Abstract
Objective
The goal of this study was to determine the neural correlates of excessive habit formation in obsessive-compulsive disorder (OCD). We aimed to (i) test for neurobiological convergence with the known pathophysiology of OCD and (ii) infer, based on abnormalities in brain activation, whether these habits arise from dysfunction in the goal-directed or habit system.
Method
Thirty-seven OCD patients and 33 controls learned to avoid shocks while undergoing a functional Magnetic Resonance Imaging (fMRI) scan. Following 4 blocks of training, we tested if the avoidance response had become a habit by removing the threat of shock and measuring continued avoidance. We tested for task-related differences in brain activity in 3 ROIs, the caudate, putamen and medial orbitofrontal cortex at a statistical threshold of p<.05, family-wise error (FWE) corrected.
Results
We observed excessive habit formation in OCD patients, which was associated with hyper-activation in the caudate. Activation in this region was also associated with subjective ratings of increased urge to perform habits. The OCD group, as a whole, showed hyper-activation in the medial orbitofrontal cortex (mOFC) during the acquisition of avoidance, however this did not relate directly to habit formation.
Conclusions
OCD patients exhibited excessive habits that were associated with hyper-activation in a key region implicated in the pathophysiology of OCD, the caudate nucleus. Prior studies suggest that this region is important for goal-directed behavior, suggesting that habit-forming biases in OCD may be a result of impairments in this system, rather than differences in the build up of stimulus-response habits themselves.
Introduction
The habit hypothesis of obsessive-compulsive disorder (OCD) suggests that the disorder reflects dysfunction in the brain systems that support automatic habits and more purposeful, goal-directed control over action (1). Habits are automatic, stimulus-driven behaviors that can arise under many conditions, the most commonly accepted of which is the over-training of simple responses (2). However, habits can also arise from failures in goal-directed control, which can render behavior habitual even very early on in training (3, 4). These two systems, habit and goal-directed, therefore each contribute to the likelihood that a habit will be performed in a given situation. In OCD, it is currently unclear which of these putative systems drives the exaggerated tendency to display habits, which has been observed regardless of whether they are working to gain rewards (5) or avoid punishments (6). However, two recent studies found deficits in goal-directed behavior during trial-by-trial learning in OCD, using paradigms that did not involve repeating simple responses (7, 8). This suggests that excessive habits in OCD could arise due to disturbances in the goal-directed, rather than the habit system. The present study aimed to test for neurobiological convergence in support for this possibility, drawing on a rich cross-species neuroscience literature, which has identified dissociable neural substrates of these two systems (9).
The medial orbitofrontal cortex (mOFC) and the caudate nucleus each contribute to goal-directed control over our behavior. Specifically, the caudate and mOFC have both been shown to subserve learning involving action-outcome contingencies (4, 10, 11). Additionally, the mOFC plays a pivotal role in tracking the current value of outcomes (12–14). Another region in the basal ganglia, the putamen, is necessary for the formation of stimulus-response habits with practice (11, 15, 16). We tested if functional activation in these three regions was associated with habit-forming biases in OCD, and in doing so aimed to reveal if dysfunction in the goal-directed or habit-learning system accounted for excessive habits in OCD.
Generally, the habit hypothesis of OCD exhibits good face validity in that habits, like compulsions, continue in spite of awareness that these actions are not useful/wanted (i.e. ego-dystonic), and are associated with the experience of an urge to perform them (6). A secondary goal of this study was to test the neurobiological validity of the OCD habit hypothesis by assessing if activation associated with habit forming in OCD overlaps with that implicated in the disorder symptomatology. The literature has broadly converged on a model of OCD that involves hyperactivity within fronto-striatal circuits (17), with effects in the orbital gyri caudate nucleus head being among the most reliable (18–20). Evidence for this model comes primarily from (now rather dated) functional brain imaging studies examining brain activity at rest (21–23), during symptom provocation (24–26), and pre- and post-treatment with psychotherapy or pharmacotherapy (22, 27–29). A more widely distributed network of regions has also been implicated in OCD in recent studies employing task-related functional Magnetic Resonance Imaging (fMRI) analysis, including the nucleus accumbens, amygdala and other parts of the prefrontal cortex (30–32). However, given that fMRI activation patterns are entirely dependent on the task employed, the results have been unsurprisingly heterogeneous and have failed to confirm activation seen during earlier studies examining task-independent activity patterns that are characteristic of OCD. We hypothesized that, if excessive habits are an appropriate model of OCD, then brain-activation associated with habit-forming in these patients should overlap with those associated with the symptomatology, specifically in the mOFC and caudate. Although less consistently implicated, there is some suggestion that the putamen may be enlarged in OCD, an effect related to age, and plausibly the chronic performance of compulsive behavior (33). We therefore also tested the possibility that aberrant activation in the putamen, perhaps reflecting over-active habit learning, would be associated with habits in OCD.
To investigate the neural basis of habit-forming biases in OCD, we used fMRI to examine changes in brain activation while patients acquired and later performed habits. To do this, we used an avoidance task that has previously been shown to be sensitive to differences in habit formation between OCD patients and controls (6). Only subjects who had not previously participated in the previously published behavioral study using this task were eligible to take part. This task was selected because avoidance rather than appetitive compulsions are characteristic of OCD. This approach therefore allowed us to model the disorder more closely, and secondarily to investigate how habit learning might relate to anxiety and explicit fears in OCD. To test for habits we used the so-called ‘outcome devaluation’ technique, where behavior is defined as a habit if it persists despite changes in the value that action produces (34), in other words, habits are behaviors that are driven by stimuli, not by motivation or ‘goals’.
