Abstract
BACKGROUND
Human papillomavirus type 16 (HPV16) E6 antibodies are a promising biomarker of oropharyngeal cancer (OPC); however, seropositivity among non-OPC cases is not well characterized.
METHODS
Pre-treatment sera from 260 (38 OPC, 222 non-OPC) incident head and neck cancers diagnosed at the University of Pittsburgh between 2003 and 2006 were tested for HPV16 (L1,E1,E2,E4,E6,E7) and non-HPV16 E6 (HPV6,11,18,33) antibodies. Sensitivity and specificity of HPV16 E6 antibodies for HPV-driven tumors was evaluated among tumors with known HPV status (n=25).
RESULTS
63.2% of OPC versus 27.5% of non-OPC cases were HPV16 seropositive; HPV16 E6 seroprevalence was 60.5% and 6.3% respectively, odds ratio 22.8 (95% confidence interval [CI] 9.8–53.1). Sensitivity and specificity of HPV16 E6 antibodies for HPV-driven OPC was 100% [95%CI:50%–100%; n=6] and 100% [95%CI:60%–100%, n=4] compared to 0% (n=2) and 0% (n=13) for non-OPC cases.
CONCLUSIONS
HPV16 antibodies were significantly more common in OPC versus non-OPC cases, particularly HPV16 E6 antibodies.
Keywords: human papillomavirus 16, HPV16, HPV16 E6, HPV antibodies, HPV seropositivity, oropharyngeal cancer, OPC, non-oropharyngeal cancer, non-OPC
INTRODUCTION
A rapid increase in the incidence of oropharyngeal cancer (OPC) has been reported in many parts of the developed world1–8, which has been attributed to a rise in the subset caused by HPV infection7. It is increasingly evident that the focal point of this epidemic is the US, where the incidence of OPC has risen by more than 200 percent over the past several decades and where it is estimated that more than 70% of OPCs are caused by HPV16 infection9.
HPV16 serology has been recently identified as a potentially promising early biomarker for OPC. A prospective European-based study by Kreimer and Johannson et al conducted with pre-diagnostic serum found that 34.8% of patients with OPC were seropositive for HPV16 E6 compared to only 0.6% of controls10. Additionally, these antibodies were present more than 10 years prior to diagnosis10.
In addition to OPC, HPV has also been implicated in causing a small proportion of head and neck squamous cell carcinomas (HNSCC) outside of the oropharynx (~15%)11. Yet, HPV16 serology has not been fully evaluated among this subset of cancers, especially within the US. Of 5 previous studies that compared HPV16 antibody profiles among participants with HNSCC using the same multiplex serology assay as Kreimer and Johannson et al10, 12–16, only 1 study was conducted within a US-based population. Likewise, only 3 of these studies had information regarding HPV tumor status12, 13, 15; thus, little is known about what proportion of individuals with HPV-driven HNSCCs mounts detectable HPV16 antibody responses and whether this proportion varies by anatomic site within the head and neck.
The main aim of this case-case analysis was to describe HPV16 seropositivity among HNSCC cases within a clinical US population and to compare HPV16 seroprevalence among OPC and non-OPC cases using the same multiplex serology assay used by Kreimer and Johannson et al10 which has demonstrated the best risk stratification capabilities of any assay to date with a specificity of 99.5% for oropharyngeal cancer. Additionally, we took advantage of existing data on a small subset (n=25) of individuals with known HPV tumor status as determined by the current clinical gold standard of concurrent HPV in situ hybridization (ISH) and p16 immunohistochemical (IHC) staining. Among this subset, our aim was to estimate what proportion of individuals with HPV-driven HNSCCs mounts an HPV16 antibody response and to determine whether this proportion differs by anatomic site.
MATERIALS AND METHODS
Study Population
Previously untreated incident cases of HNSCC were identified prospectively using an IRB-approved tissue banking study at the Department of Otolaryngology at the University of Pittsburgh Cancer Institute. Four milliliters of peripheral blood were collected without anticoagulant in red top vacutainers and allowed to coagulate for 15 to 30 minutes at room temperature. Sera were separated by centrifugation. All specimens were immediately aliquoted, frozen, and stored in a dedicated −80°C freezer. No more than one freeze-thaw cycle was allowed for each sample.
