Abstract
Objective
Transplant recipients are at risk of developing progressive multifocal leukoencephalopathy (PML), a rare demyelinating disorder caused by oligodendrocyte destruction by JC virus.
Methods
Reports of PML following transplantation were found using PubMed Entrez (1958–July 2010). A multicenter, retrospective cohort study also identified all cases of PML among transplant recipients diagnosed at Mayo Clinic, Johns Hopkins University, Washington University, and Amsterdam Academic Medical Center. At 1 institution, the incidence of posttransplantation PML was calculated.
Results
A total of 69 cases (44 solid organ, 25 bone marrow) of posttransplantation PML were found including 15 from the 4 medical centers and another 54 from the literature. The median time to development of first symptoms of PML following transplantation was longer in solid organ vs bone marrow recipients (27 vs 11 months, p = 0.0005, range of <1 to >240). Median survival following symptom onset was 6.4 months in solid organ vs 19.5 months in bone marrow recipients (p = 0.068). Case fatality was 84% (95% confidence interval [CI], 70.3–92.4%) and survival beyond 1 year was 55.7% (95% CI, 41.2–67.2%). The incidence of PML among heart and/or lung transplant recipients at 1 institution was 1.24 per 1,000 posttransplantation person-years (95% CI, 0.25–3.61). No clear association was found with any 1 immunosuppressant agent. No treatment provided demonstrable therapeutic benefit.
Interpretation
The risk of PML exists throughout the posttransplantation period. Bone marrow recipients survive longer than solid organ recipients but may have a lower median time to first symptoms of PML. Posttransplantation PML has a higher case fatality and may have a higher incidence than reported in human immunodeficiency virus (HIV) patients on highly-active antiretroviral therapy (HAART) or multiple sclerosis patients treated with natalizumab.
Progressive multifocal leukoencephalopathy (PML) is a rare, emerging central nervous system demyelinating disease caused by reactivation of JC virus.1 More than 50% of adults are infected by JC virus,2 but only certain populations are susceptible to viral changes that can lead to PML. PML was initially described in 3 patients with hematological malignancy in 1958.3 The first report of PML in a transplant recipient was in 19714 and has been followed by isolated case reports over the last nearly 4 decades.
The number and survival of transplant recipients has increased dramatically since these original reports of PML, with approximately 450,000 solid organ transplants occurring in the United States over the past 20 years.5 The risk factors, clinical spectrum, and incidence of PML among transplant recipients remain uncertain even though the transplant population is likely at a significant risk of developing PML. Comparable groups of immunosuppressed patients have found PML to be of high concern in human immunodeficiency virus (HIV) infection (0.6% of patients treated with highly-active antiretroviral therapy [HAART]),6 multiple sclerosis (0.1% of patients treated with natalizumab for 1 year),7 multiple rheumatological disorders,8 rituximab therapy,9 and more rarely in other conditions. In these settings, the case fatality of PML can be high10 and no effective treatment is recognized.
We reviewed the available literature for any cases of PML following transplantation over the past 4 decades. We also performed a retrospective multicentered cohort study of PML in transplant recipients in 4 large academic medical centers. These cases were collectively analyzed for demographic, clinical, and treatment-related features in order to characterize the spectrum of PML in solid organ and bone marrow transplant recipients.
Patients and Methods
Case Ascertainment
The institutional review board at each center approved this study. A search for all cases of PML among transplant recipients was performed for the years 1988–2008 (Mayo Clinic, Rochester, MN), 1995–2008 (The Johns Hopkins University, Baltimore, MD), 1990–2008 (Washington University, St. Louis, MO), and 1991–June 2010 (Academic Medical Center, The Netherlands). Searches were performed by diagnostic code keywords “progressive multifocal leukoencephalopathy” and “JC virus” in the transplant recipient database in Rochester and by search for transplant recipients among all identified cases of PML in clinical and pathology records in Amsterdam, St. Louis, and Baltimore. All centers perform bone marrow and living and cadaveric kidney transplantation. In Rochester, Baltimore, and St. Louis, heart, lung, liver, pancreas, and intestine transplants are also performed.
Literature Review
Reported cases of PML among solid-organ and bone marrow transplant recipients were found in PubMed in July 2010 using the keywords “progressive multifocal leukoencephalopathy” or “JC virus” and “transplantation” or “lung” or “heart” or “liver” or “kidney” or “pancreas” or “intestine” or “bone marrow.” All articles that reported cases of PML in the posttransplantation period with sufficient detail in any language were included. Translation of articles into English was performed by physicians who were native speakers of the language of the article. Reference lists from articles found by this search were also used to identify cases that were not identified by the PubMed database search.
Data Collection
Collected cases were assessed for clinical and demographic features, including age at transplantation, sex, type of transplantation, underlying diagnosis prompting transplantation, time to first neurological symptoms representing PML, presenting symptoms, time between symptom onset and PML diagnosis, survival following PML diagnosis, current and past immunosuppressant regimen, attempted treatment, outcome of PML, presence of immune reconstitution inflammatory syndrome (IRIS), and cause of death. Data retrieval was completed by July 2010 and patients still alive were censored on the date of their last clinical contact.
