almost a decade for a role for TPCs to emerge in animals with the proposal that they were the long sought after intracellular Ca2+-permeable channel regulated by NAADP (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009). Since then, many reports have supported a role for TPCs in NAADP-regulated Ca2+ release (Morgan et al., 2015) and in a variety of Ca2+-dependent physiological responses such as muscle contraction, hormone secretion, fertilization and differentiation (Kelu et al., 2015; Park et al., 2015; Parrington and Tunn, 2014; Patel, 2015). More recently however, a role for TPCs as mediators of NAADP-regulated Ca2+ release has been questioned, and instead, TPCs have been proposed to be Na+-channels regulated by phosphatidylinositol (3,5)-bisphosphate (PI(3,5)P2) (Wang et al., 2012). This suggestion has prompted a flurry of studies that have, on the one hand, reaffirmed the status of TPC proteins as integral components of NAADP-regulated Ca2+ release, while at the same time providing new insights into their role in mediating different cation currents across endo-lysosomal membranes (Morgan and Galione, 2014; Patel, 2015).
The use of several mutant Tpcn mouse lines that have been developed with the aim of knocking out Tpcn expression, has been important in confirming and revealing modes of regulation and properties of TPC proteins, and uncovering new roles for TPC proteins in physiological processes, both at the cellular and whole-organism levels (Arndt et al., 2014; Arredouani et al., 2015; Calcraft et al., 2009; Cang et al., 2013, 2014a; Capel et al., 2015; Davidson et al., 2015; Favia et al., 2014; Gerasimenko et al., 2015; Grimm et al., 2014; Lear et al., 2015; Lin et al., 2015; Lin-Moshier et al., 2012; Ruas et al., 2014, 2015a; Sakurai et al., 2015; Tsaih et al., 2014; Tugba Durlu-Kandilci et al., 2010; Wang et al., 2012). However, validation of these mutant lines as TPC knockouts has not always been as thorough as necessary to draw compelling conclusions from such studies. This is partly because some studies have not assessed the impact of gene targeting upon Tpcn expression in putative knockout lines by experimental means, and partly because they have not taken into account the possibility of alternative expressed forms of Tpcn mRNAs in the mouse.
Mouse Tpcn Genes and Proteins Structures
The TPCN gene family is a member of the superfamily of voltage-gated cation channels (Galione et al., 2009) composed of three homologs (TPCN1, TPCN2 and TPCN3) that have originated by gene duplication from a common ancestral gene (Rahman et al., 2014). TPCN3 is not present in all TPCN-containing species, being absent in species such as humans and mice due to deletion of vast relevant gene sequences (Cai and Patel, 2010).
The deduced primary structure of TPC protein isoforms, that share an overall amino acid sequence identity of 27% (for mouse TPC1 and TPC2), is organized in two domains containing six transmembrane segments each, and are therefore considered to be structurally placed between the four-domain voltage-gated cation channels and the single-domain TRP channels. Based on their domainorganization similarity with the superfamily of voltagegated cation channels (Long et al., 2005), they are thought to dimerize (forming either homodimers or heterodimers (Churamani et al., 2012; Rietdorf et al., 2011)) to form a channel with the pore being lined by transmembrane segments S5 and S6 and the N- and C-terminal tails facing the cytosol (Hooper et al., 2011) (Fig. 1).
Figure 1.
Proposed topology and structure of TPC proteins. (A) Two-domain organization and topology of TPCs. S1–S6 represent the six transmembrane helixes in each domain and P represents the pore helix. S4 contains the characteristic positively charged residues. (B) Proposed TPC dimeric structure, based on the crystal structure of a mammalian voltage-dependent Shaker family K+ channel (Long et al., 2005). Only the transmembrane helixes are represented with the monomers coloured in brown and beige. The pore forming region is shaded grey. Arrow indicates ionic flow from the lumen to the cytoplasm.
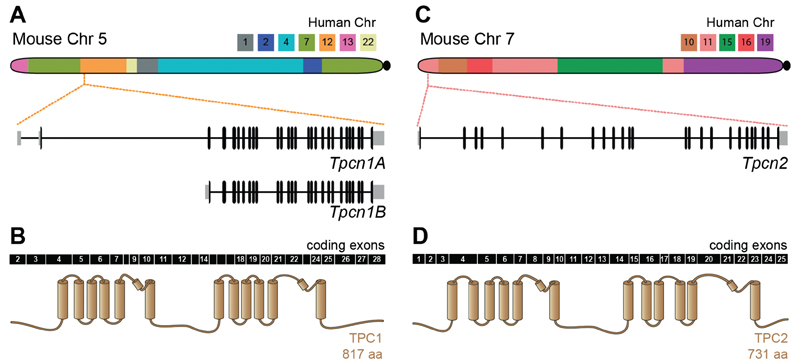
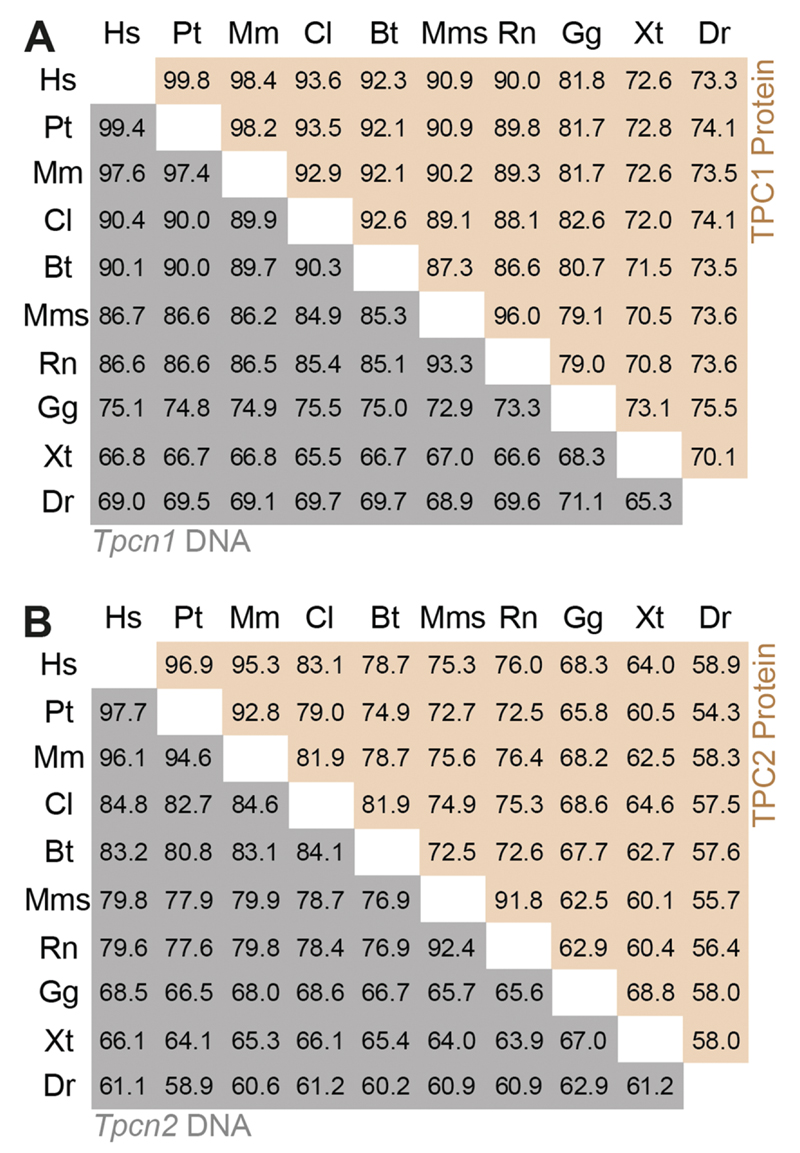
The mouse Tpcn1 gene (GeneID: 252972; MGI: 2182472) is located on Chromosome 5 within a region syntenic to human Chromosome 12. It spans approximately 54.5 kbp with a total number of 28 exons (based on mRNA RefSeq NM_145853) (Fig. 2(A)). The percentage of identity amongst Tpcn1 orthologs within the Euteleostomi (bony vertebrates) clade, range from 65.3–99.4% at the DNA level and 70.1–99.8% at the protein level (Fig. 3(A)), and in particular the mouse TPC1 protein is 90.9% identical to the human ortholog. Expression analysis for Tpcn1 based on northern blotting of rat and mouse tissue (Ishibashi et al., 2000; Zong et al., 2009) has revealed two major mRNA forms. These are likely to correspond to two isoforms, Tpcn1A and Tpcn1B; the shorter Tpcn1B isoform has been recently suggested to arise from an alternative promoter situated just upstream from exon 3 (Ruas et al., 2014). The extent of TPC1B’s contribution to TPC-mediated events has not been thoroughly evaluated but although this variant TPC1 protein does not appear to mediate PI(3,5)P2-regulated Na+ -currents (Wang et al., 2012), it has been shown to still retain some functionality in terms of NAADP-regulation of Ca2+-release (Ruas et al., 2015a).
Figure 2.
Chromosomic localization and gene structures of mouse Tpcn genes. (A, C) The major chromosomic synteny blocks between mouse and human (based on (Chinwalla et al., 2002)) are colour coded as indicated. Zoomed-in structures correspond to the exonic organization of Tpcn genes; horizontal lines correspond to intronic sequences, vertical blocks indicate exons and grey blocks correspond to UTR regions. (B, D) Coding exons for TPC1 and TPC2 are represented in black and numbered. The corresponding protein is represented in brown and the position of protein features has been aligned to the corresponding exons. Transmembrane segments are represented by cylinders.
Figure 3.
Identity tables for TPCN genes and TPC protein sequences from Euteleostomes. Identity tables for TPCN1/TPC1 (A) and TPCN2/TPC2 (B) orthologues were built based on information from HomoloGene (http://www.ncbi.nlm.nih.gov/homologene). Species names are abbreviated as indicated with underlined letters: Homo sapiens, Pan troglodytes, Macaca mulatta, Canis Lupus, Bos taurus, Mus musculus, Rattus norvegicus, Gallus gallus, Xenopus tropicalis, Danio rerio.
The mouse Tpcn2 gene (Gene ID: 233979; MGI 2385297) is located on chromosome 7, within a region syntenic to human Chromosome 11. It spans 30.0 kbp with a total number of 25 exons (based on mRNA RefSeq NM_146206) (Fig. 2(B)). The overall identity between Tpcn2 orthologs within Euteleostomi species is somewhat lower than amongst the Tpcn1 orthologs, ranging from 58.9–97.7% at the DNA level and 54.3–96.9% at the protein level (Fig. 3(B)). The mouse TPC2 protein is 75.3% identical to its human ortholog.
Gene Disruption Models
Genetically modified animals have been invaluable tools for investigating the role of specific genes in cellular pathways and whole-animal physiology, and to create models of human diseases. Amongst mammals, genetically modified mice have gained particular prominence because of the availability of mouse embryonic stem (ES) cell lines and their amenability to integration of extra genetic information into their genome, either randomly or in a targeted manner. However, this is likely to change as new gene-editing techniques, such as ZFNs (zinc finger nucleases), TALENs (transcription activator-like effector nucleases), and in particular CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9, have proven to be efficient tools that allow hereditable gene disruptions in numerous species, including different mammalian species, to be achieved both rapidly and economically (Doudna and Charpentier, 2014).