Method
Participants
Participants were 37 OCD patients and 33 controls matched at mean-level for age, handedness, smoking behavior, education and gender. A trend (p=.092) towards lower premorbid verbal IQ (using the National Adult Reading Test: NART, 35) was however observed in the OCD group, but analyses of covariance confirmed this did not drive any of the results. Participants in this study were excluded if they had participated in a previous study examining avoidance habits in OCD in our lab (6), i.e. all participants were task-naïve. Patients were free of co-morbid psychiatric diagnoses. Fourteen patients were free of psychotropic medication, the remaining 23 patients has been stabilized on medication for a minimum of six weeks prior to taking part in the study and the majority were taking selective serotonin reuptake inhibitors (SSRIs; see Supplement). For all of the results presented, there were no significant differences between medicated and unmedicated patients unless otherwise stated. Further details on participant characteristics and recruitment can be found in the Supplement.
Procedure
Avoidance Training and Habit Test
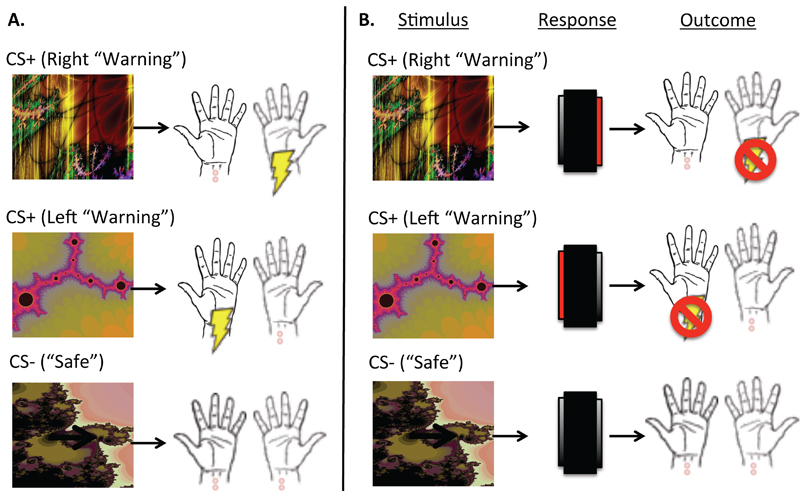
Participants completed a shock avoidance paradigm similar to one described in detail elsewhere (6) and in the supplementary materials. Participants were instructed that their goal was to avoid receiving shocks, which would be delivered to their right and left wrists following the presentation of a conditioned stimulus (CS+) (Figure 1). During fMRI scanning, we over-trained the avoidance response across 4 blocks each containing 30 trials (10 per CS), before testing for habits using outcome devaluation. Before the final block, the left shock outcome was ‘devalued’ by disconnecting the electrodes from participants’ left wrists. The shock to the right wrist remained threatening or ‘valued’. Importantly, up until this point, subjects had identical training with both left and right CSs that, in the final block, were defined as devalued or valued by the connection status of wrist electrodes. Subjects were informed on-screen that they could no longer be shocked to the left wrist and their only goal was to avoid the remaining shock (which was on the right). The task proceeded for one block constituting the habit test. To prevent new learning during the test, shocks were no longer delivered to any CS.
Figure 1. Task Schematic.
Panel A depicts the Pavlovian (stimulus-outcome) contingencies, which were 100% deterministic. There were two Warning CSs (CS+); one predicted a shock to the left wrist, and another predicted a shock to the right wrist. A Safe CS (CS-) never predicted shock.
Panel B depicts the avoidance contingencies, including stimuli, responses and outcomes. When top (right warning) predictive CS+ appears on screen, it indicates that a shock to the right wrist is imminent. If participants press on the right side of the foot-box (highlighted in red) while this CS+ is on-screen, they will avoid this shock. Likewise pressing on the left side of the foot-box when the middle (left warning) CS+ appears cancels an otherwise imminent shock to the left wrist. The safe stimulus always remained safe, regardless of responding.
To better clarify how habits in OCD relate to implicit and explicit fear and belief, we collected supporting data including skin conductance responses (SCRs), explicit contingency knowledge and subjective ratings of shock expectancy, shock unpleasantness, urge to perform habits and attempts to suppress habits. See also Supplement (Table S2). Participants completed an additional and unrelated experiment in the same session (after this task had been completed), the results of which will be published elsewhere.