Between 2003 and 2006, a total of 1,213 individuals with head and neck cancer were treated at the University of Pittsburgh Cancer Institute, of which 736 (61%) were enrolled as part of the tissue banking study (453 incident and 283 prevalent cases). Of the 453 incident cases of head and neck cancer, all incident HNSCC cases that had a serum sample collected prior to treatment or within 7 days post-treatment and for which the serum specimen was still available for testing were included in this current analysis (N=260). Incident HNSCC cases: i) that declined to provide a serum sample; ii) for whom a serum sample was not collected within 7 days post-treatment and iii) for whom a serum sample was collected, but the sample was no longer available for testing were excluded from this study (N=193). The protocol was approved by the University of Pittsburgh Institutional Review Board; all participants provided written informed consent.
Laboratory Methods
Serologic Testing
Frozen serum samples were sent on dry ice to the German Cancer Research Center (DKFZ, Heidelberg, Germany). Serology testing was performed using multiplex assays by laboratory staff who were blinded to the cancer status of the participants14, 17–19. Antigens were affinity-purified, bacterially expressed fusion proteins with N-terminal Glutathione S-transferase. Samples were analyzed for HPV16 antibodies to the major capsid protein (L1), the early oncoproteins (E6, E7), and other early proteins (E1, E2, E4). Additionally, seroreactivity against the E6 protein from the following HPV types was also assessed; HPV6, HPV11, HPV18, and HPV33. Antibody levels were quantified as median fluorescence intensity (MFI) and dichotomized as positive or negative based on defined cutpoints. For all proteins with the exception of HPV16 E6, MFI cut-offs were based on 5 standard deviations above the mean antibody level among 371 HPV DNA-negative, Korean female self-reported virgins, after iterative exclusion of outliers, as previously described20. The following MFI cutoffs were used for the HPV16 proteins: L1, 331; E1, 150; E2, 550; E4, 1940; E7, 972. The following MFI cutoffs were used for the E6 proteins of non-HPV16 types: HPV6, 250; HPV11, 265; HPV18, 600; and HPV33, 501. For HPV16 E6, the cutoff for seropositivity was elevated from the standard cutoff of 484 to 1,000 MFI. Previous work from our group demonstrated that increasing the seropositivity cutoff to 1,000 results in an increased specificity for oropharyngeal cancer without a concurrent decrease in sensitivity10.
HPV tumor testing methods
As part of clinical management, information regarding HPV tumor status was available for a subset of participants (n=25). Paraffin tumor tissue sections were evaluated for HPV expression using a combination of p16 overexpression and HPV DNA in situ hybridization (ISH) methods as previously described21. Briefly, immunohistochemical (IHC) evaluation of p16 overexpression was conducted on deparaffinized tissue sections using the monoclonal antibody p16INK4 (BD Pharmingen, dilution 1:200; San Diego, CA); p16 immunoreactivity in ≥70% of cells was considered positive22. ISH was performed with a probe set specific for HPV types: HPV 6, 11, 16, 18, 31, 33, 35, 45, 51, and 52 (Dako Cytomation, Carpinteria, CA). Cases dual positive for p16 and HPV ISH were considered HPV-driven; those dual negative were considered HPV-negative and those with discordant results were considered inconclusive and thus, were not included within this analysis.
Statistical Analyses
Characteristics of the HNSCC cases were evaluated by OPC versus non-OPC; a chi-square test was used to evaluate differences by OPC status. The proportion of cases seropositive for HPV16 proteins (L1, E1, E2, E4, E6 and E7) and E6 proteins from non-HPV16 types (HPV6, 11, 18, 33) was calculated separately by OPC versus non-OPC; odds ratios (OR) and 95% confidence intervals (CI) were calculated in univariate analyses using logistic regression. Due to cross-reactivity of HPV16 E6 antibodies with E6 proteins of certain phylogenetically related non-HPV16 types, an additional analysis was conducted restricting to individuals seronegative for HPV16 E6. Univariate logistic regression was used to evaluate determinants of HPV16 E6 seropositivity by OPC status. Patient characteristics evaluated included age, smoking status, alcohol consumption and tumor stage (I–II vs. III–IV). Among individuals with known HPV tumor status, the proportion of HPV-driven cases seropositive for HPV16 E6 (sensitivity) and the proportion of HPV-negative cases seronegative for HPV16 E6 (specificity) was calculated by OPC versus non-OPC; 95% CIs for sensitivity and specificity estimates were calculated using a modified Wald method. All-cause mortality for HPV16 E6 seropositive and seronegative OPC and non-OPC cases was evaluated by Cox proportional hazards regression; years since cancer diagnosis was used as the time variable. The adjusted hazard ratio (aHR) was calculated by including age at diagnosis, tumor stage (I–II vs. III–IV), treatment and smoking history into the model. A sensitivity analysis using time since treatment as the time variable provided similar results (data not shown). All analyses were performed by STATA-SE Version 12.1.