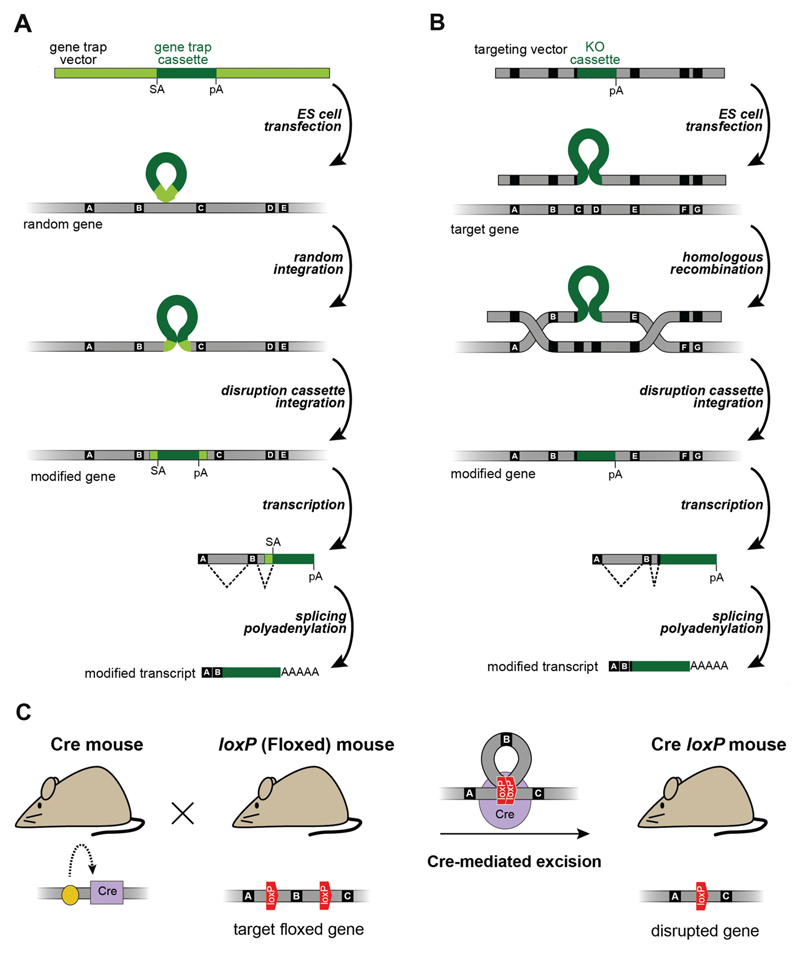
High-throughput strategies have also been employed to generate a vast amount of different mouse ES cell lines with disruptions in specific genes and with the potential to cover all mouse genes (Guan et al., 2010). These initiatives have employed several gene-disruption strategies such as:
-
(a)
gene trap, where transcription-disruptive cassettes, carrying splice acceptor (SA) sequences and polyadenylation (pA) signals, are inserted randomly into introns and result in the premature termination of translation and the consequent generation of truncated proteins (Fig. 4(A));
-
(b)
gene-targeted approaches, by replacement of specific exonic sequences with disruptive cassettes for premature termination (Fig. 4(B)); or
-
(c)
targeted deletion of exonic sequences, using site-specific recombination strategies such as the Cre/loxP system, resulting in removal of exons containing the initiation codon, or creation of premature translation termination caused by frameshift induced by exon removal (Fig. 4(C)).
Figure 4.
General strategies used for gene disruption in Tpcn genes. (A, B) Gene trapping (A) and homologous recombination (B) approaches for development of mutant mouse lines. Introns are represented in grey, exons are represented in black, and labelled with letters and disruptive sequences are in green. SA (splice acceptor sequence), pA (poly-adenylation signal). (C) Strategy for development of mutant mice using a Cre-mediated excision of exonic sequences. Yellow circle represents the promoter driving Cre expression.
These resources have resulted in a large number of ES cell lines for creation of mutant mouse lines characterized by loss-of-function, partial and conditional expression, and gene-expression reporters.
Several different ES cell or mouse lines carrying mutations in Tpcn1 and Tpcn2 alleles have been created either by consortium-led or independent initiatives. The mutant lines that have been used so far are described below, including the predicted consequences of such gene disruption strategies on Tpcn expression. Wherever possible, we will follow the guidelines for nomenclature of mouse mutants set by the International Committee on Standardized Genetic Nomenclature for Mice (http://www.informatics.jax.org/mgihome/nomen/gene.shtml).
Tpcn1 Mutant Lines
Tpcn1Gt(XG716)Byg
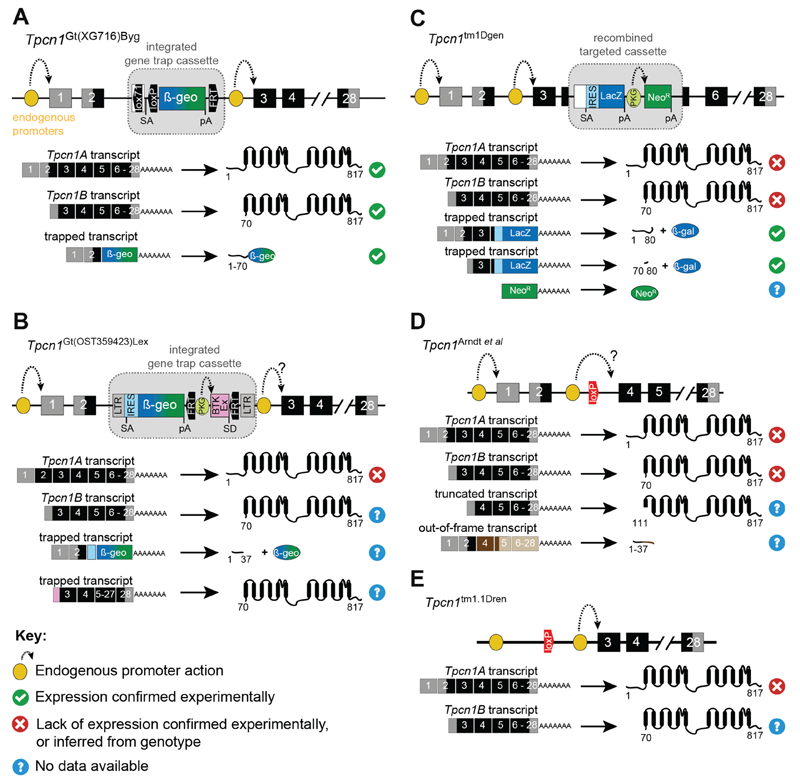
This ES cell line created by BayGenomics carries a mutant Tpcn1 allele by gene-trap insertion in the intron between exons 2 and 3 and it was used to create homozygote mice for the mutant allele (Ruas et al., 2014) (Fig. 5(A)). The gene-trap cassette carried in this line is a promoterless gene trap containing a reporter/selection gene (β-geo, a fusion between β-galactosidase (lacZ) and neomycin transferase (NeoR) coding sequences) flanked by a SA sequence and a pA signal. When recognized by the splicing machinery, the presence of the exogenous SA will result in production of a trapped fusion transcript of exons 1–2 together with the reporter β-geo sequence; the presence of the pA signal will ensure termination of the transcript just downstream from the reporter sequence. Expression of this trapped transcript is driven by the endogenous Tpcn1 promoter and can therefore be used to track spatial and temporal expression of Tpcn1. Additionally, the presence of lox sites flanking the SA sequence can be used to modify the trapped Tpcn1 gene by a Floxin strategy (Singla et al., 2010). Using this recombination-mediated cassette exchange, the SA sequence can either be removed (reverting the trapped allele to its wild-type expression) or exchanged for other exogenous sequences.
Figure 5.
Gene structure of Tpcn1 mutant mouse lines. The top section of each panel contains the gene structures of published Tpcn1 mutant mouse lines. Symbols for mutant alleles are based on MGI database nomenclature, except for Tpcn1Arndt et al. which is indicated by the publication reference where it was first described. (A) Gene trap line Tpcn1Gt(XG716)Byg (gene trap vector pGT1Lxf inserted in intron upstream from exon 3). (B) Gene trap line Tpcn1Gt(OST359423)Lex (gene trap vector VICTR 37, inserted in intron upstream from exon 3). (C) Targeted homologous recombination line Tpcn1tm1Dgen (targeted deletion of exons 4–5 with knock out cassette). (D) Cre loxP line Tpcn1Arndt et al. (Cre-mediated excision of floxed exon 3). (E) Tpcn1tm1.1Dren (Cre-mediated excision of floxed exon 2 containing the initiation codon for TPC1A). Predicted transcripts and proteins expressed from the mutant alleles are shown below the gene structures, on the right and left, respectively. Exons are represented by numbered boxes (grey – non-coding exons; black – coding exons; dark brown – coding exons for TPC-unrelated sequences; light brown – non-coding exons). Predicted proteins are depicted in: black (TPCs), brown (TPC unrelated), blue (β-gal), green (NeoR) or blue/green (β-geo); numbers represent first and last amino acid residues. BTK (cDNA for Bruton agammaglobulinemia tyrosine kinase), En2I (intron 1 of mouse En2 gene), FRT (flippase recognition target sequence), IRES (internal ribosomal entry site), LacZ (cDNA for β-galactosidase), LTR (long terminal repeats), loxP (loxP sequence), lox71 (mutant LoxP sequence), NeoR (cDNA for neomycin transferase), pA (poly-adenylation signal), PKGP (promoter of mouse Pkg gene), SA (splice acceptor), SD (splice donor).
Characterization of Tpcn1 expression in several tissues from homozygote mice carrying this gene trap mutation revealed, however, that expression of Tpcn1 is not greatly affected (Ruas et al., 2014); although the exogenous SA sequence is recognized by the endogenous splicing machinery (resulting in the production of the predicted trapped transcript) this process is not very efficient allowing therefore parallel production of the full-length transcript (Tpcn1A) (Ruas et al., 2014). Additionally, it was found that a further naturally occurring Tpcn1 transcript (Tpcn1B) could be initiated from sequences downstream from the gene trap vector insertion site; this was presumed to be regulated by a promoter situated in the long intron upstream of exon 3 (Fig. 2(A)) (Ruas et al., 2014).
Tpcn1Gt(OST359423)Lex
ES cells carrying this mutant Tpcn1 allele were developed by Lexicon Genetics, and mice derived from them were used in two studies (Davidson et al., 2015; Lin-Moshier et al., 2012). This Tpcn1 mutant line carries a gene-trap cassette inserted in the intron between exons 2 and 3 (Fig. 5(B)). This gene-trap cassette contains two modules: (i) the first module is promoterless and functioning in a similar way to the example above. It is expected to generate therefore a trapped Tpcn1 transcript composed of exons 1–2 fused to the β-geo reporter; this fusion transcript is translated into two separate polypeptides due to the presence of an internal ribosomal entry site (IRES). (ii) The second module (flanked by FRT sites) contains an exogenous promoter (PKG promoter) and exon (BTK exon), and a splice donor (SD) sequence.
Transcription from this module is driven by the PKG promoter and is dependent on the endogenous pA signal in Tpcn1, allowing for a pA-trap transcript to be formed composed of the BTK exon and Tpcn1 sequences starting at exon 3. Translation from this pA-trapped transcript could potentially result in production of TPC1B, provided that its open reading frame is in the same reading frame as that of Tpcn1B. The presence of FRT sites flanking this pA-trap module can be used in Flp-mediated excision or exchange of the FRT-flanked sequence.
Although no published data is available related to the expression of the predicted trapped transcripts, the effect of this gene-trap insertion on TPC1 expression was assessed by immunoblotting of liver homogenates and it was found that the major immunoreactive protein form was significantly reduced in the sample from Tpcn1Gt(OST359423)Lex animals (Hooper et al., 2015). The effect of this gene trap insertion on Tpcn1B expression (either from the alternative endogenous promoter, or from the pA-trap transcript) is still not clear as detection of this less abundant form might require more sensitive methods of detection such as RT-PCR (Ruas et al., 2015b).
Tpcn1tm1Dgen
Mice carrying this mutant allele have been used in several studies (Arredouani et al., 2015; Favia et al., 2014; Lear et al., 2015; Ruas et al., 2014, 2015a). This Tpcn1 mutant line characterized by a targeted deletion of sequences from exons 4–5 was developed by Deltagen (Fig. 5(C)). Expression from this mutant Tpcn1 allele is predicted to result in truncated Tpcn1 fusion transcripts composed of exons 1–3 (or exon 3 alone dependent on which endogenous promoter is driving expression) and a reporter LacZ sequence contained within the targeted cassette. Translation from these transcripts will result in small TPC1 N-terminal sequences and β-galactosidase (β-gal). Additionally, a NeoR sequence is predicted to be expressed from the exogenous PKG promoter.