Data Analysis
Behavior
Behavioral data were analyzed using ANOVA for parametric data and Mann-Whitney U, Chi-square and Spearman’s rho correlations. In the habit test, which followed outcome devaluation, we compared the number of avoidance responses performed to the Devalued and Valued CSs. We also measured false alarms to the Safe CS. OCD patients were divided into two Habit Groups: “Habit” and “No Habit”, determined by whether or not they made any response to the Devalued CS during the habit test. In subsequent analyses we compared the two groups defined by this distinction. During training, as left and right CSs each predicted an avoidable shock, we collapsed these into one factor, Warning (i.e. CS+). We analyzed accuracy during training in terms of percentage correct avoidance responses over each of the 4 experimental blocks. Results are presented as significant at p<.05 and trends are defined as 0.1>p>0.05.
fMRI
Based on prior literature on habit learning in healthy humans, and the known neurobiological profile of OCD, we examined anatomically defined bilateral a priori regions of interest (ROI), the medial orbitofrontal cortex (mOFC) (11–13), caudate (10, 11) and putamen (11, 16). We used Pickatlas toolbox in SPM8 (36) to define ROIs according to the anatomical automatic labeling (AAL) atlas. Activation within these ROIs was deemed significant at p<0.05, corrected for family-wise error (FWE) at the voxel level, and for testing across multiple ROIs (p<.05/3). Results from whole brain exploratory analyses are presented at p<.001, uncorrected, with a minimum cluster size of 10 voxels. Whilst we discuss these results to some extent, we caution that replication is needed.
First level analyses of the habit test data from the final block, modeled the three CSs (Valued, Devalued and Safe) along with the six movement parameters produced during realignment (further details in supplement). The Habit Group was defined as patients who had formed habits and those who had not (No Habit Group), based on their responding to the devalued CS. Whilst behavioral responses to the devalued CS allow us to discern if a habit has formed, neural responses to this CS are confounded by the experience of the devaluation procedure, and the associated differences in behavioral responding, urge to respond and attempts to suppress responding. Therefore, to capture the neural signature of habits, we examined the contrast of Valued-Safe, which captures the neural activation associated with unperturbed habitual responding. This analysis therefore relies on the reasonable assumption that if avoidance responding to one CS+ (i.e. devalued) has become habitual, then so has responding to the other CS+ (i.e. valued), given the equivalence of contingencies and training duration.
In the first level analysis for training data from the first 4 blocks, we modeled the three CSs (Left, Right, and Safe), Block (1,2,3 and 4) and the six movement parameters for each block. To assess brain regions involved in the development of habits over time, we used a mixed factor generalized linear model, where we tested for an interaction between CS (Warning (collapsed Left + Right), Safe) and Block (1–4), where Block was a parametric modulator. We then tested for group differences at the second level. This allowed us to examine changes in activation that progressed with over-training, or the putative ‘stamping in’ of habits.
Results
Habit Test
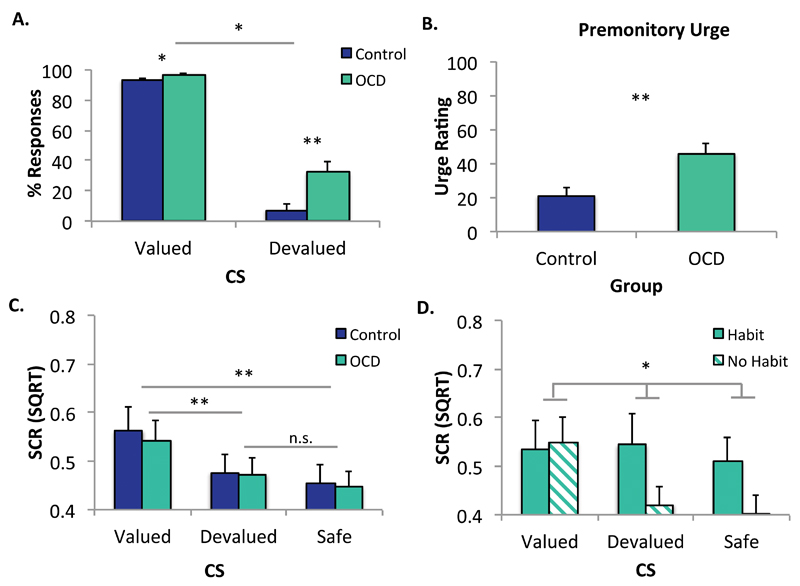
OCD patients showed increased habits relative to healthy controls, replicating previous findings using both avoidance (6), and appetitive (5, 7) paradigms. There was a significant main effect of Group (OCD, Control) on the number of responses overall, F(1,68)=10.691, p=.002 and an interaction between group and CS (Valued, Devalued) during the devaluation test, F(1,68)=5.408, p=.023 (Figure 2A). Simple effects analyses revealed that OCD patients compared to controls responded at a significantly higher rate to the devalued CS, F(1,69)=8.139, p=.006, and to the valued CS, F(1,69)=4.896, p=.030. However, the significant interaction indicates that the group difference was greater for the devalued compared to the valued CS, and responding to the valued and devalued CSs were not significantly correlated Spearman’s r=-.276, p=.099. The trend was in the opposite direction to what would be predicted by a disinhibition account, such that the greater the habits, the fewer the responses to the valued CS.
Figure 2. Habit Test: Behavioral Data.
** p<.01; *p<.05; n.s. non-significant
Error bars denote standard error of the mean (SEM)
Panel A depicts the percentage of accurate avoidance responses made to the Devalued and Valued stimuli. In line with previous research, OCD patients developed more habits than Controls, evidenced by greater responding to the Devalued CS (p<.006). Overall, OCD patients (n=37) responded more to both the Devalued and Valued CSs relative to controls (n=33). However, a significant interaction between Group (OCD, Control) and CS (Valued, Devalued), F(1,69)=5.335, p=.024, indicated that the difference was greater for the Devalued compared to the Valued CS (p<.037).