RESULTS
Participant Characteristics
Of the 260 participants, 85.4% (N=222) were diagnosed with cancers outside of the oropharynx (non-OPC) (Table 1). 94% of non-OPC tumors originated from either the oral cavity or larynx; the three most common non-OPC tumor sites were oral tongue (30.6%, N=68), floor of mouth (11.7%, N=36) and supraglottis (11.3%, N=25). Of the 260 participants, 14.6% (N=38) were diagnosed with oropharyngeal cancer (OPC); the vast majority (94.8%) of oropharyngeal tumors arose from either the tonsil (63.2%, n=24) or base of tongue (31.6%, n=12) (Supplemental Table 1).
Table 1.
Participant characteristics, by non-OPC versus OPC.
| Characteristics | Non-OPC
|
OPC
|
p-value | ||
|---|---|---|---|---|---|
| N= 222 | N= 38 | ||||
|
| |||||
| N | % | N | % | ||
| Age at Diagnosis (years) | |||||
| Median (IQR) | 62 | 53–73 | 53.5 | 51–60 | 0.002 |
| Gender | |||||
| Male | 159 | 71.6 | 32 | 84.2 | 0.11 |
| Female | 63 | 28.4 | 6 | 15.8 | |
| Race | |||||
| Caucasian | 213 | 96 | 38 | 100.0 | NE |
| Asian | 2 | 0.9 | 0 | 0.0 | |
| African American | 7 | 3.2 | 0 | 0.0 | |
| Smoking Historya | |||||
| No | 47 | 21.2 | 13 | 34.2 | 0.07 |
| Yes | 172 | 77.8 | 25 | 65.8 | |
| Alcohol History | |||||
| No | 81 | 37.5 | 8 | 21.1 | 0.06 |
| Yes | 135 | 62.5 | 30 | 79.0 | |
| Tumor Grade | |||||
| Moderate | 139 | 62.6 | 19 | 50.0 | 0.10 |
| Not Evaluated | 31 | 14 | 9 | 23.7 | |
| Poor | 33 | 14.9 | 10 | 26.3 | |
| Unknown | 1 | 0.5 | 0 | 0.0 | |
| Well | 18 | 8.1 | 0 | 0.0 | |
| Tumor Stage | |||||
| I | 59 | 26.6 | 3 | 7.9 | 0.14 |
| II | 34 | 15.3 | 4 | 10.5 | |
| III | 45 | 20.3 | 11 | 29.0 | |
| IVA | 81 | 36.5 | 18 | 47.4 | |
| IVB | 3 | 1.4 | 1 | 2.6 | |
| IVC | 0 | 0.0 | 1 | 2.6 | |
| Treatment | |||||
| Surgery | 125 | 56.3 | 6 | 15.8 | <0.001 |
| Surgery + Radiation + Chemob | 52 | 23.4 | 13 | 34.2 | |
| Surgery + Radiationb | 31 | 14.0 | 6 | 15.8 | |
| Radiation + Chemob | 11 | 5.0 | 13 | 34.2 | |
| Radiationb | 2 | 1.0 | 0 | 0.0 | |
| Surgery + Chemob | 1 | 0.5 | 0 | 0.0 | |
Does not sum to 100% due to missing data (n=3)
Includes participants who additionally received other treatments (i.e.: experimental vaccine, brachy, EGFR inhibitor; n=16 total)
Compared to non-OPC cancer cases, participants with OPC were significantly younger at diagnosis (median age 53.5 versus 62.0 years, P=0.002) and more likely to receive multimodality treatment (84.2% versus 42.8% receiving two or more modes of treatment, P<0.001); Table 1. A greater proportion of OPC cancer patients also tended to be non-smokers (34.2% versus 21.1%, P=0.07) and to have a history of alcohol use (79.0% versus 62.5%, P=0.06) compared to participants with non-OPC. Cases of OPC and non-OPC were similar in terms of gender, race, tumor grade and stage.