Criteria for Diagnosis of PML and PML-IRIS
Different levels of certainty were applied to the diagnosis of PML in this study. When JC virus was identified in brain tissue by pathological examination or polymerase chain reaction in the cerebrospinal fluid (CSF PCR), the diagnosis was considered to be confirmed. When a patient had characteristic features on magnetic resonance imaging (MRI) of the brain, neurological deficits, and the clinical diagnosis of PML by the treating physician, with exclusion of other possible etiologies, the case was considered to be suspected. Suspected cases were not tested for JC virus in the CSF and did not undergo brain tissue examination.
Since there is no consensus on the criteria for IRIS in patients undergoing transplantation, the following were considered diagnostic of PML-IRIS in posttransplantation patients. These criteria are modified from the criteria used for IRIS in the setting of HIV infection treated with HAART.11 A neurologist assessed each of the clinical cases from the present series to determine if the patient fit the criteria for PML-IRIS.
Symptoms consistent with an inflammatory reaction in lesions of PML that lead to clinical deterioration and/or enhancement on MRI of the brain.
Symptoms appear while on immunosuppressant therapy that is reduced or changed.
These symptoms could not be explained by a newly acquired infection, by the expected clinical course of a previously recognized infectious agent, or by side effects of therapy.
Statistical Analysis
Calculation of Incidence
At the Mayo Clinic Transplant Registry, a neurologist has retrospectively reviewed each heart and/or lung transplant recipient's chart in detail (F.J.M.: lung and heart-lung; D.v.d.B.: heart), including all patients from the beginning of cardiothoracic transplantation at the Mayo Clinic (1988). The total number of person-years from cardiothoracic transplant recipients (heart, single-lung or double-lung, and combined heart-lung) during follow-up time was used to calculate the incidence rate of PML among cardiothoracic transplant recipients. The censoring date was last clinical follow-up on or before 31 March 2009 for lung recipients and 31 Oct 2006 for heart recipients. The total number of posttransplantation person-years was used as the total time at risk. Incidence rate estimates were obtained using a Poisson model, and corresponding confidence intervals were computed exactly.12 Potential trends in the incidence rate of PML over calendar-time were investigated using a Poisson linear regression model incorporating a dichotomization of time and fitted via generalized estimating equations.13,14
Data Analysis
Basic statistical measures included mean, median, range, and 95% confidence intervals (CIs). The programming language R version 2.8.1 (R Foundation for Statistical Computing, Vienna, Austria)15 and the statistical software STATA/IC 10 for Windows16 were used to perform all statistical computations. Statistical comparisons of means were conducted using 2-sample t tests, while 2-sample continuity-corrected Z tests were used to compare proportions.11,17 CIs for means were obtained by inverting 1-sample 2-sided t tests, while CIs for all proportions were constructed using the score interval along with Yates' continuity correction.11,17 Tests of equality of medians were performed using a chi-square statistic as in the Mood-Brown median test; in all cases, a Wilcoxon-Mann-Whitney rank-sum test for detecting a location shift between 2 distributions was also performed and yielded concordant results.11,18 Estimates of the survival curve were obtained using the Kaplan-Meier estimator.19 CIs for the median survival time using data subject to loss to follow-up were computed using the method of Crowley and Brookmeyer.20 Hazard rates were estimated using a smoothing approach based on the Epanechnikov kernel, including local bandwidth selection and boundary correction.21,22 All tests of hypothesis were performed under 2-sided alternatives.
Results
All centers reported cases of PML in their posttransplantation populations (Academic Medical Center, n = 3; Johns Hopkins University, n = 3; Mayo Clinic, n = 6; and Washington University, n = 3). Twelve patients with PML in this study received solid organ transplantation and 3 received bone marrow transplantation (Table 1). Confirmed categorization occurred in 12 patients and was made by CSF PCR (n = 10), brain biopsy (n = 1), and autopsy (n = 1). Suspected categorization occurred in 3 patients who were diagnosed before the routine use of PCR for JC virus testing and when the families declined autopsy. Brain imaging and clinical findings were considered to be PML by the treating physicians. Immunosuppressive drugs varied by patient, type of organ transplant, center, and time period, with the most common regimen being multiple drug therapy including prednisone. When considering the immunosuppressive drugs a patient was on at the time of PML symptom onset, in this cohort, no 1 drug was taken by all patients. At the time of symptom onset, patients were exposed to prednisone (n = 13), cyclosporine (n = 8), mycophenolate mofetil (n = 7), tacrolimus (n = 5), azathioprine (n = 3), sirolimus (n = 2), rituximab (n = 3), alemtuzumab (n = 1), muromonab (n = 1), leflunomide (n = 1), thalidomide (n = 1), methotrexate (n = 1), OKT3 (n = 1), and alkylating chemotherapy (n = 1). Five cases have been published previously either in aggregated cohort studies (see Table 1; cases 5–7)23,24 or as isolated case reports (cases 4 and 10).25,26 All cases reported from these centers are HIV seronegative.