Panel B depicts urge to respond ratings. OCD patients reported a greater urge to respond relative to healthy controls, U=345, Z=-3.191, p=.001.
Panel C depicts SCR data. There were no differences between OCD (n= 36) and Controls (n=31) in SCR (note 2 controls and 1 patient were excluded from this analysis due to insufficient SCR data). There was a main effect of CS, F(2,130)=16.163, p<.001. Conditioned fear responses extinguished to the Devalued CS to a level equivalent to the Safe CS, p=.181. Responses to the Valued CS remained elevated relative to the Devalued, p<.002 and Safe CSs, p<.001.
Panel D depicts SCR data between Habit (n=15) and No Habit (n=21; 1 subject excluded due to insufficient SCR data) groups from within the OCD group. There was a significant interaction between CS and Habit Group, F(2,68)=4.818, p=.011. While the No Habit group had a significant main effect of CS, F(2,40)=9.934, p=.001, the Habit group did not, F<1.
Supporting Data
Explicit contingency knowledge was equivalent across groups, F<1 (6). OCD patients and controls reported a greater urge to respond to the devalued CS relative to healthy controls, U=345, Z=-3.191, p=.001 (Figure 2B), and this urge correlated with the number of responses made to the Devalued CS by OCD patients, Spearman’s r(37)=.668, p<.001. There were no differences in SCR between OCD patients and Controls (F<1), but those patients who formed habits showed inferior discrimination between the three CSs (devalued, valued and safe) compared to those who did not form habits during the habit test (CS by Group interaction: p=.011), but not during training (F<1.8). More detailed analyses are presented in the supplement along with expectancy and suppression data.
fMRI
Habit Test
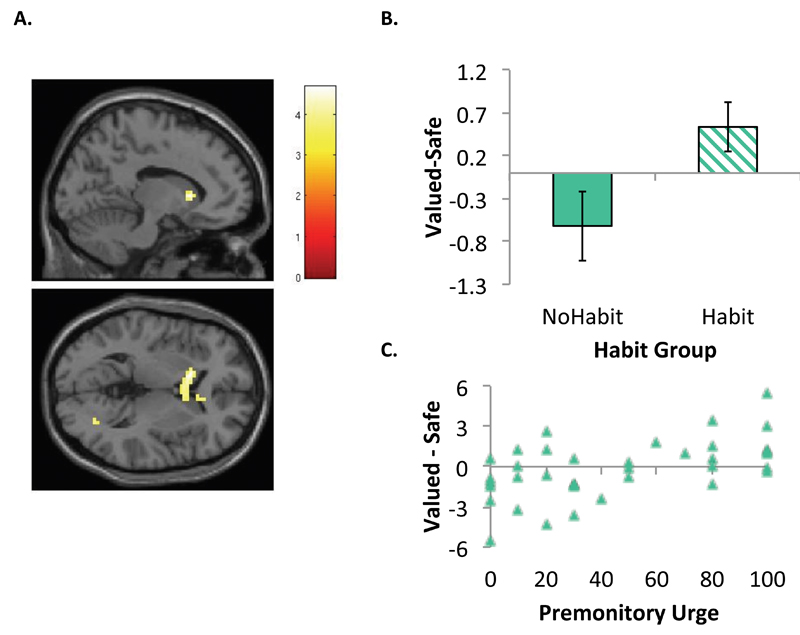
Activation associated with habitual responding in OCD patients was captured using the contrast of Valued – Safe CSs, comparing Habit Groups (Habit n=15, No Habit n=22). OCD patients exhibiting habits during the test showed hyper-activation in the left caudate nucleus [-12,17,4] (extending to the right) relative to those who did not (Figure 3), T(35)=4.68, p<.05 FWE-corrected ROI. Using the urge to respond as a regressor in an independent analysis at the second level, replacing the binary Habit Group factor, we found that within the OCD group, there was a positive relationship between activation in the right caudate [6,8,1] for this same contrast, T(35)=3.81, p<.001, uncorrected. This pattern was also observed in the left caudate at a more liberal threshold of p<.005. There was no such relationship in controls. Whilst there were no significant differences between Study Groups (OCD, Control) in activation to the Valued-Safe contrast, the OCD Habit group showed significantly greater activation of the right caudate [15,26,1] relative to controls (p<.001, uncorrected). Conversely, when comparing OCD No Habit to controls, we found hypo-activation in the right caudate (p<.05 FWE-corrected, ROI).
Figure 3. Comparison of OCD patients who did and did not develop habits.
Panel A depicts the interaction between Group (Habit n=15, No Habit n=22), and CS (Valued, Safe) during the devaluation test in the left caudate at T(35)=4.68, FWE corrected level, p<.05 using a bilateral caudate ROI.
Panel B is a plot of the first eigenvariate of the Valued-Safe contrast extracted from left caudate cluster [-12,17,4] (pictured top right). Patients with habits show significant hyper-activation of the caudate relative to those who did not exhibit habits.
Panel C is a plot showing the parametric association between activity in the right caudate [6,8,1] and the self-reported urge to respond in OCD patients for the Valued-Safe contrast, T(35)=3.81, p<.001. This pattern was also observed in the left caudate at a more liberal threshold of p<.005.