Seroreactivity Against HPV16 Proteins, OPC versus Non-OPC
Seroreactivity against HPV16 proteins was common among cases of OPC and non-OPC (Table 2); overall 32.7% (85 out of 260) of participants were seropositive for at least 1 of the HPV16 proteins tested (L1, E1, E2, E4, E6 and E7). Participants with OPC had a 4.5 times greater odds (95% CI: 2.2–9.3) of being seroreactive against at least 1 HPV16 protein compared to non-OPC cases; seroprevalence was 63.2% and 27.5% for OPC and non-OPC cases, respectively. Of the HPV16 proteins tested, seroreactivity against HPV16 E6 was most common among OPC cases. Participants with OPC had a 22.8 times greater odds (95% CI: 9.8–53.1) of being seroreactive against HPV16 E6; 60.5% (23 out of 38) of participants with OPC tested seropositive for HPV16 E6 compared to 6.3% (14 out of 222) of participants with non-OPC. The 14 HPV16 E6 seropositive non-OPC cases were classified as originating from the following anatomic sites: floor of mouth, n=5; oral tongue, n=4; and 1 case each of gingiva (upper); hypopharynx (NOS); larynx (overlap lesion); larynx (supraglottis); pharynx (NOS). Following a detailed chart review, none of the 14 HPV16 seropositive non-OPC cases had evidence of being misclassified cases of OPC.
Table 2.
Association of HPV16 L1, E1, E2, E4 and E7 seropositivity with oropharyngeal cancer, by non-OPC versus OPC.
| HPV16 Proteins | Non-OPC
|
OPC
|
||||
|---|---|---|---|---|---|---|
| Total | No. Positive (%) | OR | Total | No. Positive (%) | OR (95% CI) | |
| Any | 222 | 61 (27.5) | Ref | 38 | 24 (63.2) | 4.5 (2.2–9.3) |
| HPV16 L1 | 222 | 33 (14.9) | Ref | 38 | 21 (55.3) | 7.1 (3.4–14.8) |
| HPV16 E1 | 222 | 9 (4.1) | Ref | 38 | 14 (36.8) | 13.8 (5.4–35.3) |
| HPV16 E2 | 222 | 10 (4.5) | Ref | 38 | 17 (44.7) | 17.2 (7.0–42.2) |
| HPV16 E4 | 222 | 5 (2.3) | Ref | 38 | 13 (34.2) | 22.6 (7.4–68.6) |
| HPV16 E6 | 222 | 14 (6.3)a | Ref | 38 | 23 (60.5) | 22.8 (9.8–53.1) |
| HPV16 E7 | 222 | 16 (7.2) | Ref | 38 | 18 (47.4) | 11.6 (5.1–26.2) |
| Any 2 | 222 | 6 (2.7) | Ref | 38 | 3 (7.9) | 5.8 (1.3–25.5) |
| Any 3 | 222 | 3 (1.4) | Ref | 38 | 5 (13.2) | 19.2 (4.1–88.7) |
| Any 4 | 222 | 2 (0.9) | Ref | 38 | 0 (0.0) | NE |
| Any 5 | 222 | 2 (0.9) | Ref | 38 | 6 (15.8) | 34.5 (6.4–187.1) |
| All 6 | 222 | 0 (0.0) | Ref | 38 | 9 (23.7) | NE |
The 14 HPV16 E6 non-OPC cases were classified as follows: mouth (floor, NOS), n=5; tongue (NOS), n=4; and 1 case each of gum (upper); hypopharynx (NOS); larynx (overlap lesion); larynx (supraglottis); pharynx (NOS).
Age, gender, smoking and alcohol consumption were not associated with HPV16 E6 seropositivity for either cases of OPC or non-OPC. Only increased tumor stage (III-IV versus I-II) was significantly associated with HPV16 E6 seropositivity; OR 10.3 (95% CI: 1.3–80.3) and 14.7 (95% CI: 1.5–139.8) for cases of non-OPC and OPC, respectively (Supplemental Table 2).
HPV16 L1 seroreactivity, a sign of cumulative exposure to HPV16, was commonly observed among both OPC and non-OPC cases. Among non-OPC cases, seroreactivity against HPV16 L1 was the most common of all HPV16 antigens tested (14.9%, 33 out of 222). Yet, HPV16 L1 seroprevalence was still highest among OPC cases 55.3% (21 out of 38); OR 7.1, 95% CI: 3.4–14.8.
OPC cases were also more likely than non-OPC cases to be seroreactive against multiple HPV16 proteins. Of the 24 HPV16 seropositive OPC cases, 23 (95.8%) were seroreactive against 2 or more HPV16 proteins compared to only 21.3% of non-OPC cases (13 out of 61); P<0.0001. Among OPC cases, seroreactivity against all 6 HPV16 proteins was most common, 23.4% (9 out of 38) of OPC cases were seroreactive against L1, E1, E2, E4, E6 and E7 compared to none of the non-OPC cases; P<0.0001.