As the deleted sequences in this line are shared between Tpcn1A and Tpcn1B, Tpcn1tm1Dgen is expected to behave as a true TPC1 knockout line. Indeed, lack of Tpcn1 expression from this mutant allele has been confirmed both by immunoblotting and RT-PCR in several tissues and cell preparations (Ruas et al., 2014, 2015a).
Tpcn1Arndt et al.
This Tpcn1 mutant line used in two studies (Arndt et al., 2014; Sakurai et al., 2015) is characterized by a Cre/lox-mediated deletion of exon 3 (Arndt et al., 2014) (Fig. 5(D)). Expression from this Tpcn1 mutant allele is predicted to generate a transcript containing sequences from exons 1–2 fused to exons 4–28 creating a frameshift transcript relative to the wild-type with a premature stop codon in exon 5; this has the potential to result in a polypeptide with a N-terminal section corresponding to amino acid residues 1–37 of TPC1 (exons 1–2) and a C-terminal section composed of 74 amino acid residues (exons 4–5) with a sequence unrelated to TPC1 due to the frameshift caused by removal of exon 3. However, it is not known whether the transcript from which this polypeptide would originate escapes nonsense mediate mRNA decay (NMD), induced by the premature termination codon (Popp and Maquat, 2013). Additionally, it is not known whether deletion of exon 3 still allows transcription to be driven by the alternative endogenous promoter producing a transcript that could result in a TPC1 protein with an N-terminal truncation of 110 amino acid residues.
The status of TPC1 expression in this line was assessed by immunotechniques using an antibody recognizing the C-terminal region of TPC1; no detectable expression of TPC1 was observed by western blotting (kidney and sperm), or by electron microscopy (EM) and immunocy-tochemistry in testis (Arndt et al., 2014). However, additional RT-PCR data would be beneficial to completely rule out possible low expression of the 5ʹ-truncated Tpcn1 transcript in this Tpcn1 mutant line.
Tpcn1tm1.1Dren
This Tpcn1 mutant line carries a deletion of exons 1–2 originated by a Cre/lox-mediated strategy (Wang et al., 2012) (Fig. 5(E)). This mutation is predicted to knockout expression of Tpcn1A by removal of the exon containing the initiation codon, but is predicted not to affect expression of Tpcn1B driven by the alternative promoter. However, no data is available to assess the Tpcn1 expression status in animals carrying this mutant allele that were used in (Cang et al., 2013, 2014a; Wang et al., 2012).
Tpcn2 Mutant Lines
Tpcn2Gt(YHD437)Byg
An ES cell line for this mutant Tpcn2 allele was obtained from BayGenomics for production of a mutant mouse line (Calcraft et al., 2009) that has been used in several studies (Arredouani et al., 2015; Calcraft et al., 2009; Capel et al., 2015; Favia et al., 2014; Gerasimenko et al., 2015; Lear et al., 2015; Lin et al., 2015; Ruas et al., 2015a; Tsaih et al., 2014; Tugba Durlu-Kandilci et al., 2010). The Tpcn2Gt(YHD437)Byg allele carries a gene trap cassette in the intron downstream from exon 1 (the first codon exon) (Fig. 6(A)). The properties of this gene trap cassette are similar to the ones described for Tpcn1Gt(XG716)Byg. The predicted transcripts resulting from the gene trap integration in Tpcn2 correspond to the trapped transcript containing sequences from exon 1 fused to the β-geo reporter sequence.
Figure 6.
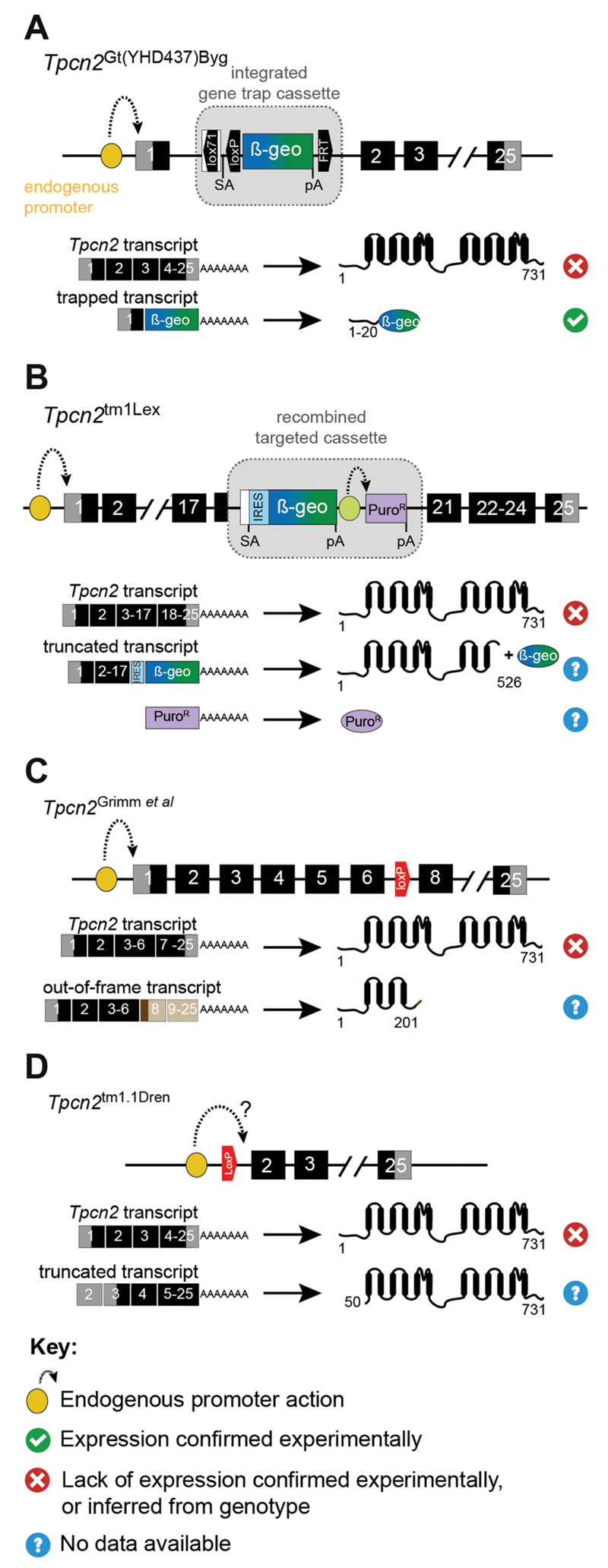
Gene structure of Tpcn2 mutant mouse lines. Organization of figure as for Figure 5. Symbols for mutant alleles are based on MGI database nomenclature, except for Tpcn2Grimm et al. which is indicated by the publication reference where it was first described. (A) Gene trap line Tpcn2Gt(YHD437)Byg (gene trap vector pGT0Lxf, inserted in intron upstream from exon 1). (B) Targeted homologous recombination line Tpcn2tm1Lex (targeted deletion of exons 18–20 with knock out cassette from a pKO vector). (C) Cre loxP line Tpcn2Grimm et al. (Cre-mediated excision of floxed exon 7). (D) Cre loxP line Tpcn2tm1.1Dren (Cre-mediated excision of floxed exon 1 containing the initiation codon). PuroR (cDNA for puromycin N-acetyl-transferase); all other abbreviations and symbols as in Figure 5.
Expression of the trapped transcript was confirmed in livers from Tpcn2Gt(YHD437)Byg mice (Calcraft et al., 2009) and no wild-type transcript that could have arisen from gene-trap skipping was detected in any of the tissues analysed by RT-PCR (liver, kidney, retroperitoneal fat, heart, bladder detrusor, bone derived macrophages and MEFs (Calcraft et al., 2009; Capel et al., 2015; Ruas et al., 2015a; Tsaih et al., 2014; Tugba Durlu-Kandilci et al., 2010)) confirming that this line is a TPC2 knockout.
Tpcn2tm1Lex
A mouse line carrying this mutant allele was created by Lexicon Genetics using a gene-targeted approach to delete sequences from exons 18–20 by recombination with a disruption cassette (Fig. 6(B)). Expression from this mutant allele is expected to knockout expression of wild-type Tpcn2 leaving residual expression of Tpcn2 sequences from exons 1 to part of exon 18 and β-geo, driven by the endogenous promoter. Additional expression of puromycin N-acetyl-transferase (PuroR) is expected from an exogenous promoter carried in the disruption cassette. No data is available for the expression profile of this Tpcn2 mutant mouse line used in (Lin-Moshier et al., 2012).
Tpcn2Grimm et al.
This mouse line used in (Grimm et al., 2014; Sakurai et al., 2015) carries a Cre/lox-mediated deletion of Tpcn2 exon 7 (Fig. 6(C)) (Grimm et al., 2014). It is expected to knockout expression of wild-type Tpcn2 (by internal exon removal) and has the potential to express a transcript containing sequences from exons 1–6 fused to exons 8–25 creating a premature stop codon in exon 8 due to a frameshift induced by exon 7 removal. If refractory to NMD this transcript would result in a polypeptide composed of amino acid residues 1–201 of TPC2 with a few extra amino acid residues unrelated to TPC2 sequence due to the frameshift caused by exon removal.
Lack of full-length TPC2 was demonstrated by immunoblotting and immunofluorescence in hepatocytes and MEFs (Grimm et al., 2014). However, no data related to the expression of the N-terminal part of TPC2 is available.
Tpcn2tm1.1Dren
This mouse line carries a Cre/lox-mediated deletion of exon 1 in Tpcn2 (Fig. 6(D)) (Wang et al., 2012) and is therefore expected to knockout expression of wild-type Tpcn2 by removal of the initiation codon. Whether expression driven by the endogenous promoter still allows transcription of a 5ʹ-truncated Tpcn2 and in-frame translation initiating at the first alternative start codon in exon 3 is not clear. Although the authors alluded to the possibility of such a transcript being produced (Wang et al., 2012), no data was provided regarding Tpcn2 expression for this mutant allele (Cang et al., 2013; Wang et al., 2012).
Use of Tpcn Mutant Lines
None of the Tpcn mutant lines described above present visible gross abnormalities at the whole organism level but, nevertheless, important phenotypic changes and physiological abnormalities have been detected in these animals and in organs, tissues or cell preparation derived from them. These abnormalities have been instrumental both in the elucidation of molecular mechanisms of TPC-mediated signalling and in implicating this signalling pathway in broader cellular contexts, in ways that we will discuss below.
Regulation by NAADP
Since the initial proposal that NAADP targeted an unidentified Ca2+-release channel in acidic organelles, the effort to identify such a channel culminated in publications from independent groups proposing that TPCs were the targets of NAADP action (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009). This was based on measurements of NAADP-mediated cytosolic Ca2+ signals in cells lines where TPC expression/activity was manipulated by overexpression, knockdown and by the use of dominant-negative mutants. Since then, results from many subsequent studies continue to provide evidence that TPCs are regulated by NAADP (Morgan and Galione, 2014; Morgan et al., 2015).