Acquisition of Avoidance
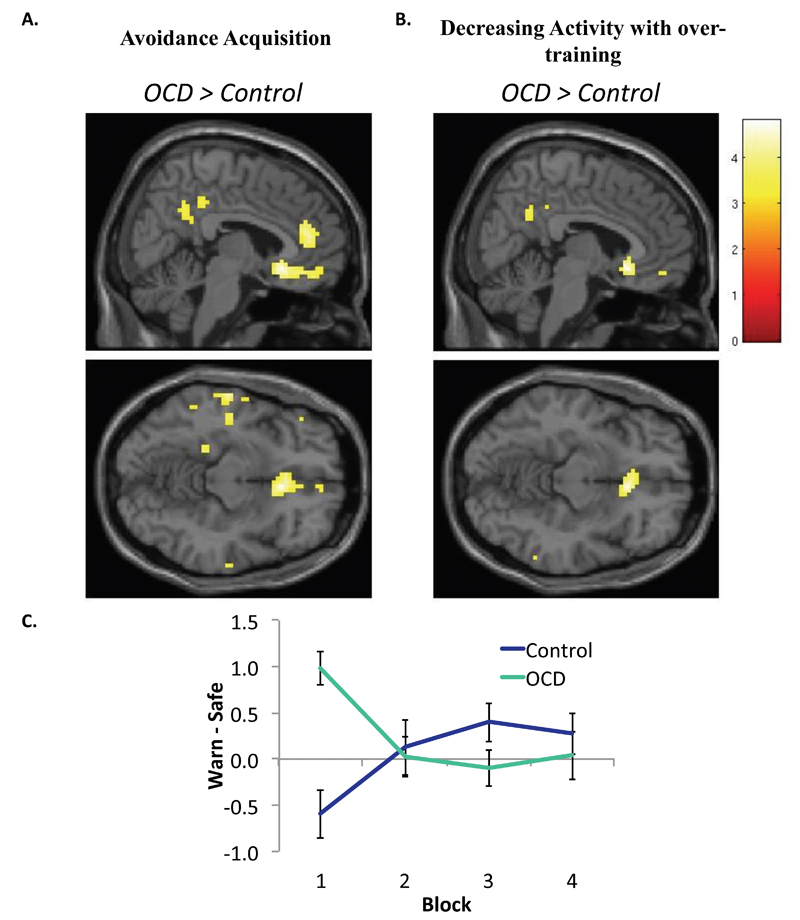
We tested for differences between OCD and Controls in activity associated with the acquisition of avoidance (i.e. Block 1) using a contrast of CS (Warning, Safe). We found significant hyper-activation in the mOFC in OCD patients relative to controls, T(68)=4.96 [6,23,−11], (FWE p<.05 corrected ROI) (Figure 4A). A more extensive set of regions were hyper-active in OCD during this initial learning at p<.001, uncorrected (Table S2). Controls did not show greater activation than OCD patients in any region. There were no significant differences between Habit and No Habit patients.
Figure 4. Group differences during avoidance acquisition and over-training.
Panel A shows areas of hyper-activation in OCD patients relative to Controls during the initial acquisition of avoidance, i.e. Block 1. There was a significant difference in the medial OFC, T(1,68)=4.96 [6,23,-11], (FWE p<.05 corrected mOFC ROI).
Panel B depicts a significant interaction between Group (OCD, Control) and Stimulus (Warning, Safe) and Block (1,2,3,4) in the medial OFC T(68)=4.82, [(FWE p<.05 corrected mOFC ROI). The peak voxel was also at [6,23,-11].
Panel C is a plot of the mean eigenvariate for the contrast of Warn – Safe plotted across time for each of the groups using the mOFC cluster displayed above at p<.001 uncorrected. This graph reveals a pattern of hyper-activation in OCD patients during initial acquisition that decreases over time. Control participants show initial hypo-activation, which increases with extended training.
Results are displayed at p<.001 uncorrected. Error bars denote SEM.
Over-Training of Avoidance
To test if over-training was associated with changes in brain activation, we compared changes in BOLD activation between OCD patients and controls across blocks. There was a significant interaction with group in the mOFC, T(68)=4.82 (FWE p<.05 corrected ROI) (Figure 4B), such that OCD patients showed a decrease in activation over successive blocks, whereas controls showed an increasing pattern in this region (Figures 4C). The peak of this interaction was the same as that showing initial hyper-activation in OCD patients during block 1 [6,23,-11]. Other regions showing a similar pattern at p<.001 uncorrected are presented in the supplement (Table S3).
We compared Habit Groups on this contrast to test if differences in activation during training foreshadowed the expression of habits. There were no effects in our a priori ROIs. However, at p<.001 uncorrected, we found a significant interaction in the right precuneus, T(1,35)=4.11, Z=3.68 ([18,-49,34], cluster extent (Ke)=14) and right superior occipital gyrus T(1,35)=4.42, Z=3.91 ([27,-94,16], Ke=22). In both of these regions, patients in the No Habit group showed a decreasing pattern of activation, while those who later formed habits did not (Figure S2).