Seroreactivity Against non-HPV16 E6 Proteins, OPC versus Non-OPC
Seroreactivity against E6 proteins from non-HPV16 types was less common (Table 3); overall 7.7% (20 out of 260) participants were seropositive for at least 1 of the non-HPV16 E6 proteins tested (HPV6, 11, 18, 33). Compared to non-OPC cancer cases, participants with OPC had a 9.6 greater odds (95% CI: 3.7–25.4) of being seroreactive against non-HPV16 E6 proteins; 29.0% (11 out of 38) of OPC and 4.1% (9 out of 222) of non-OPC cancer cases were seroreactive against at least 1 non-HPV16 E6 protein. OPC cases had an 11.1 (95% CI: 2.5–48.5) and 14.5 (95% CI: 4.1–51.2) fold greater odds of being seroreactive against E6 proteins from HPV11 and HPV33 than non-OPC cases, respectively. Due to cross-reactivity of HPV16 E6 antibodies with E6 proteins of certain phylogenetically related non-HPV16 types, an additional analysis was conducted restricting to HPV16 E6 seronegatives. None of the associations remained statistically significant following restriction of the analyses to HPV16 E6 seronegatives.
Table 3.
Association of non-HPV16 type E6 seropositivity with oropharyngeal cancer, by non-OPC versus OPC.
| HPV E6 Proteins | Non-OPC
|
OPC
|
|||||
|---|---|---|---|---|---|---|---|
| Total | No. Positive (%) | OR | Total | No. Positive (%) | OR (95%CI) | ORa (95%CI) | |
| Any | 222 | 9 (4.1) | Ref | 38 | 11 (29.0) | 9.6 (3.7–25.4) | 2.4 (0.3–21.4) |
| HPV6 E6 | 222 | 3 (1.4) | Ref | 38 | 0 (0.0) | NE | NE |
| HPV11 E6 | 222 | 3 (1.4) | Ref | 38 | 5 (13.2) | 11.1 (2.5–48.5) | 4.9 (0.5–50.0) |
| HPV18 E6 | 222 | 1 (0.5) | Ref | 38 | 1 (2.6) | 6.0 (0.4–97.6) | 14.8 (0.9–249.1) |
| HPV33 E6 | 222 | 4 (1.8) | Ref | 38 | 8 (21.1) | 14.5 (4.1–51.2) | NEb |
Restricted to HPV16 E6 seronegatives
Of the 4 HPV33 E6 seropositive non-OPC cases, 3 were seropositive for HPV16 E6. Of the 8 HPV33 E6 seropositive OPC cases, all were HPV16 E6 seropositive.
Sensitivity and Specificity of HPV16 E6 Seropositivity for HPV-driven Cancer
Of the 260 cases of HNSCC, 9.6% (n=25) had both p16 immunohistochemistry (IHC) and HPV in situ hybridization (ISH) test results; 15 non-OPC and 10 OPC (Table 4). 13.3% (n=2 out of 15) of the non-OPC cases were HPV-driven compared to 60% (n=6 out of 10) of OPC cases. All 6 HPV-driven OPC cases were seropositive for HPV16 E6 (sensitivity=100% [95% CI: 50%–100%]) and all the HPV-negative OPC cases were HPV16 E6 seronegative (n=4; specificity=100% [95% CI: 60%–100%]). Neither the HPV-driven (n=2) nor the HPV-negative (n=13) non-OPC cases had detectable HPV16 E6 antibodies.
Table 4.
Sensitivity of HPV16 E6 for HPV-driven head and neck cancer, by non-OPC versus OPC.