One of the original publications tested the requirement for endogenous TPC2 in NAADP-mediated events in pancreatic β cells, a cell type where NAADP had previously been shown to induce Ca2+ release from acidic stores (Masgrau et al., 2003; Mitchell et al., 2003; Naylor et al., 2009; Yamasaki et al., 2004). By using pancreatic β cells isolated from Tpcn2Gt(YHD437)Byg animals the authors showed an essential requirement for TPC2 in the production of oscillatory Ca2+-activated plasma membrane inward currents elicited by NAADP (Calcraft et al., 2009) and, more recently, in the production of the underlying trigger NAADP-induced Ca2+ transients (Arredouani et al., 2015), suggesting therefore that TPC2 mediates native NAADP-induced Ca2+ responses in this cell type. Further studies using different cell types derived from the same Tpcn2 mouse line have consolidated the requirement for TPC2 in NAADP-mediated responses, such as in the contractile responses of smooth muscle in the bladder detrusor and in ventricular myocytes (Capel et al., 2015; Tugba Durlu-Kandilci et al., 2010) and Ca2+ signals in bone marrow-derived macrophages, mouse embryonic fibroblast (MEFs), pancreatic acinar cells and cardiomyocytes (Capel et al., 2015; Gerasimenko et al., 2015; Ruas et al., 2015a). Furthermore, results obtained from MEFs derived from Tpcn1tm1Dgen animals demonstrated that endogenous TPC1 also contributes to NAADP-induced Ca2+ signals in this cell type and that ablation of both TPC1 and TPC2 (using Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg-derived MEFs) is necessary to abolish NAADP-induced Ca2+ signals that can be restored by re-expression of wild-type but not by permeability-mutant TPCs (Ruas et al., 2015a).
In addition to monitoring cytosolic Ca2+ signals, several groups have performed electrophysiological studies in endo-lysosomal preparations of MEFs (or other cell types) derived from several mouse lines. The use of Tpcn1tm1Dgen, Tpcn2Gt(YHD437)Byg, Tpcn2Grimm et al. and Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg has provided evidence for the requirement of TPCs in NAADP-induced Ca2+- permeable currents across endo-lysosomal membranes (Grimm et al., 2014; Ruas et al., 2015a). In particular, TPC2 seems to be the predominant channel mediating these Ca2+-permeable currents (Ruas et al., 2015a), possibly reflecting a prevalent localization of mouse TPC2 in the organelle preparations used for the measurements.
In contrast, other studies using a different mouse line designed to knockout both TPC1 and TPC2 (Tpcn1tm1.1Dren/Tpcn2tm1.1Dren) seemed to suggest that TPCs are not required for regulation of Ca2+ responses, dependent on NAADP signalling, in pancreatic β cells (Wang et al., 2012). These results contrast with those obtained with Tpcn2Gt(YHD437)Byg animals where NAADP responses were ablated (Arredouani et al., 2015; Calcraft et al., 2009). This stark contrast is consistent with the possibility that truncated TPC proteins are still present in Tpcn1tm1.1Dren/Tpcn2tm1.1Dren animals (Figs. 5 and 6) that could still support NAADP-mediated Ca2+ release. Although the authors have shown that these N-terminal truncated forms of TPC1 and TPC2 were not able to promote Na+-permeable currents mediated by PI(3,5)P2 (see below) (Wang et al., 2012), they were empirically determined to support NAADP-mediated Ca2+ signals, even when expressed at lower levels than the equivalent wild-type proteins (Ruas et al., 2015a). These results raise questions regarding the validity of the Tpcn1tm1.1Dren/Tpcn2tm1.1Dren mouse line as a TPC1/2 null line.
Although TPCs are clearly essential components of the NAADP-signalling pathway, the precise mechanism whereby NAADP activates TPCs is not yet fully understood (e.g., are TPC directly activated by NAADP?). The first study proposing TPCs as targets for NAADP action demonstrated that membrane preparations from HEK cells overexpressing TPC2 showed increased [32P]NAADP binding that co-immunoprecipitated with TPC2 (Calcraft et al., 2009). Rather than being direct targets for NAADP, it has been proposed that TPCs associate with accessory NAADP-binding protein(s) allowing for NAADP-mediated regulation. Indeed, in sea urchin egg preparations, TPC-immunoprecipitates contain [32P]NAADP-binding protein(s) (Ruas et al., 2010) that are of smaller molecular weight than TPCs, as assessed by electrophoretic mobility of proteins photoaffinity labelled with [32P]N3-NAADP (Walseth et al., 2012a); similar results were obtained by photoaffinity labelling of mammalian samples (Lin-Moshier et al., 2012; Walseth et al., 2012b).
The use of TPC-null liver homogenates derived from Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg animals has clearly shown that TPCs are not necessary for NAADP-binding detected by radioligand-binding assays using [32P]NAADP (Ruas et al., 2015a). A similar conclusion was suggested by earlier studies using samples from Tpcn1Gt(OST359423)Lex and Tpcn2tm1Lex mice in photoaffinity labelling with [32P]N3-NAADP, where the pattern of labelled proteins was undistinguishable from wild-type samples (Lin-Moshier et al., 2012). However, at present, one cannot formally exclude the possibility that NAADP might still bind directly to TPCs, as a contribution of these, potentially less abundant, proteins for NAADP binding might not be detected in assays using complex protein samples.
Regulation by PI(3,5)P2
The phosphoinositide PI(3,5)P2 is an endo-lysosome-specific lipid and considered to be a non-selective endolysosomal channel modulator (McCartney et al., 2014) similar to the role played by PI(4,5)P2 at the plasma membrane (Mori and Inoue, 2014). Indeed, PI(3,5)P2 has been shown to modulate currents across endo-lysosomal membranes that were originally attributed to TRPML1 (Dong et al., 2010; Feng et al., 2014).
Recently, TPCs have also been proposed to be targets for PI(3,5)P2 regulation (Boccaccio et al., 2014; Cang et al., 2014a, 2014b; Grimm et al., 2014; Jha et al., 2014; Pitt et al., 2014; Ruas et al., 2015a; Sakurai et al., 2015; Wang et al., 2012) although the extent of their contribution to the overall currents mediated by this phosphoinositide differ amongst some studies.
The first report describing PI(3,5)P2 regulation of TPCs used endo-lysosomes from bone-marrow derived macrophages from Tpcn1tm1.1Dren/Tpcn2tm1.1Dren animals to propose that TPCs were essential for PI(3,5)P2-activated currents across endo-lysosomal membranes mainly mediated by Na+ (Wang et al., 2012). These findings were corroborated by a further study using endo-lysosomal preparations from peritoneal resident macrophages from the same mouse line (Cang et al., 2013). However, other studies using endo-lysosomes from MEFs derived from Tpcn2Grimm et al. (Grimm et al., 2014) and Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg (Ruas et al., 2015a) mouse lines have indicated that although TPCs contribute to PI(3,5)P2-regulated currents, there is still a substantial residual current that is TPC-independent, presumably mediated by TRPML1. It is therefore likely that PI(3,5)P2 action on TPC activity is more akin to a permissive modulator rather than of a specific activator.
Membrane Potential
The electrical potential across cellular membranes controls ionic movements and, in turn, a variety of cellular processes, often via effects on channels such as voltage-gated Ca2+ channels (VGCC) and TRP channels. TPC proteins share a similar domain organization of six transmembrane helixes with these voltage-regulated channels and, in particular, the characteristic basic residues of the voltagesensor transmembrane S4 (Catterall, 2010; Nilius et al., 2005) are also present in TPCs (Fig. 1). Some studies have provided evidence for voltage regulation and gating of TPC1 (Cang et al., 2014a; Pitt et al., 2014; Rybalchenko et al., 2012) and TPC3 (Cang et al., 2014b) in lipid bilayers or expressed heterologously in cell lines, and this voltage gating is affected by mutations in the voltage sensor S4 (Cang et al., 2014a). Remarkably, this regulatory property of TPC1 was shown to confer electrical excitability to endo-lysosomal membranes when this TPC isoform was heterologously expressed in cell lines (Cang et al., 2014a).
Interestingly, a similar voltage-gated Na+ conductance was detected in endo-lysosomal preparations from wild-type mice cardiac myocytes and kidney cells, but not from peritoneal macrophages (Cang et al., 2014a). Using myocyte preparations from Tpcn1tm1.1Dren mice, the voltage-gated Na+ current was no longer observed but could be rescued by re-expression of TPC1, indicating that TPC1 is responsible for the voltage-gated Na+ currents seen in wild-type cells. Importantly, results obtained from endo-lysosomes of cardiac myocytes from mutant Tpcn1tm1.1Dren mice show that TPC1 is also necessary for the electrical excitability observed in the equivalent wild-type cells.
Luminal pH and Ca2+ Homeostasis
Endo-lysosomes maintain their acidic luminal pH via the action of the vacuolar-type H+-ATPase (V-ATPase) with counter-ion currents being important for dissipation of transmembrane voltage created by proton pumping (Mindell, 2012). Rather than being static, the luminal pH of endo-lysosomes can be dynamically regulated and NAADP-mediated signaling has been shown to induce alkalinization of luminal pH in sea urchin eggs (Morgan and Galione, 2007a, 2007b; Morgan et al., 2013), pancreatic acinar cells (Cosker et al., 2010) and guinea pig atrial myocytes (Collins et al., 2011). Consistent with this, TPC overexpression has been suggested to induce alkalinization of luminal pH, in cell lines such as HeLa and MEG-01 (Ambrosio et al., 2015; Lu et al., 2013), with opposite effects being observed upon down regulation of TPC expression (Ambrosio et al., 2015).
In contrast, studies of MEFs from the mutant mice lines Tpcn2Gt(YHD437)Byg, Tpcn1tm1Dgen (Ruas et al., 2014), Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg (Ruas et al., 2015a), Tpcn2Grimm et al. (Grimm et al., 2014) and of macrophages from Tpcn1tm1.1Dren/Tpcn2tm1.1Dren (Cang et al., 2013) seem to indicate that TPC proteins are not essential for maintenance of steady-state endo-lysosomal luminal pH which was unaffected. On the other hand, removal of TPC2 in myoblasts from Tpcn2Gt(YHD437)Byg mice resulted in alkalinization of the luminal pH and an impairment of lysosomal protease activity (Lin et al., 2015). Similar conflicting results were obtained regarding the possible involvement of TPCs in the dynamic regulation of luminal pH upon certain stimuli; while wild-type endo-lysosomes from macrophages maintain their luminal pH during starvation, in Tpcn1tm1.1Dren/Tpcn2tm1.1Dren preparations a luminal alkalinization is observed upon nutrient removal (Cang et al., 2013). However, another study has found no differences, in luminal pH or in pH-dependent proteolytic maturation of cathepsin D and L, between MEFs from Tpcn2Grimm et al. and wild-type mice either in normal or starvation conditions (Grimm et al., 2014).
Removal of TPC channels could also be expected to influence endo-lysosomal luminal ion homeostasis by, for example, reduction of cation leakage. While elevated luminal Ca2+ was observed in MEFs from Tpcn2Grimm et al., as assessed by cytosolic Ca2+ signals induced by treatment with bafilomycin A1 (a V-ATPase inhibitor) or nigericin (a protonophore) (Grimm et al., 2014), no increased Ca2+ signals were observed in MEFs from Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg treated with nigericin or GPN (lysosomotropic agent) (Ruas et al., 2015a).
Based on the disparate results regarding the effects of TPC removal on the maintenance of luminal pH and Ca2+ storage, it is difficult therefore to estimate the overall contribution of TPCs for luminal ionic homeostasis. It is however possible that TPC’s contribution might vary between different tissues and/or cell types.
Trafficking and Fusion
Given the localization of TPCs in organelles of the endolysosomal system and the importance of Ca2+ signals for organelle fusion required for trafficking processes, it is of no surprise that a link between TPCs and endo-lysosomal trafficking events has been investigated. Manipulation of TPC expression and disruption of NAADP signalling have been shown to affect several cellular processes dependent on the endo-lysosomal system, such as retrograde transport from the plasma membrane to the Golgi and to lysosomes (Grimm et al., 2014; Ruas et al., 2010, 2014), endocytosis of filoviruses (Sakurai et al., 2015), exocytosis of cytolytic proteins from cytotoxic T lymphocytes (Davis et al., 2012), pigment deposition in Xenopus oocytes (Lin-Moshier et al., 2014), and aberrant trafficking in Parkinson’s disease (Hockey et al., 2015). Additionally, proteomic analysis of TPC2-immunoprecipitates has revealed physical interactions between TPCs and various trafficking regulatory proteins (Grimm et al., 2014; Lin-Moshier et al., 2014), and interaction of TPC2 with Rab GTPases was highlighted to be important for TPC2’s role in some trafficking events (Lin-Moshier et al., 2014).