Psycho-Physiological Interaction (PPI) analysis
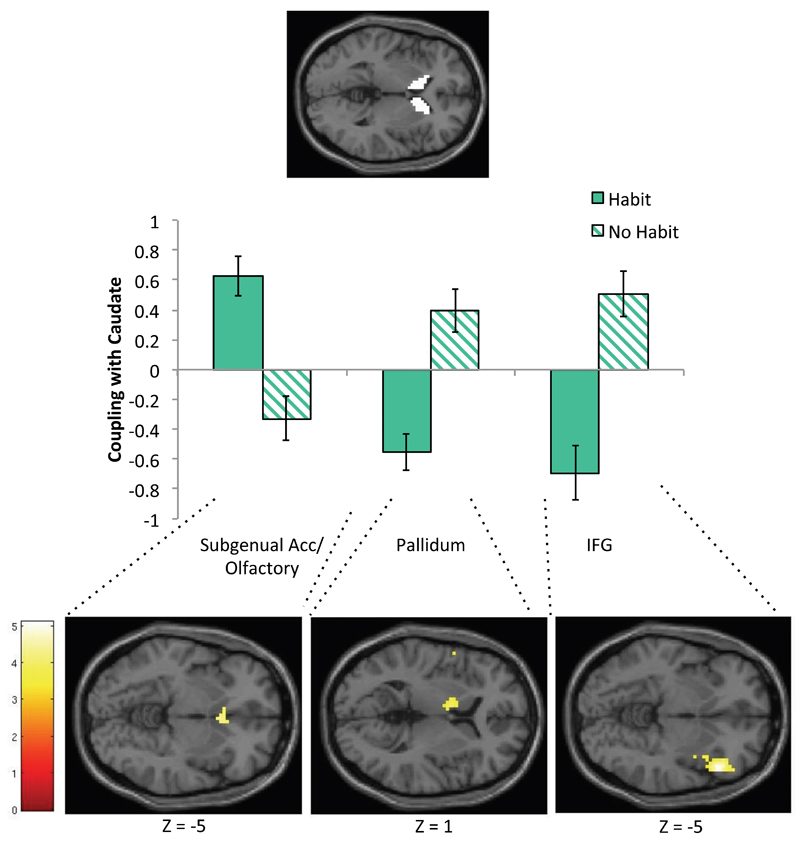
We conducted post hoc PPI analyses to interrogate if the caudate, which was hyperactive in OCD patients who formed habits and correlated with the self-reported urge to respond, showed abnormal neuronal connectivity during the acquisition of avoidance. To do this, we tested for functional connectivity between activation in the bilateral caudate (ROI) and the whole brain during Block 1 (Warning-Safe). There was a significant difference in neural coupling between the Habit and No Habit groups, such that in the No Habit Group, there was positive coupling between the caudate and the right inferior frontal gyrus (rIFG) (cluster corrected at FWE, p<.05) and left pallidum (p<.001, uncorrected) (Figure 5) during the early acquisition of avoidance, and negative coupling with activation in the subgenual ACC/olfactory cortex (Brodmann Area 25) (p<.001, uncorrected). This cluster was rostral to the mOFC cluster observed to be hyperactive in OCD patients (relative to controls) during this stage, but overlapped in 2 voxels (p<.05, FWE-corrected ROI of mOFC cluster where OCD patients showed hyper-activity during avoidance acquisition). This pattern was reversed for patients in the habit group (Table S5, Figure 5), such that they exhibited negative coupling between caudate and the rIFG and pallidum, and positive coupling with subgenual ACC/olfactory cortex. There were no differences between patients overall and controls.
Figure 5. Regions where neural coupling between the caudate differed between Habit and No Habit groups.
PPI analysis results at p<.001 uncorrected. The psychological variable was CS (Warning – Safe) during acquisition of avoidance, i.e. Block 1, and the physiological variable (PPI seed) was activity for this contrast in an anatomical caudate ROI. Eigenvariates from clusters showing interaction effects (between PPI and Habit group) are plotted below. The panel below displays the clusters showing significant psychophysiological interactions in olfactory/subgenual ACC (T(35)=4.4), pallidum (T(35)=3.91), and rIFG (T(35)=5.11), at p<.001 uncorrected.
Discussion
Habits in OCD were associated with hyper-activation in the caudate nucleus. During the performance of habits by those patients who displayed them there was greater caudate activation, than in controls and in OCD patients not forming habits. Independent analysis revealed that, across the entire OCD group, greater activation in this region was correlated with the self-reported urge to perform these habits; there was no such relationship in controls.
Translational work in rodents and humans has previously revealed that the caudate is necessary for goal-directed control over action. Lesions to this region in rodents render behavior habitual after only moderate training (4), cocaine-induced habitual responding is associated with increased excitability in the rodent homologue of the caudate (37), and in humans white matter connectivity between the caudate and the mOFC is predictive of improved goal-directed control over action (11) on a task that reveals habit biases in OCD (5). Studies examining dynamic mechanisms of associative learning (rather than devaluation) suggest an important role for the caudate in linking outcomes to actions, i.e. contingency learning (10, 38, 39). However, in the present study, as explicit contingency knowledge was matched across groups we would not expect the direction of activity in our study to mirror these results. Rather, our results may reflect difficulties in translating explicit contingency knowledge into action preferences, similar to a recent study by Corbit and colleagues (37). Hyper-activation in the caudate is one of the most consistent neurobiological markers of OCD symptomatology (with mOFC hyper-activation being the other) (18, 19), and our data therefore lend strong support to a model of OCD centered on failures in goal-directed control over actions, resulting in compulsive habits.