| Non-OPC Tumor Status (N=15)
|
OPC Tumor Status (N=10)
|
|||
|---|---|---|---|---|
| HPV-Drivena (N=2) | HPV-Negativeb (N=13) | HPV-Drivena (N=6) | HPV-Negativeb (N=4) | |
|
| ||||
| HPV16 E6 Serology | N (%) | N (%) | N (%) | N (%) |
| Seropositive | 0 (0.0) | 0 (0.0) | 6 (100) | 0 (0.0) |
| Seronegative | 2 (100) | 13 (100) | 0 (0.0) | 4 (100) |
HPV ISH and p16 double positive
HPV ISH and p16 double negative
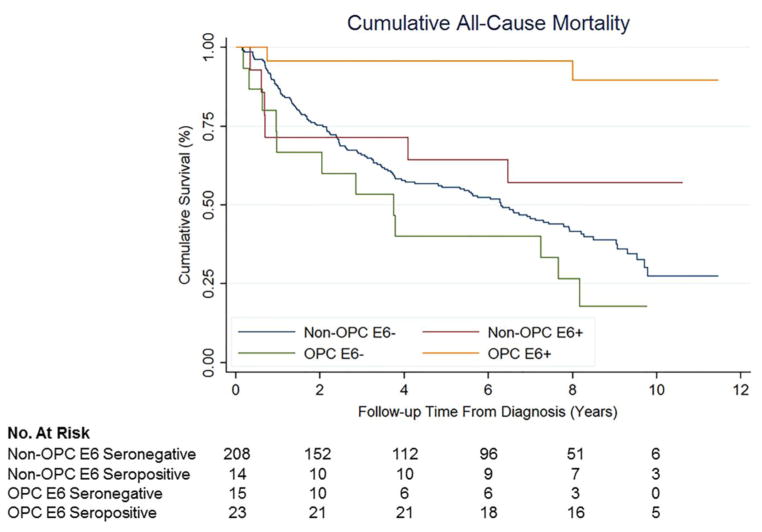
Cumulative Survival All-Cause Mortality
5-year survival rates were highest among HPV16 E6 seropositive patients; 91.3% and 64.3% for HPV16 E6 seropositive OPC and non-OPC patients, respectively. 5-year survival for HPV16 E6 seronegative non-OPC and OPC patients was 50.0% and 40.0%, respectively (Figure 1). Compared to non-OPC HPV16 E6 seronegative patients, HPV16 E6 seronegative OPC was associated with more than a 2 fold increased hazard of death (aHR, 2.4 [95% CI: 1.3–4.6; P=0.006]) while HPV16 E6 seropositive OPC was associated with a 90% reduction (aHR, 0.1 [95% CI: 0.02–0.5; P=0.005]) (Table 5). No significant differences in all-cause mortality were observed for non-OPC cases by HPV16 E6 serostatus; aHR for HPV16 E6 seropositive versus seronegative non-OPC cases was 0.6 (95% CI: 0.2–1.3; P=0.16).
Figure 1.
Cumulative all-cause mortality for non-OPC HPV16 E6 seronegative cases (blue), non-OPC HPV16 E6 seropositive cases (red), OPC HPV16 E6 seronegative cases (green) and OPC HPV16 E6 seropositive cases (yellow).
Table 5.
Cumulative survival all-cause mortality, by OPC status and HPV16 E6 serology
| Cancer Type | Hazard Ratio (95% CI) | p-value | Hazard Ratioa (95% CI) | p-value |
|---|---|---|---|---|
| non-OPC HPV16 E6 Seronegative | Ref | - | Ref | - |
| non-OPC HPV16 E6 Seropositive | 0.6 (0.3–1.4) | 0.25 | 0.6 (0.2–1.3) | 0.16 |
| OPC HPV16 E6 Seronegative | 1.6 (0.9–2.9) | 0.13 | 2.4 (1.3–4.6) | 0.006 |
| OPC HPV16 E6 Seropositive | 0.1 (0.02–0.4) | 0.001 | 0.1 (0.02–0.5) | 0.005 |
Adjusted for age at diagnosis, stage (I–II vs. III–IV), treatment and smoking history
DISCUSSION
In our case-case analysis of 260 incident HNSCCs, participants with OPC were significantly more likely than participants with non-OPC to be seroreactive against HPV16; the majority (63.2%) of OPC cases were seropositive for at least 1 of the HPV16 proteins tested compared to a minority (27.5%) of non-OPC cases. Of the HPV16 proteins tested, seroreactivity against HPV16 E6 was most common among OPC cases; the majority of OPC cases were seropositive for HPV16 E6 (60.5%) compared to 6.3% of non-OPC cases, respectively. Among HPV16 seropositive cases, individuals with OPC were more likely than non-OPC cases to be seroreactive against multiple HPV16 proteins; 23.4% of HPV16 seropositive OPC cases were seroreactive against all 6 proteins tested compared to none of the HPV16 seropositive non-OPC cases. Among the subset of 25 cases with known HPV tumor status, estimated sensitivity and specificity of HPV16 E6 serology for HPV-driven OPC was 100% and 100%, respectively, compared to 0% and 0% for non-OPC cases. All participants (n=6) with confirmed HPV-driven OPC tumors were seropositive for HPV16 E6 compared to none of the participants with HPV-negative OPC tumors (n=4); all non-OPC cases were HPV16 E6 seronegative regardless of HPV status of the tumor. In terms of survival, compared to HPV16 E6 seronegative non-OPCs, HPV16 E6 seropositivity was associated with a significantly reduced hazard of death among OPC patients and a suggestive reduction among non-OPCs. Among HPV16 E6 seronegative cases, OPC was associated with more than a 2 fold increased hazard of death compared to non-OPC.