MEFs derived from mutant TPC mouse lines have been used to highlight different contributions of TPC1 and TPC2 towards diverse retrograde transport pathways without, however, affecting gross endo-lysosomal morphology (Grimm et al., 2014; Ruas et al., 2014). For example, Tpcn1tm1Dgen MEFs were defective in trafficking of cholera toxin subunit B from the plasma membrane to the Golgi, a pathway that involves trafficking via TPC1-containing organelles such as early and recycling endosomes, whereas Tpcn2Gt(YHD437)Byg MEFs showed normal trafficking (Ruas et al., 2014). On the other hand Tpcn2Gt(YHD437)Byg MEFs showed slower kinetics of ligand-induced PDGFRβ (platelet-derived growth factor receptor β) degradation in comparison to wild-type or Tpcn1tm1Dgen MEFs, suggesting an impairment of trafficking from the plasma membrane to lysosomes, the main organelle where TPC2 is present (Ruas et al., 2014).
Different contributions of TPC1 and TPC2 were also unmasked in other studies using MEFs and hepatocytes: those from Tpcn2Grimm et al. cells, but not those from Tpcn1Arndt et al. show an aberrant accumulation of EGF (epidermal growth factor) in late endosomes/lysosomes after being trafficked from the plasma membrane (Grimm et al., 2014; Sakurai et al., 2015), a phenotype that could be rescued by re-instatement of wild-type TPC2 expression (Grimm et al., 2014). Tpcn2Grimm et al. MEFs also showed slower kinetics of EGF degradation compared to wild-type cells (Grimm et al., 2014). Investigating further into the reasons behind aberrant accumulation of EGF in late endosomes/lysosomes of cells derived from Tpcn2Grimm et al. animals, the authors proposed that this was due to impaired fusion between late organelles of the endo-lysosomal system. So, while MEFs treated with the “fast” Ca2+ chelator BAPTA-AM (to block local Ca2+ signals necessary for fusion events) mimicked the EGF accumulation seen in Tpcn2Grimm et al. cells, the mutant cells were refractory to BAPTA treatment. Additionally, formation of enlarged Rab5-positive vesicles induced by the constitutively active Rab5(Q79L) mutant was normal in Tpcn2Grimm et al. MEFs indicating that fusion events between early endosomes seem to be unchanged by TPC2 removal (Grimm et al., 2014). Defects in fusion events between late organelles of the endocytic pathway in Tpcn2Grimm et al. cells were also proposed to be the cause of accumulation of LDL-cholesterol in the late endocytic organelles, accounting for hepatic cholesterol overload and liver damage observed in the mutant mice fed with a cholesterol-rich diet (discussed below) (Grimm et al., 2014).
Recently, studies using MEFs lacking TPC1 (Tpcn1Arndt et al.) or TPC2 (Tpcn2Grimm et al.) have shown that these mutant cells are resistant to infection by Ebola virus, and expression of TPC1 or TPC2 rescued infectivity (Sakurai et al., 2015). It was further suggested that this was due to defects in viral delivery into the cytoplasm that occurs after virus-endosome fusion. As such, although viral particles still localized in Lamp1-positive vesicles in Tpcn1Arndt et al. or Tpcn2Grimm et al. MEFs, the virus capsid release into the cytoplasm was arrested in these cells (Sakurai et al., 2015). These results further support a role for TPCs in endosome maturation via vesicular fusion and trafficking.
Acrosome Reaction
NAADP signalling has been implicated in the process of fertilization in echinoderms (Santella et al., 2012) and in various aspects of sperm biology in both echinoderms and mammals. NAADP and NAADP-synthesizing activity have been detected in sea urchin sperm (Billington et al., 2002; Churchill et al., 2003; Vasudevan et al., 2008, 2010) and capacitated human sperm (Sánchez-Tusie et al., 2014). Sperm also contain acidic Ca2+ stores that are targets for NAADP action and binding (Arndt et al., 2014; Sánchez-Tusie et al., 2014) with the proposal that the store corresponds to the acrosome (Arndt et al., 2014; Vasudevan et al., 2010). The acrosome is a secretory vesicle located in the sperm head involved in an egg-induced exocytosis of hydrolyzing enzymes. Acrosome exocytosis is triggered by an increase in cytosolic Ca2+, within the sperm, elicited by opening of store-operated channels at the plasma membrane and IP3R in internal membranes (Buffone et al., 2014). More recently however, NAADP-mediated signalling was proposed to be involved in acrosome exocytosis under physiological conditions, possibly via Ca2+-induced Ca2+ -release mechanisms targeting IP3Rs (Arndt et al., 2014).
Both Tpcn1 and Tpcn2 are expressed in testicular tissue, and TPC1 in the sperm is localized in a membrane between the the subacrosomal space and the plasma membrane, likely to correspond to the acrosome outer membrane (Arndt et al., 2014). Tpc1Arndt et al. mice showed no obvious differences in either testis and epididymal spermatozoa morphology, or acrosome shape. However, sperm from mutant mice were not competent for acrosomal reaction mediated by NAADP, although spontaneous or Ca2+-induced acrosomal reactions were not affected. Additionally, total sperm counts from the epididymis were reduced in mutant mice, and although these mice were fertile with no obvious fertility impairment regarding offspring number per litter and sex distribution, they showed longer time intervals between litters (Arndt et al., 2014).
Another observation resulting from this study was the apparent sub-Mendelian proportion of homozygote Tpc1Arndt et al. mice obtained from heterozygote crosses, in favour of a wild-type genotype, suggesting a subfertile reproductive phenotype (Arndt et al., 2014). However, another study using Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg reported the expected Mendelian proportion of homozygote double mutant mice derived from heterozygote dihybrid crosses (Ruas et al., 2015a). It is possible that the different impact of TPC1 disruption on fertility, reported by these two studies, might be due to an influence of the background strain in which the mice are kept (129SV;C57BL/6 (Arndt et al., 2014) vs 129P2;C57BL/6 (Ruas et al., 2015a)) and/or that different compensation mechanisms operate in the case where both TPC1 and TPC2 expression is disrupted.
Nutrient Handling and Cell Metabolism
Glucose
Control of circulating glucose levels relies on a very fine-tuned and multi-leveled mechanism to keep glucose levels within a narrow physiological range. Central to this is the secretion of insulin by pancreatic β cells to promote glucose uptake by tissues across the whole organism. Insulin secretion is triggered by a cascade of events initiated by an increase in intracellular ATP and inhibition of ATP-sensitive K+ channels (KATP) at the plasma membrane. This ultimately results in membrane depolarization, subsequent opening of voltage-gated Ca2+ channels and an increase in cytosolic Ca2+ that induces secretion of insulin from secretory granules (Rutter et al., 2015). NAADP-mediated signaling has been proposed to contribute to Ca2+ signals in pancreatic β cells (Johnson and Misler, 2002; Kim et al., 2008; Masgrau et al., 2003; Mitchell et al., 2003; Naylor et al., 2009; Park et al., 2013; Shawl et al., 2012; Yamasaki et al., 2004) via gating of a Ca2+- activated plasma membrane cation channel contributing to the glucose-induced depolarization of membrane potential (Arredouani et al., 2015). Furthermore Tpcn2 has been suggested to be the causative gene in a quantitative trait locus for β-cell dysfunction in rats (Tsaih et al., 2014).
Studies using pancreatic β cells from Tpcn2Gt(YHD437)Byg mice have demonstrated a requirement for TPC2 in NAADP-evoked inward currents (Calcraft et al., 2009) and further studies using this mutant mouse line have confirmed the requirement for TPC2 in both NAADP-evoked Ca2+ transients and downstream plasma membrane currents (Arredouani et al., 2015). Extending their study to glucose-induced Ca2+ signals in pancreatic β cells from Tpcn1tm1Dgen and Tpcn2Gt(YHD437)Byg mice, the authors showed a significant role for TPC1 and TPC2 in this glucose-induced response. Furthermore, the hypoglycaemic agent and KATP channel inhibitor tolbutamide, was shown to rely on NAADP signalling to evoke an increase in intracellular Ca2+ and that consequently β cells from Tpcn2Gt(YHD437)Byg mice were refractory to tolbutamide treatment (Arredouani et al., 2015). Together these results point to a requirement for TPCs in glucose-induced Ca2+ signals in pancreatic β cells. However, a different conclusion was drawn from studies using pancreatic β cells from Tpcn1tm1.1Dren/Tpcn2tm1.1Dren where no differences were observed in NAADP- or glucose-induced Ca2+ signals, although these Ca2+ signals were still inhibited by the NAADP antagonist Ned-19 (Wang et al., 2012). This lack of effect of TPC disruption on Ca2+ responses has been suggested to be due to possible residual expression of TPC sequences in this mutant line (Morgan and Galione, 2014), as discussed above (Figs. 5(E) and 6(D)).
Further evidence seems to suggest that TPCs are indeed important players in pancreatic β cell function; intact pancreata of Tpcn1tm1Dgen or Tpcn2Gt(YHD437)Byg perfused with glucose showed reduced insulin secretion compared to wild-type (Arredouani et al., 2015). Accordingly, circulating insulin levels after glucose challenge were subnormal in Tpcn2Gt(YHD437)Byg animals (Tsaih et al., 2014). Additionally, although Tpcn2Gt(YHD437)Byg animals show normal glucose handling in a glucose-tolerance test (Arredouani et al., 2015; Tsaih et al., 2014), Tpcn1tm1Dgen mice show a mild diabetic trait characterized by reduced glucose clearance after a glucose challenge, when compared to wildtype animals (Arredouani et al., 2015).
Lipid
Lipids are important nutrients used for several cellular processes, such as signalling pathways, maintenance of cell membranes, and energy storage in the form of triglycerides in white adipose tissue (WAT). Due to the negative impact of obesity on human health much attention has been devoted to the regulation of lipid metabolism, and recently a role for brown adipose tissue (BAT) in obesity control has been emerging via its role in energy dissipation in the form of heat (Elattar and Satyanarayana, 2015).
Following from the observation that, on a cholesterol-rich diet, Tpcn2Grimm et al. mice showed accumulation of large gallstones in gallbladders and a non-alcoholic fatty liver disease phenotype, resulting from poor intracellular cholesterol transport (Grimm et al., 2014), these mice were further analysed for gene expression changes associated with lipid metabolism, under high-cholesterol diet. While expression of genes involved in cholesterol ester synthesis, bile acid synthesis and efflux were up-regulated, those involved in cholesterol synthesis and uptake, and bile acid uptake were down-regulated in Tpcn2Grimm et al. mice (Grimm et al., 2014), consistent with a compensatory mechanism operating in these animals. Furthermore, circulating levels of proteins associated with liver damage and levels of lipoproteins were also increased in Tpcn2Grimm et al. mice on a high-cholesterol diet compared to wild-types. However, no signs of obesity were found in these mutant mice even under a cholesterol-rich diet (Grimm et al., 2014).
A further study using Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg mice revealed that these animals showed a late-onset obesity between 6 and 9 months of age, compared to wild-types, under a standard diet (Lear et al., 2015). The cause for this phenotype was attributed to an impaired thermogenic activity of BAT caused by reduced lipid availability in BAT from Tpcn1tm1Dgen/Tpcn2Gt(YHD437)Byg mice. This conclusion was based on the observations that although food intake, mitochondrial respiratory chain function and uncoupling protein-1 expression were unaffected by loss of Tpcn1/2 expression, BAT maximal temperature, lean mass-adjusted oxygen consumption, phosphorylated hormonesensitive lipase expression, lipid density and expression of β-adrenergic receptors were all lower in the mutant mice (Lear et al., 2015).
mTORC1-Dependent Pathways/Autophagy
One of the sensing mechanisms for nutrient abundance converge on a signaling hub relying on the action of the protein kinase mTOR (mammalian target of rapamycin) in the mTORCl protein complex. Several environmental cues such as levels of oxygen, energy levels, growth factors and amino acids activate this complex by recruitment of mTORCl to lysosomes (Laplante and Sabatini, 2012). TPCs have been suggested to function as nutrient sensors linked to mTOR action; not only do TPCs and mTOR physically interact with each other (Cang et al., 2013; Lin et al., 2015) but TPC activity is also inhibited by mTOR (Cang et al., 2013; Jha et al., 2014) and more generally by ATP (Cang et al., 2013; Jha et al., 2014), likely involving the additional action of other protein kinases (Jha et al., 2014).