OCD patients showed initial hyper-activation in the mOFC during avoidance learning, which reduced with extended training, whereas control subjects showed the opposite pattern. A post hoc PPI analysis revealed that during the acquisition of avoidance, positive coupling between the caudate and the subgenual ACC (partially overlapping with the mOFC cluster) was observed in patients who later demonstrated habits, but a negative coupling was observed in those who did not. The role of the subgenual ACC in habit forming has been sparsely studied, but two studies have shown that its likely homologue, the infralimbic cortex, must be intact for habits to persist in rodents (40, 41), suggesting that excessive connectivity between this region and the caudate may be one possible way that goal-directed control is compromised in OCD. Other differences in BOLD activity associated with habit formation within the OCD group were observed in the precuneus and superior occipital gyrus, which were sensitive to extended training, and functional connectivity between the caudate and the rIFG and pallidum during early learning. These results require replication, but suggest the possibility that a more distributed network may be involved in habit-forming biases in OCD.
Excessive habit formation in OCD was not related to differences in activation in the putamen. This region is critical for habit formation in rodents, such that lesions to the homologous dorsolateral striatum allow animals to remain goal-directed despite overtraining (15). Moreover, a similar dependency has been observed in healthy humans, such that the formation of habits is associated with white matter connectivity strength between the putamen and premotor cortex (11), and changes in putamen activity over time (16) (although directionality of the latter association has been inconsistent and may not relate to cue-evoked responses, but rather responses in general (42–44)). Although applying the usual caveat when interpreting null effects, the present data suggest that acquisition of automatic action tendencies may not be affected in OCD. Rather, habit biases in OCD appear to emerge as a result of failures in goal-directed control, associated with caudate (and possibly mOFC) hyper-activity. This conclusion dovetails with recent data showing that model-based instrumental learning, which is a constituent of goal-directed control, is impaired in OCD, and reliant on the structural integrity of the mOFC and caudate, but not the putamen (8).
The present study investigated avoidance, rather than appetitive, habits in order to determine how conditioned and explicit fear relates to habit formation in OCD. As this is the first study to have examined the neural correlates of avoidance habits, whether these results can be generalized to appetitive habit forming, which is similarly over-active in OCD (5), is an open question. The study of avoidance is critical in OCD, however, as prior studies have shown that aberrant fear conditioning processes are characteristic of OCD. For example, patients exhibit a pattern of hypo-activation in the ventromedial PFC (partially subsuming mOFC) and caudate during fear conditioning (45). The results of the present study converge with these finding in terms of localization, but diverge with respect to directionality, a difference presumably associated with passive fear learning vs. avoidance (46).
Whilst we observed no differences in SCR between OCD and controls, those patients who formed habits did not show differential SCRs to the stimuli (valued, devalued and safe) during the habit test (results presented in the Supplement). This could reflect overgeneralization of fear, which has been shown to relate to maladaptive instrumental avoidance (47). However, as these patients were able to discriminate during learning, this effect is likely a consequence rather than a cause of habitual responding. Taken together with the findings of Milad and colleagues (45), and studies suggesting that stress and anxiety contribute to habit biases in healthy people (48, 49), it is likely that a complex interaction between fear learning and habits may be critical to understanding the pathogenesis of OCD.
The findings in the caudate pertain primarily to patients who have formed habits versus those who have not, rather than representing a difference between patients and controls. As such, it is possible that with further training, for example, a similar pattern might be observed in control subjects who form habits. This is a question for future research. If this is the case, our results suggest that habit forming and associated caudate hyperactivity is hastened in OCD, rather than this process being qualitatively different.
The majority of our medicated patients were taking serotonergic medication (mainly SSRIs), which previous work has shown affects avoidance responding and inhibition in healthy humans (50). However, we found no evidence for differences between medicated and unmedicated patients (matched for symptom severity) in terms of behavior and almost all brain activation contrasts, indicating that medication effects did not drive our results.
Conclusions
These data implicate dysfunction in regions that support goal-directed control over action in excessive habit formation in OCD. These data also add convergent support to the habit hypothesis of OCD, such that it exhibits excellent neurobiological convergence with the known pathophysiology of OCD.
Supplementary Material
Disclosures and Acknowledgements
This research was funded by a Wellcome Trust grant (089589/Z/09/Z) awarded to TW Robbins, BJ Everitt, AC Roberts, JW Dalley and BJ Sahakian. Work was completed at the Behavioural and Clinical Neuroscience Institute, which is supported by a joint award from the Medical Research Council and Wellcome Trust (G00001354).
Dr Gillan is supported by a Sir Henry Wellcome Postdoctoral Fellowship (101521/Z/12/Z). Dr Apergis-Schoute and Dr Morein-Zamir are supported by the Wellcome Trust grant above (089589/Z/09/Z). Dr Urcelay reports no competing interests. Dr Sule reports no competing interests. Professor Fineberg receives grant/research funding from Cephalon, GlaxoSmithKline, and Lundbeck and is on the speaker’s bureaus of AstraZeneca and Lundbeck. Professor Sahakian has consulted for Boehringer-Ingelheim, Cambridge Cognition, Eli Lilly, GlaxoSmithKline, Novartis, and S hire; she has received honoraria for Grand Rounds in Psychiatry at Massachusetts General Hospital (CME credits) and for speaking at the International Conference on Cognitive Dysfunction in Schizophrenia and Mood Disorders; she was on the Medical Research Council Neurosciences and Mental Health Board and on the Science Coordination Team for the Foresight Project on Mental Capital and Wellbeing; she has been on Panel LS5 for the European Research Council; and she receives an honorarium from the Journal of Psychological Medicine. Dr. Robbins has consulted for Cambridge Cognition, Lundbeck, Pfizer, and the Scientific Advisory Board and has received research grants from GlaxoSmithKline and Lundbeck.