Five previous studies have compared HPV16 antibody profiles among participants with head and neck cancer using a multiplex serology assay used by Kreimer and Johannson et al, an assay that has demonstrated the best risk stratification capabilities of any assay to date10, 12–15. In these studies, HPV16 E6 seroprevalence ranged from 3% (Central Europe)14 to 64% (US)12 for OPC cases and 1% (Western Europe)10 to 6% (Brazil)13 for non-OPC cases. This variation in HPV16 E6 seroprevalence by study is most likely a reflection of the wide geographic variation in the portion of HNSCCs attributable to HPV infection; the proportion of OPCs due to HPV infection has been estimated to be as low as 15% in Central and South America and as high as 60% in North America11. Thus, when comparing HPV16 E6 seroprevalence estimates between studies, it is important to consider the underlying population from which those estimates were generated. Only 1 of the 5 previous studies was conducted within a US population and therefore, is most directly comparable to our current analysis12. In a study of 170 oral cavity and 74 OPCs recruited from two US hospitals, Smith et al12 reported that 9.4% of oral cavity and 63.5% of OPCs were seropositive for E6 and/or E7 proteins of HPV types 16, 18 or 33. Comparison between our current study and the previous study is difficult given that Smith et al12 did not report data on HPV16 E6 serology separately and that our study did not assess for seroreactivity against the E7 proteins of HPV18 or HPV33. However, 12.6% (28 out of 222) of the non-OPC and 63.2% (24 out of 38) of the OPCs in our study were seroreactive against E6 proteins from either HPV16, 18 or 33; a finding in line with that reported by Smith et al12. Despite methodological differences in assignment of HPV tumor status, a similar trend in decreased sensitivity among non-OPC compared to OPC cases was also noted among both studies. Smith et al12 reported that of 34 participants with HPV-driven OPC tumors, all were seropositive for HPV16 E6 and/or E7 positive (sensitivity=100%) compared to 5 out of 19 participants with HPV-driven non-OPC tumors (sensitivity=26%); (n.b. specificity was not reported). Although based on a small number of HPV-driven tumors (n=8), here we report a similar sensitivity for HPV-driven OPC and non-OPC, 100% (6 out of 6) and 0% (0 out of 2), respectively. Thus, data from this study as well as the study by Smith et al12 suggest that, among HPV-driven HNSCCs, the oropharynx may be uniquely suited to induce antibody responses to HPV infection compared to other sites within the head and neck given the proximity of the oropharynx to the lymphatic system.
This theory is further bolstered by our finding that of the individuals with detectable HPV16 antibodies, OPC cases were approximately 5 times more likely than non-OPC cases to mount antibody responses against multiple HPV16 proteins. Of the 24 HPV16 seropositive OPC cases, 95.8% (n=23) had detectable antibodies against at least 2 or more HPV16 proteins and 23.7% were seroreactive against all 6 HPV16 proteins tested (L1, E1, E2, E4, E6, E7). In comparison, of the 61 HPV16 seropositive non-OPC cases, only 21.3% (n=13) were seroreactive against at least 2 or more HPV16 proteins and no individual had detectable antibodies against all 6 HPV16 proteins.
In terms of survival, results of this current study are in line with our previous findings10 that HPV16 E6 seropositivity is associated with a reduced hazard of death among OPC patients. It is well documented that HPV-positive OPC patients have significantly improved overall survival compared to HPV-negative cases23. Based on our sensitivity data, the increased survival among HPV16 E6 seropositive OPCs is most likely the result of HPV16 E6 antibodies marking the HPV-positive subset of OPC. Our data was also suggestive of a reduced hazard of death for HPV16 E6 seropositive non-OPCs, although most likely due to the small number cases (N=14 [6.3%]), this finding did not reach statistical significance. Interesting, among HPV16 E6 seronegative cases, OPC was associated with more than a 2 fold increased hazard of death compared to non-OPC.
Although it is unclear as to why, this may be due to non-OPC sites being surgically salvageable if primary therapy fails, which is not the case for OPC. Although our findings are consistent with previous work in other US populations, it is important to note that our study included a subsample of head and neck patients seen at the University of Pittsburgh Cancer Institute between 2003 and 2008. Therefore, our findings may lack generalizability. Additionally, our findings must be interpreted with caution given our limited sample size. Comparisons between OPC and non-OPC were based on a small number of OPC cases (n=38). Likewise, our estimates of sensitivity and specificity were based on a small subset of previously tested tumors (n=25) which represented 10% of our full cohort; thus our sensitivity and specificity estimates lack precision. However, our study also had several strengths. We analyzed a large number of serum samples from HNSCC cases from a well-documented population of individuals seen at one medical center. This gave us access to numerous demographic and clinical variables for analysis as well as allowed us to conduct detailed chart reviews when necessary to confirm clinical details. For the serological analyses, we used the same highly specific multiplex serology assay used by Kreimer and Johannson et al in the first prospective study of the HPV16 E6 marker10 and for assignment of HPV tumor status, we used the current clinical gold standard of HPV ISH and p16 IHC staining.