Studies using Tpcn1tm1.1Dren/Tpcn2tm1.1Dren mice support a model that places TPCs downstream from mTOR action; in macrophages, TPCs are the main targets accountable for inhibition of endo-lysosomal currents by ATP, via mTOR, and in hepatocytes from this TPC mutant line mTOR function is not affected as concluded by normal nutrient-driven translocation of mTOR to lysosomes and insulin-stimulated phosphorylation of the mTOR target, Rps6kb1 (ribosomal protein S6 kinase, polypeptide 1; p70S6K) (Cang et al., 2013). However, studies using Tpcn2Gt(YHD437)Byg mice have placed TPC2 upstream from mTOR, as myotubes derived from these mutant mice showed a reduced mTOR activity as detected by phosphorylation of mTOR itself, Rps6kb1 and its target Rps6 under nutrient availability, and reactivation of mTOR under prolonged starvation was delayed in comparison to wild-type animals (Lin et al., 2015).
One of the cellular responses to nutrient availability mediated by active mTORC1 on lysosomal membranes is inhibition of autophagy, an intracellular degradation system that delivers cytoplasmic content to lysosomes for degradation and re-cycling via formation of precursor autophagosome organelles (Laplante and Sabatini, 2012). TPC- and NAADP-mediated signaling have been implicated in autophagic responses, as suggested by a physical interaction between TPCs with several autophagy regulators (Cang et al., 2013; Lin et al., 2015; Lin-Moshier et al., 2014) and by a correlation between levels of autophagosomes and levels of TPC1/2 expression or NAADP signaling status (Gómez-Suaga et al., 2012; Lu et al., 2013; Neely Kayala et al., 2012; Pereira et al., 2011).
Tpcn1tm1.1Dren/Tpcn2tm1.1Dren mice were used to test for the influence of TPCs in the autophagic response to nutrient deprivation. Based on the levels of the autophagic marker LC3-II (lipidated form of microtubule-associated protein l light chain 3) no differences were observed in liver, heart or macrophages of the mutant mice when compared to wild-types under fed or starved conditions, although a decreased amino acid efflux from lysosomes, due to protein degradation as a response to starvation, was observed in Tpcn1tm1.1Dren/Tpcn2tm1.1Dren mice (Cang et al., 2013). Fittingly, these mutant mice have much reduced exercise endurance after fasting, as assessed by a strengthdemanding treadmill test (Cang et al., 2013).
Another study using the mutant mouse line Tpcn2Gt(YHD437)Byg, suggested that TPC2 plays a role in down-regulation of autophagy in skeletal muscle. Using several autophagic markers and readouts (levels of active AKT – protein kinase regulator of autophagy, active p62/SQSTM1 – an ubiquitin-binding autophagic adaptor, lysosomal protein Lamp1, LC3-II and autophagosome numbers) the authors observed increased basal and starvation-induced autophagic signals in the tibialis anterior of Tpcn2Gt(YHD437)Byg animals. In line with the in vivo data, myotubes in culture derived from neonate myoblasts of Tpcn2Gt(YHD437)Byg animals also showed an increased autophagic flux relative to wild-types, that could be reversed by expression of TPC2 (Lin et al., 2015). Appropriately, these mutant mice present skeletal muscle atrophy in the tibialis anterior and gastrocnemius muscles as assessed by several muscle mass markers (weight and myofiber cross-sectional area), that might explain a decreased muscle endurance that was further exacerbated under starvation (Lin et al., 2015). Additionally, tissues from aged Tpcn2Gt(YHD437)Byg animals showed several anomalies, such as increased number of vacuoles in spleen, high levels of lipofuscin in liver and enhanced fibrosis in cardiomyocytes (Lin et al., 2015), implicating TPC2 in autophagy termination with consequences for cellular homeostasis.
Neoangeogenesis
Many signalling cascades triggered by binding of physiological agonists to their plasma membrane receptors rely on Ca2+ signals mediated, to different extents, by NAADP and TPCs (Galione et al., 2010), and more recently NAADP/TPC-mediated signalling has been implicated in responses to VEGF- (vascular endothelial growth factor) and to its receptor VEGFR2 in human umbilical vein endothelial cells, and in vivo as assessed by a matrigel plug assay in mice (Favia et al., 2014).
VEGF induces cell proliferation and migration, events that are important in angiogenesis. Testing for the contribution of TPCs in the regulation of VEGF-induced angiogenesis in vivo, a recent report has shown a specific requirement for TPC2 in this process. While in wild-type and Tpcn1tm1Dgen mice subcutaneous VEGF-matrigel plugs showed an intense vascularization after a 5-day period, in Tpcn2Gt(YHD437)Byg mice no such vascularization was observed (Favia et al., 2014). This indicates that VEGF-induced cell migration from surrounding tissues and formation of vascular structures connected to mouse blood vessels requires TPC2, suggesting that TPC2 could be a potential future therapeutic target in anti-angiogenic strategies.
Heart Function
Excitation-contraction coupling in the cardiac muscle is governed by cytosolic Ca2+ transients established by co-ordinated Ca2+ fluxes though the sarcolemma and membranes of internal Ca2+ stores, the major one being the sarcoplasmic reticulum. Not surprisingly, dysregulation of the mechanisms that control these Ca2+ signals is the major contributor to heart dysfunction conditions such as arrhythmias, declining myocardial contractility, hypertrophy and infarction (Røe et al., 2015).
Several observations suggest that NAADP-mediated signalling is operational in the heart: heart and/or cardiomyocyte preparations possess
-
(i)
NAADP-synthesising enzymatic activity (Chini and Dousa, 1995; Lewis et al., 2012; Macgregor et al., 2007),
-
(ii)
NAADP-binding protein(s) (Bak et al., 2001),
-
(iii)
NAADP-regulated Ca2+ stores (Bak et al., 2001; Capel et al., 2015; Collins et al., 2011; Davidson et al., 2015; Macgregor et al., 2007; Mojžišová et al., 2000; Nebel et al., 2013) and
-
(iv)
a direct correlation between manipulation of NAADP signalling and effects on cardiomyocyte contraction have been described (Capel et al., 2015; Macgregor et al., 2007; Nebel et al., 2013).
Further work is establishing NAADP-regulated pathways as a key player in the physiology and pathophysiology of the heart, in particular during stimulation of the β-adrenergic pathway. β-adrenergic receptors, a class of stimulatory G protein-coupled receptors, play a crucial role in cardiac contractility via downstream cAMP-dependent phosphorylation of key proteins involved in the generation of Ca2+ signals and acute β-adrenergic stimulation can result in arrhythmias triggered by increased Ca2+ transients. Not surprisingly, this pathway represents a major pharmacological target used in cardiovascular therapies (Capote et al., 2015). The observation that cardiomyocyte contraction during continued β-adrenergic stimulation, evoked by the agonist isoproterenol, was inhibited by treatment with self-desensitising concentrations of NAADP, suggested a role for NAADP signalling in the β-adrenergic stimulation of contractions (Macgregor et al., 2007). Indeed, an increase of endogenous levels of NAADP was shown to be coupled to β-adrenergic receptor stimulation (Lewis et al., 2012; Macgregor et al., 2007). Additionally, spontaneous diastolic Ca2+ transients induced by acute β-adrenergic receptor stimulation with isoproterenol were also blocked by a NAADP antagonist – BZ194 (Dammermann et al., 2009) – and in vivo, this agent was also able to reduce isoproterenol-induced arrhythmias in mice (Nebel et al., 2013). Recent results obtained from Tpcn2Gt(YHD437)Byg mice indicate a role for TPC2 as an effector of NAADP action in the β-adrenergic responses detailed above. Increases in Ca2+ transients and contractions following β-adrenergic receptor stimulation were greatly reduced in cardiomyocytes and hearts from Tpcn2Gt(YHD437)Byg mice when compared to wild-type controls (Capel et al., 2015). Moreover, Tpcn2Gt(YHD437)Byg mice subjected to a ventricular burst pacing protocol were less prone to arrhythmias and less sensitive to isoproterenol in induction of ventricular tachycardia or fibrillation (Capel et al., 2015). Additionally, Tpcn2Gt(YHD437)Byg mice were also protected against chronic effects of β-adrenergic stimulation when compared to wild-type controls; after a fourteen-day period of isoproterenol treatment, mice lacking TPC2 showed less hypertrophy, and improved cardiac function as assessed by echocardiography, morphometry and histology (Capel et al., 2015).
Another pathological pathway where NAADP-mediated signalling seems to be playing a role is the one involved in reperfusion injury. After cardiac ischaemia, reperfusion therapies can, paradoxically, result in additional cardiac damage due to increased Ca2+ signals that result in mitochondrial Ca2+ overload, with subsequent opening of the mitochondrial permeability transition pore followed by cell death (Mozaffari et al., 2013). Recently it was shown that in isolated cardiomyocytes, inhibition of NAADP signalling suppressed the ischaemia/reperfusion-induced Ca2+ oscillations with concomitant increase in cell survival (Davidson et al., 2015). Additionally, the size of the myocardial infarct region from mice subjected to an experimental ischaemia/reperfusion protocol was significantly smaller in the animal group treated with the NAADP antagonist Ned-K (Davidson et al., 2015). Significantly, results obtained from experiments using Tpcn1Gt(OST359423)Lex mice, revealed a role for TPC1 in events leading to ischaemia/reperfusion injury; when compared to wild-type animals, Tpcn1Gt(OST359423)Lex mice were protected against ischaemia/reperfusion injury, revealed by significantly smaller myocardial infarction areas (Davidson et al., 2015).
Concluding Remarks
Mutant Tpcn mouse lines have been an invaluable addition to the “tool kit” available for the investigation of TPC- and NAADP-mediated signalling pathways. In particular, it is now possible to study the role of this signalling pathway at the whole-organism level, which is a significant step forward from cell-based studies where disruption of this signalling pathway has been achieved either pharmacologically, or genetically, using RNA interference approaches or by the use of dominant-negative mutants.
The studies here described have been fundamental in supporting the notion that, as endo-lysosomal cation channels, TPCs play an important role in processes relying on trafficking events and fusion. Additionally, they appear to behave as a signalling hub where multiple patho/physiological processes converge, with TPC1 and TPC2 having different and specific contributions.
The use of different mutant Tpcn mouse lines has in some cases generated conflicting results, in particular in the studies dealing with involvement of TPCs in NAADP-mediated signalling and contribution of TPCs for pancreatic β cell physiology. Although it is possible that the discrepancies could be the result of different experimental conditions, they hint at the possibility that some disruption strategies might still allow for residual expression of functional TPC sequences. In addition, expressed nonfunctional truncated TPC sequences could still be involved in protein–protein interactions (either with another TPC isoform or with other interacting proteins) with unpredicted downstream effects. All these possible scenarios highlight the need for thorough characterization of the mutant lines regarding Tpcn expression.
An important question to address in the future is whether the detected tissue- and organ-specific abnormalities, such as those of pancreatic-, adipose-tissue and heart functions, are a direct consequence of loss of Tpcn expression in that organ, or an indirect effect of this loss in other tissues in the body, or due to compensatory mechanisms operating during embryonic development. In this respect, the development of tissue-specific and/or conditional knockout mice will be beneficial.