References
- 1.Gillan CM, Robbins TW. Goal-directed learning in obsessive-compulsive disorder. Philosophical Transactions of the Royal Society: B. doi: 10.1098/rstb.2013.0475. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson A. Actions and Habits: The Development of Behavioural Autonomy. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1985;308:67–78. [Google Scholar]
- 3.de Wit S, Ridderinkhof KR, Fletcher PC, Dickinson A. Resolution of outcome-induced response conflict by humans after extended training. Psychol Res. 2013;77:780–793. doi: 10.1007/s00426-012-0467-3. [DOI] [PubMed] [Google Scholar]
- 4.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 5.Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, de Wit S. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, Fineberg NA, Sahakian BJ, Robbins TW. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:631–638. doi: 10.1016/j.biopsych.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillan CM, Morein-Zamir S, Kaser M, Fineberg NA, Sule A, Sahakian BJ, Cardinal RN, Robbins TW. Counterfactual processing of economic action-outcome alternatives in obsessive-compulsive disorder: further evidence of impaired goal-directed behavior. Biol Psychiatry. 2014;75:639–646. doi: 10.1016/j.biopsych.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voon V, Derbyshire K, Ruck C, Irvine M, Worbe Y, Enander J, Schreiber L, Gillan C, Fineberg N, Sahakian B, Robbins T, et al. Disorders of compulsivity: a common bias towards learning habits. Molecular Psychiatry. doi: 10.1038/mp.2014.44. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka SC, Balleine BW, O’Doherty JP. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci. 2008;28:6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal Connectivity Underlies Individual Differences in the Balance between Habitual and Goal-Directed Action Control. Journal of Neuroscience. 2012;32:12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci. 2009;29:11330–11338. doi: 10.1523/JNEUROSCI.1639-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nature communications. 2013;4:2264–2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 16.Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Research-Neuroimaging. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 20.Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- 21.Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- 22.Baxter LR, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 23.Baxter LR, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in nondepressed patients with obsessive-compulsive disorder. Am J Psychiatry. 1988;145:1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- 24.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 25.Schienle A, Schäfer A, Stark R, Walter B, Vaitl D. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int J Psychophysiol. 2005;57:69–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Cottraux J, Gérard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, Galy G, Millet P, Labbé C, Lavenne F, Bouvard M, et al. A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res. 1996;60:101–112. doi: 10.1016/0165-1781(96)02697-2. [DOI] [PubMed] [Google Scholar]
- 27.Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, Alazraki A, Selin CE, Ferng HK, Munford P, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- 28.Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, Rapoport SI, Rapoport JL, Grady CL. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry. 1992;49:690–694. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JM, Stoessel PW, Baxter LR, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- 30.Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry. 2011;69:867–874. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Cannistraro PA, Wright CI, Wedig MM, Martis B, Shin LM, Wilhelm S, Rauch SL. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56:916–920. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Remijnse PL, Nielen MMA, van Balkom AJLM, Hendriks GJ, Hoogendijk WJ, Uylings HBM, Veltman DJ. Differential frontal-striatal and paralimbic activity during reversal learning in major depressive disorder and obsessive-compulsive disorder. Psychological Medicine. 2009;39:1503–1518. doi: 10.1017/S0033291708005072. [DOI] [PubMed] [Google Scholar]
- 33.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, Hoexter MQ, et al. Multicenter Voxel-Based Morphometry Mega-Analysis of Structural Brain Scans in Obsessive-Compulsive Disorder. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 34.Adams C. Post-conditioning devaluation of an instrumental reinforcer has no effect on extinction performance. Quarterly Journal of Experimental Psychology. 1980;32:447–458. [Google Scholar]
- 35.Nelson HE. National adult reading test (NART): Test manual. Windsor: NFER-Nelson; 1982. [Google Scholar]
- 36.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 37.Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39:1893–1901. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liljeholm M, Tricomi E, O’Doherty JP, Balleine BW. Neural Correlates of Instrumental Contingency Learning: Differential Effects of Action-Reward Conjunction and Disjunction. Journal of Neuroscience. 2011;31:2474–2480. doi: 10.1523/JNEUROSCI.3354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 40.Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carelli RM, Wolske M, West MO. Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci. 1997;17:1804–1814. doi: 10.1523/JNEUROSCI.17-05-01804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- 46.Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3:33. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Meurs B, Wigget N, Wicker I, Lissek S. Maladaptive Behavioral Consequences of Conditioned Fear-Generalization: A Pronounced, Yet Sparsely Studied, Feature of Anxiety Pathology. Behavior, Research and Therapy. doi: 10.1016/j.brat.2014.03.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proc Natl Acad Sci U S A. 2013;110:20941–20946. doi: 10.1073/pnas.1312011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci. 2009;29:7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends in Cognitive Sciences. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.