In conclusion, compared to HNSCCs arising from anatomic sites outside of the oropharynx, OPCs were significantly more likely to be seroreactive against HPV proteins, in particular HPV16 E6 (>60% of OPCs were seropositive). The higher seroprevalence of HPV16 E6 antibodies among OPC patients is most likely due to the higher proportion of OPCs attributable to HPV infection as well as the proximity of the oropharynx to lymphatic system. Taken together, our results suggest that HPV16 E6 antibodies may have potential clinical utility as a marker for OPC, yet perhaps limited utility for non-OPC. However, larger studies are needed to more precisely estimate the sensitivity of HPV16 E6 serology for both HPV-driven OPC and non-OPC and; thus, will be important for determining the potential clinical utility of this biomarker for detection of HPV-driven HNSCCs.
Highlights.
We tested 260 incident head and neck cancers for HPV antibodies
63.2% of OPC and 27.5% non-OPC cases were seroreactive against HPV16 proteins
HPV16 E6 antibodies were most common, 60.5% and 6.3% for OPC versus non-OPC
A subset of cases had known HPV status (N=25)
100% (N=6) and 0% (N=2) of HPV-driven OPC and non-OPC cases had E6 antibodies
Acknowledgments
This work was supported by the following grant awarded to Robert L. Ferris: 1P50 CA097190
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Additional Information
These data were partially presented in poster format (abstract number: P002) at the American Head and Neck Society Translational Research Meeting in Boston Massachusetts, April 2015.
All authors have contributed substantially to conception and design, acquisition of data, and/or analysis and interpretation of data; drafting of the article and/or revising it critically for important intellectual content; and all authors gave final approval of the version submitted to Cancer Epidemiology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert L. Ferris, Email: ferrrl@upmc.edu.
Tim Waterboer, Email: t.waterboer@dkfz-heidelberg.de.
References
- 1.Hocking JS, Stein A, Conway EL, et al. Head and neck cancer in Australia between 1982 and 2005 show increasing incidence of potentially HPV-associated oropharyngeal cancers. Br J Cancer. 2011;104(5):886–91. doi: 10.1038/sj.bjc.6606091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomberg M, Nielsen A, Munk C, Kjaer SK. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129(3):733–41. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VM, Cundall-Curry D, Bridger MW. Trends in the incidence rates of tonsil and base of tongue cancer in England, 1985–2006. Ann R Coll Surg Engl. 2010;92(8):655–9. doi: 10.1308/003588410X12699663904871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syrjanen S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57(5):449–55. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioka A, Tsukuma H, Ajiki W, Oshima A. Trends in head and neck cancer incidence in Japan during 1965–1999. Jpn J Clin Oncol. 2005;35(1):45–7. doi: 10.1093/jjco/hyi004. [DOI] [PubMed] [Google Scholar]
- 6.Braakhuis BJ, Visser O, Leemans CR. Oral and oropharyngeal cancer in The Netherlands between 1989 and 2006: Increasing incidence, but not in young adults. Oral Oncol. 2009;45(9):e85–9. doi: 10.1016/j.oraloncology.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–9. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–15. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–31. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 12.Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol. 2012;2012:571862. doi: 10.1155/2012/571862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez RV, Levi JE, Eluf-Neto J, et al. Human papillomavirus (HPV) 16 and the prognosis of head and neck cancer in a geographical region with a low prevalence of HPV infection. Cancer Causes Control. 2014;25(4):461–71. doi: 10.1007/s10552-014-0348-8. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro KB, Levi JE, Pawlita M, et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int J Epidemiol. 2011;40(2):489–502. doi: 10.1093/ije/dyq249. [DOI] [PubMed] [Google Scholar]
- 15.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105(8):536–45. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 16.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50(5):370–9. doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309(1–2):200–4. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 19.Sitas F, Egger S, Urban MI, et al. InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers. J Natl Cancer Inst. 2012;104(2):147–58. doi: 10.1093/jnci/djr499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clifford GM, Shin HR, Oh JK, et al. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1874–9. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 21.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28(36):5294–300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–73. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 23.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]