Acknowledgments
This work was supported by the The Wellcome Trust and Medical Research Council, UK. We thank Dr. Anthony Morgan and Dr. Lianne Davis for comments on the manuscript.
Abbreviations
- AKT
protein kinase B
- p62/SQSTM1
sequestosome 1 protein
- ATP
adenosine triphosphate
- BAPTA-AM
1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid—acetoxymethyl ester
- BAT
brown adipose tissue
- β-gal
β-galactosidase
- β-geo
fusion of β-gal with neomycin transferase
- BTK
Bruton agammaglobulinemia tyrosine kinase
- cAMP
cyclic adenosine monophosphate
- CRISPR
clustered regularly interspaced short palin-dromic repeats
- EGF
epidermal growth factor
- EM
electron microscopy
- ES
cell embryonic stem cell
- GPN
glycyl-L-phenylalanine 2-naphthylamide
- HEK
cells human embryonic kidney 293 cell line
- HeLa
cells human epithelial cell line
- IP3Rs
inositol 1,4,5-trisphosphate receptors
- IRES
internal ribosomal entry site
- KATP
channels ATP-sensitive K+ channels
- kbp
kilo base pairs
- LacZ
gene coding for β-gal
- Lamp1
Lysosomal-associated membrane protein 1
- LC3
microtubule-associated protein 1 light chain 3
- LDL
Low-density lipoprotein
- MEFs
mouse embryonic fibroblasts
- MEG-01 cells
human megakaryoblastic cell line
- MGI
mouse Genomics Informatics
- mTOR
mammalian target of rapamycin
- mTORC1
mammalian target of rapamycin complex 1
- NAADP
nicotinic acid adenine dinucleotide phosphate
- NAADP/AM
NAADP-acetoxymethyl ester
- N3-NAADP
azido-NAADP
- NeoR
coding sequence for neomycin transferase
- NMD
nonsense mediate mRNA decay
- p62/SQSTM1
sequestosome 1 protein
- pA
polyadenylation
- PDGFRβ
platelet-derived growth factor receptor β
- PI(3,5)P2
phosphatidylinositol (3,5)-bisphosphate
- PI(4,5)P2
phosphatidylinositol (4,5)-bisphosphate
- PKG
phosphoglycerate kinase
- PuroR
puromycin N-acetyl-transferase
- Rab GTPases
member of the Ras superfamily of monomeric G proteins
- Rps6
ribosomal protein S6
- Rps6kb1
ribosomal protein S6 kinase, polypeptide 1
- RT-PCR
reverse transcription polymerase chain reaction
- SA
splice acceptor
- SD
splice donor
- SR
sarcoplasmic reticulum
- TALENs
transcription activator-like effector nucleases
- TPC
two-pore channel (protein)
- TPCN/Tpcn
two-pore channel (gene)
- TRP
channels transient receptor potential channels
- TRPML1
transient receptor potential cation channel, mucolipin subfamily, member 1
- V-ATPase
vacuolar-type H+-ATPase
- VEGF
vascular endothelial growth factor
- VGCC
voltage-gated Ca2+ channels
- WAT
white adipose tissue
- ZFNs
zinc finger nucleases
Biographies
 Margarida Ruas was awarded her Ph.D. for the study of the tumour suppressor protein p16/INK4a, under the supervision of Gordon Peters at the ICRF London Institute. Her first post-doctoral position was held at the lab of Tony Kouzarides at the Gurdon Institute, Cambridge, UK, studying the role of the oncogene EMSY as a platform for association of chromatin regulatory proteins. Since joining the lab of Antony Galione, she held a Todd-Bird Junior Research Fellowship and focus her research on the molecular identification and characterization of signalling proteins involved in the NAADP-mediated Ca2+-release pathway.
Margarida Ruas was awarded her Ph.D. for the study of the tumour suppressor protein p16/INK4a, under the supervision of Gordon Peters at the ICRF London Institute. Her first post-doctoral position was held at the lab of Tony Kouzarides at the Gurdon Institute, Cambridge, UK, studying the role of the oncogene EMSY as a platform for association of chromatin regulatory proteins. Since joining the lab of Antony Galione, she held a Todd-Bird Junior Research Fellowship and focus her research on the molecular identification and characterization of signalling proteins involved in the NAADP-mediated Ca2+-release pathway.
 Antony Galione was educated at Trinity College, Cambridge, UK, and received a B.A. in Natural Sciences and a Ph.D. on the role of Ca2+ oscillations in cell activation in Michael Berridge’s lab. After a short spell at UCL working on mammalian fertilisation with Michael Whitaker, he went to Johns Hopkins University as a Harkness Fellow studying the role of Ca2+ signals in early development. Since joining the Department of Pharmacology, Oxford, he has been successively a Beit Memorial Fellow, Wellcome Trust Career Development Fellow and Wellcome Trust Senior Fellow. He holds the Professorship of Pharmacology at Oxford and was elected as a Fellow of the Academy of Medical Sciences in 2010 for his contributions to the advancement of medical science. His primary work has been concerned with the mechanisms and roles of cADPR and NAADP as intracellular messengers.
Antony Galione was educated at Trinity College, Cambridge, UK, and received a B.A. in Natural Sciences and a Ph.D. on the role of Ca2+ oscillations in cell activation in Michael Berridge’s lab. After a short spell at UCL working on mammalian fertilisation with Michael Whitaker, he went to Johns Hopkins University as a Harkness Fellow studying the role of Ca2+ signals in early development. Since joining the Department of Pharmacology, Oxford, he has been successively a Beit Memorial Fellow, Wellcome Trust Career Development Fellow and Wellcome Trust Senior Fellow. He holds the Professorship of Pharmacology at Oxford and was elected as a Fellow of the Academy of Medical Sciences in 2010 for his contributions to the advancement of medical science. His primary work has been concerned with the mechanisms and roles of cADPR and NAADP as intracellular messengers.
 John Parrington obtained a B.A. in Zoology at the University of Cambridge, UK, and his Ph.D. from the University of London working at the ICRF London Institute. After a post-doctoral research period at the MRC National Institute for Medical Research, John was awarded an MRC Career Development Fellowship followed by an MRC Senior NonClinical Fellowship at University College, London. He subsequently joined the Department of Pharmacology and he is now an Associate Professor in Molecular and Cellular Pharmacology and a Tutorial Fellow in Medicine at Worcester College, Oxford. John’s principal research interest is in using molecular approaches to study how Ca2+ signalling governs key physiological events.
John Parrington obtained a B.A. in Zoology at the University of Cambridge, UK, and his Ph.D. from the University of London working at the ICRF London Institute. After a post-doctoral research period at the MRC National Institute for Medical Research, John was awarded an MRC Career Development Fellowship followed by an MRC Senior NonClinical Fellowship at University College, London. He subsequently joined the Department of Pharmacology and he is now an Associate Professor in Molecular and Cellular Pharmacology and a Tutorial Fellow in Medicine at Worcester College, Oxford. John’s principal research interest is in using molecular approaches to study how Ca2+ signalling governs key physiological events.
Footnotes
Note Added in Proof
Since acceptance of this manuscript a report describing a new pancreatic β cell-specific Tpcn2 mutant mouse line has been published: Cane, M. C., Parrington, J., Rorsman, P., Galione, A., and Rutter, G. A. (2015). The two pore channel TPC2 is dispensable in pancreatic β-cells for normal Ca2+ dynamics and insulin secretion. Cell Calcium Doi: 10.1016/j.ceca.2015.12.004. [Epub ahead of print].
Conflict of Interest
There is no conflict of interest.
References
- Ambrosio AL, Boyle JA, Pietro SMD. TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release. Mol Biol Cell. 2015;26:3263–3274. doi: 10.1091/mbc.E15-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt L, Castonguay J, Arlt E, Meyer D, Hassan S, Borth H, Zierler S, Wennemuth G, Breit A, Biel M, Wahl-Schott C, et al. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Mol Biol Cell. 2014;25:948–964. doi: 10.1091/mbc.E13-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani A, Ruas M, Collins SC, Parkesh R, Clough F, Pillinger T, Coltart G, Rietdorf K, Royle A, Johnson P, Braun M, et al. NAADP and endolysosomal two-pore channels modulate membrane excitability and stimulus-secretion coupling in mouse pancreatic β cells. J Biol Chem. 2015;290:21376–21392. doi: 10.1074/jbc.M115.671248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak J, Billington RA, Timar G, Dutton AC, Genazzani AA. NAADP receptors are present and functional in the heart. Curr Biol. 2001;11:987–990. doi: 10.1016/s0960-9822(01)00269-x. [DOI] [PubMed] [Google Scholar]
- Billington RA, Ho A, Genazzani AA. Nicotinic acid adenine dinucleotide phosphate (NAADP) is present at micromolar concentrations in sea urchin spermatozoa. J Physiol. 2002;544:107–112. doi: 10.1113/jphysiol.2002.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaccio A, Scholz-Starke J, Hamamoto S, Larisch N, Festa M, Gutla PVK, Costa A, Dietrich P, Uozumi N, Carpaneto A. The phosphoinositide PI(3,5)P2 mediates activation of mammalian but not plant TPC proteins: Functional expression of endolysosomal channels in yeast and plant cells. Cell Mol Life Sci. 2014;71:4275–4283. doi: 10.1007/s00018-014-1623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Hirohashi N, Gerton GL. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biol Reprod. 2014;90:112. doi: 10.1095/biolreprod.114.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol Biol Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, Lin P, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Aranda K, Ren D. A non-inactivating high-voltage-activated two-pore Na+ channel that supports ultra-long action potentials and membrane bistability. Nat Commun. 2014b;5:5015. doi: 10.1038/ncomms6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Bekele B, Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol. 2014a;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo Y, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel RA, Bolton EL, Lin WK, Aston D, Wang Y, Liu W, Wang X, Burton R-AB, Bloor-Young D, Shade K-T, Ruas M, et al. Two pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-adrenoceptor signaling in the heart. J Biol Chem. 2015;290:30087–30098. doi: 10.1074/jbc.M115.684076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capote LA, Mendez Perez R, Lymperopoulos A. GPCR signaling and cardiac function. Eur J Pharmacol. 2015;763(Part B):143–148. doi: 10.1016/j.ejphar.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Ion channel voltage sensors: Structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Dousa TP. Enzymatic-synthesis and degradation of nicotinate adenine dinucleotide phosphate (NAADP), a Ca2+-releasing agonist, in rat tissues. Biochem Biophys Res Commun. 1995;209:167–174. doi: 10.1006/bbrc.1995.1485. [DOI] [PubMed] [Google Scholar]
- Chinwalla AT, Cook LL, Delehaunty KD, Fewell GA, Fulton LA, Fulton RS, Graves TA, Hillier LW, Mardis ER, McPherson JD, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Churamani D, Hooper R, Brailoiu E, Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, O’Neill JS, Masgrau R, Patel S, Thomas JM, Genazzani AA, Galione A. Sperm deliver a new second messenger: NAADP. Curr Biol. 2003;13:125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- Collins TP, Bayliss R, Churchill GC, Galione A, Terrar DA. NAADP influences excitation–contraction coupling by releasing calcium from lysosomes in atrial myocytes. Cell Calcium. 2011;50:449–458. doi: 10.1016/j.ceca.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Cosker F, Cheviron N, Yamasaki M, Menteyne A, Lund FE, Moutin M-J, Galione A, Cancela J-M. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem. 2010;285:38251–38259. doi: 10.1074/jbc.M110.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, Hohenegger M, et al. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc Natl Acad Sci. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Foote K, Kunuthur S, Gosain R, Tan N, Tyser R, Zhao YJ, Graeff R, Ganesan A, Duchen MR, Patel S, et al. Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc Res. 2015;108:357–366. doi: 10.1093/cvr/cvv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LC, Morgan AJ, Chen J-L, Snead CM, Bloor-Young D, Shenderov E, Stanton-Humphreys MN, Conway SJ, Churchill GC, Parrington J, Cerundolo V, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Elattar S, Satyanarayana A. Can brown fat win the battle against white fat? J Cell Physiol. 2015;230:2311–2317. doi: 10.1002/jcp.24986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia A, Desideri M, Gambara G, D’Alessio A, Ruas M, Esposito B, Bufalo DD, Parrington J, Ziparo E, Palombi F, Galione A, et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2–dependent Ca2+ signaling. Proc Natl Acad Sci. 2014;111:E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Huang Y, Lu Y, Xiong J, Wong C-O, Yang P, Xia J, Chen D, Du G, Venkatachalam K, Xia X, et al. Drosophila TRPML forms PI(3,5)P2-activated cation channels in both endolysosomes and plasma membrane. J Biol Chem. 2014;289:4262–4272. doi: 10.1074/jbc.M113.506501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, Zhu MX. The acid test: The discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2+ release channels. Pflüg Arch—Eur J Physiol. 2009;458:869–876. doi: 10.1007/s00424-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Morgan AJ, Arredouani A, Davis LC, Rietdorf K, Ruas M, Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem Soc Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Charlesworth RM, Sherwood MW, Ferdek PE, Mikoshiba K, Parrington J, Petersen OH, Gerasimenko OV. Both RyRs and TPCs are required for NAADP-induced intracellular Ca2+ release. Cell Calcium. 2015;58:237–45. doi: 10.1016/j.ceca.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P, Churchill GC, Patel S, Hilfiker S. A link between LRRK2, autophagy and NAADP-mediated endolysosomal calcium signalling. Biochem Soc Trans. 2012;40:1140–1146. doi: 10.1042/BST20120138. [DOI] [PubMed] [Google Scholar]
- Grimm C, Holdt LM, Chen C-C, Hassan S, Müller C, Jörs S, Cuny H, Kissing S, Schröder B, Butz E, Northoff B, et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- Guan C, Ye C, Yang X, Gao J. A review of current large-scale mouse knockout efforts. Genesis. 2010;48:73–85. doi: 10.1002/dvg.20594. [DOI] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper R, Churamani D, Davidson SM, Lin-Moshier Y, Walseth TF, Patel S, Marchant JS. TPC1 knockout knocks out TPC1. Mol Cell Biol. 2015;35:1882–1883. doi: 10.1128/MCB.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Hoegg-Beiler MB, Vogt J. Departure gate of acidic Ca2+ confirmed. EMBO J. 2015;34:1737–1739. doi: 10.15252/embj.201591884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proc Natl Acad Sci. 2002;99:14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelu JJ, Chan HLH, Webb SE, Cheng AHH, Ruas M, Parrington J, Galione A, Miller AL. Two-Pore Channel 2 activity is required for slow muscle cell-generated Ca2+ signalling during myogenesis in intact zebrafish. Int J Dev Biol. 2015;59:313–325. doi: 10.1387/ijdb.150206am. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B-J, Park K-H, Yim C-Y, Takasawa S, Okamoto H, Im M-J, Kim U-H. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic Islets. Diabetes. 2008;57:868–878. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear PV, González-Touceda D, Porteiro Couto B, Viaño P, Guymer V, Remzova E, Tunn R, Chalasani A, García-Caballero T, Hargreaves IP, Tynan PW, et al. Absence of intracellular ion channels TPC1 and TPC2 leads to mature-onset obesity in male mice, due to impaired Lipid availability for thermogenesis in brown adipose tissue. Endocrinology. 2015;156:975–986. doi: 10.1210/en.2014-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AM, Aley PK, Roomi A, Thomas JM, Masgrau R, Garnham C, Shipman K, Paramore C, Bloor-Young D, Sanders LEL, Terrar DA, et al. ß-Adrenergic receptor signaling increases NAADP and cADPR levels in the heart. Biochem Biophys Res Commun. 2012;427:326–329. doi: 10.1016/j.bbrc.2012.09.054. [DOI] [PubMed] [Google Scholar]
- Lin P-H, Duann P, Komazaki S, Park KH, Li H, Sun M, Sermersheim M, Gumpper K, Parrington J, Galione A, Evans AM, et al. Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J Biol Chem. 2015;290:3377–3389. doi: 10.1074/jbc.M114.608471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage-dependent shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lu Y, Hao B-X, Graeff R, Wong CWM, Wu W-T, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J Biol Chem. 2013;288:24247–24263. doi: 10.1074/jbc.M113.484253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Macgregor A, Yamasaki M, Rakovic S, Sanders L, Parkesh R, Churchill GC, Galione A, Terrar DA. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J Biol Chem. 2007;282:15302–15311. doi: 10.1074/jbc.M611167200. [DOI] [PubMed] [Google Scholar]
- Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJH, Galione A. NAADP: A new second messenger for glucose-induced Ca2+ responses in clonal pancreatic β cells. Curr Biol. 2003;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- McCartney AJ, Zhang Y, Weisman LS. Phosphatidylinositol 3,5-bisphosphate: Low abundance, high significance. BioEssays. 2014;36:52–64. doi: 10.1002/bies.201300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Lai FA, Rutter GA. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2+ release from insulin-containing vesicles in living pancreatic β-cells (MIN6) J Biol Chem. 2003;278:11057–11064. doi: 10.1074/jbc.M210257200. [DOI] [PubMed] [Google Scholar]
- Mojžišová A, Križanová O, Žáčiková L’, Komínková V, Ondriaš K. Effect of nicotinic acid adenine dinucleotide phosphate on ryanodine calcium release channel in heart. Pflüg Arch. 2000;441:674–677. doi: 10.1007/s004240000465. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Fertilization and nicotinic acid adenine dinucleotide phosphate induce pH changes in acidic Ca2+ stores in Ssea urchin eggs. J Biol Chem. 2007a;282:37730–37737. doi: 10.1074/jbc.M704630200. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007b;402:301. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Two-pore channels (TPCs): Current controversies. BioEssays. 2014;36:173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Ruas M, Galione A. TPC: The NAADP discovery channel? Biochem Soc Trans. 2015;43:384–389. doi: 10.1042/BST20140300. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Wagner SKTY, Lewis AM, Parrington J, Churchill GC, Galione A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MX, Inoue R. New experimental trends for phosphoinositides research on ion transporter/channel regulation. J Pharmacol Sci. 2014;126:186–197. doi: 10.1254/jphs.14r14cp. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–196. [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel M, Schwoerer AP, Warszta D, Siebrands CC, Limbrock A-C, Swarbrick JM, Fliegert R, Weber K, Bruhn S, Hohenegger M, Geisler A, et al. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by β-adrenergic stimulation. J Biol Chem. 2013;288:16017–16030. doi: 10.1074/jbc.M112.441246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely Kayala KM, Dickinson GD, Minassian A, Walls KC, Green KN, LaFerla FM. Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: Implications for lysosomal function. Brain Res. 2012;1489:8–16. doi: 10.1016/j.brainres.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: A voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-H, Kim B-J, Shawl AI, Han M-K, Lee HC, Kim U-H. Autocrine/paracrine function of nicotinic acid adenine dinucleotide phosphate (NAADP) for glucose homeostasis in pancreatic β-cells and adipocytes. J Biol Chem. 2013;288:35548–35558. doi: 10.1074/jbc.M113.489278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-H, Kim KN, Park D-R, Jang KY, Kim U-H. Role of nicotinic acid adenine dinucleotide phosphate (NAADP) in keratinocyte differentiation. J Invest Dermatol. 2015;135:1692–1694. doi: 10.1038/jid.2015.43. [DOI] [PubMed] [Google Scholar]
- Parrington J, Tunn R. Ca2+ signals, NAADP and two-pore channels: Role in cellular differentiation. Acta Physiol. 2014;211:285–296. doi: 10.1111/apha.12298. [DOI] [PubMed] [Google Scholar]
- Patel S. Function and dysfunction of two-pore channels. Sci Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- Pereira GJS, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, Piacentini M, et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Lam AKM, Rietdorf K, Galione A, Sitsapesan R. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+ Sci Signal. 2014;7:ra46. doi: 10.1126/scisignal.2004854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp MW-L, Maquat LE. Organizing principles of mammalian nonsense-mediated mRNA decay. Annu Rev Genet. 2013;47:139–165. doi: 10.1146/annurev-genet-111212-133424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal. 2014;7:ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J Biol Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røe ÅT, Frisk M, Louch WE. Targeting cardiomyocyte Ca2+ homeostasis in heart failure. Curr Pharm Des. 2015;21:431–448. doi: 10.2174/138161282104141204124129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Galione A, Parrington J. Reply to “TPC1 knockout knocks out TPC1.”. Mol Cell Biol. 2015b;35:1884–1884. doi: 10.1128/MCB.00083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Chuang K-T, Davis LC, Al-Douri A, Tynan PW, Tunn R, Teboul L, Galione A, Parrington J. TPC1 has two variant isoforms and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol Cell Biol. 2014;34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen C-C, Morgan AJ, Chuang K-T, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Biel M, et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015a;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter GA, Pullen TJ, Hodson DJ, Martinez-Sanchez A. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J Biol Chem. 2012;287:20407–20416. doi: 10.1074/jbc.M112.359612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Tusie AA, Vasudevan SR, Churchill GC, Nishigaki T, Treviño CL. Characterization of NAADP-mediated calcium signaling in human spermatozoa. Biochem Biophys Res Commun. 2014;443:531–536. doi: 10.1016/j.bbrc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Santella L, Vasilev F, Chun JT. Fertilization in echinoderms. Biochem Biophys Res Commun. 2012;425:588–594. doi: 10.1016/j.bbrc.2012.07.159. [DOI] [PubMed] [Google Scholar]
- Shawl AI, Park K-H, Kim B-J, Higashida C, Higashida H, Kim U-H. Involvement of actin filament in the generation of Ca2+ mobilizing messengers in glucose-induced Ca2+ signaling in pancreatic β-cells. Islets. 2012;4:145–151. doi: 10.4161/isl.19490. [DOI] [PubMed] [Google Scholar]
- Singla V, Hunkapiller J, Santos N, Seol AD, Norman AR, Wakenight P, Skarnes WC, Reiter JF. Floxin, a resource for genetically engineering mouse ESCs. Nat Methods. 2010;7:50–52. doi: 10.1038/nmeth.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaih S-W, Holl K, Jia S, Kaldunski M, Tschannen M, He H, Andrae JW, Li S-H, Stoddard A, Wiederhold A, Parrington J, et al. Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) Investigators. Identification of a novel gene for diabetic traits in rats, mice, and humans. In: Hoffmann RG, Simpson P, Jacob H, Hessner M, Solberg Woods LC, editors. Genetics. Vol. 198. 2014. pp. 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugba Durlu-Kandilci N, Ruas M, Chuang K-T, Brading A, Parrington J, Galione A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)—and agonist-evoked contractions of smooth muscle. J Biol Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan SR, Galione A, Churchill GC. Sperm express a Ca2+-regulated NAADP synthase. Biochem J. 2008;411:63. doi: 10.1042/BJ20071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan SR, Lewis AM, Chan JW, Machin CL, Sinha D, Galione A, Churchill GC. The calcium-mobilizing messenger nicotinic acid adenine dinucleotide phosphate participates in sperm activation by mediating the acrosome reaction. J Biol Chem. 2010;285:18262–18269. doi: 10.1074/jbc.M109.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Lin-Moshier Y, Weber K, Marchant JS, Slama JT, Guse AH. Nicotinic acid adenine dinucleotide 2-phosphate (NAADP) binding proteins in T-lymphocytes. Messenger. 2012b;1:86–94. doi: 10.1166/msr.2012.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Lin-Moshier Y, Jain P, Ruas M, Parrington J, Galione A, Marchant JS, Slama JT. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J Biol Chem. 2012a;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong X, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu M, et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Masgrau R, Morgan AJ, Churchill GC, Patel S, Ashcroft SJH, Galione A. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and β cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]