Table 1. Clinical Characteristics of PML Cases Following Transplantation from 4 Academic Medical Centers.
| Case Number | Age/Sex | Underlying Diagnosis | Transplantation Type | Presenting Features | Time to PML Symptom Onset from Transplantation (mo) | Time to Diagnosis from Symptom Onset (mo) | Method of Diagnosis | Survival Following PML Diagnosis (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 42/F | Hepatitis C, alcohol abuse | Liver | HA, hand clumsiness, dysarthria | 1 | 2 | CSF | Still alive (155+) |
| 2 | 50/M | Mantle cell lymphoma | Bone marrow | Irritability, memory loss, tremors | 14 | 9 | Suspected | 34 |
| 3 | 56/F | Cryptogenic cirrhosis | Liver | Cortical blindness, seizures, hemiparesis | <1a | 9 | Autopsy | 9 |
| 4 | 47/M | Histiocytosis X | Lung | Encephalopathy | 63 | 1 | Suspected | 64 |
| 5 | 47/F | Amyloidosis | Heart | Cortical blindness | 15 | 3 | Brain biopsyb | 13 |
| 6 | 64/M | Dilated cardiomyopathy | Heart | Encephalopathy | 55 | 1 | Suspected | 43 |
| 7 | 39/F | Idiopathic pulmonary fibrosis | Lung | Ataxia | 42 | 3 | CSF | 51 |
| 8 | 68/M | Cryptogenic cirrhosis | Liver | Hemiparesis | 8 | <1 | CSF | 9 |
| 9 | 56/F | Hepatitis C | Liver | Hemiparesis, hemianesthesia | 116 | 8 | CSF | 124 |
| 10 | 68/M | Chronic lymphocytic leukemia transformed to B-cell lymphoma | Bone Marrow | Ataxia, aphasia | 3 | 1 | CSF, brain biopsy | 5 |
| 11 | 55/M | Chronic membranous nephropathy | Kidney | Ataxia, dysarthria | 32 | 2 | CSF | 34 |
| 12 | 62/F | Chronic obstructive pulmonary disease | Bilateral lung | Memory loss, ataxia, falls | 27 | <1 | CSF | 16 |
| 13 | 45/M | Hypertensive nephropathy | Kidney | Cognitive slowing, hemiparesis | 202 | 1 | CSF | 204 |
| 14 | 43/M | Acute myeloid leukemia | Bone marrow | Diplopia | 20 | 1 | CSF | Still alive (44+) |
| 15 | 40/M | Focal segmental glomerulosclerosis | Kidney | Monoparesis | 48 | 12 | CSF | Still alive (13+) |
Patient was diagnosed with PRES in life; symptoms may have represented PRES or PML.
CSF PCR negative for JC virus in life.
CSF = cerebrospinal fluid; HA = headache; PCR = polymerase chain reaction; PML = progressive multifocal leukoencephalopathy; PRES = posterior reversible encephalopathy syndrome.
The incidence of PML in the heart and/or lung posttransplantation population was 3 cases in 2,428 total posttransplantation person years or an incidence rate of 1.24 per 1,000 posttransplantation person years (95% CI, 0.25–3.61). There is no significant difference in incidence rate of PML before and after 1998 in this group (rate ratio comparing pre-1998 and post-1998 = 0.154, p = 0.13). The total proportion of posttransplantation patients developing PML was 3 out of 427 cardiothoracic patients or 0.7% (95% CI, 0.18–2.21%). The baseline characteristics of this group have been previously reported.23,27 Follow-up is greater than 98% in this cohort, which includes 24 children.
A comprehensive literature search identified 33 additional published cases of solid organ transplant recipients with PML (Fig 1). Six were derived from the non-English medical literature. A total of 32 patients were reported in detail, including 3 lung,28–30 4 heart,31–34 6 liver,35–40 and 19 kidney4,41–57 recipients (Fig 2). Among kidney transplants, graft was from a deceased donor (n = 9), living donor (n = 7), combined living and dead donors (n = 1), and unstated (n = 2). There have been no reports of PML following pancreatic or intestinal transplant. All solid organ cases from the literature fulfilled the confirmed categorization, diagnosed by autopsy (n = 13), brain biopsy (n = 10), or CSF PCR (n = 9). Case reports were distributed throughout the study time-frame with more cases being reported in recent years: 1970–1979 (n = 5), 1980–1989 (n = 9), 1990–1999 (n = 7), and 2000–2009 (n = 12). There is no significant difference in the proportion of women, mean age of transplantation, time to symptom onset of PML posttransplantation, time from symptom onset to diagnosis of PML, or time from symptom onset to death between the current series of 12 solid organ transplant recipients and the 32 cases reported (Table 2).
Figure 1.
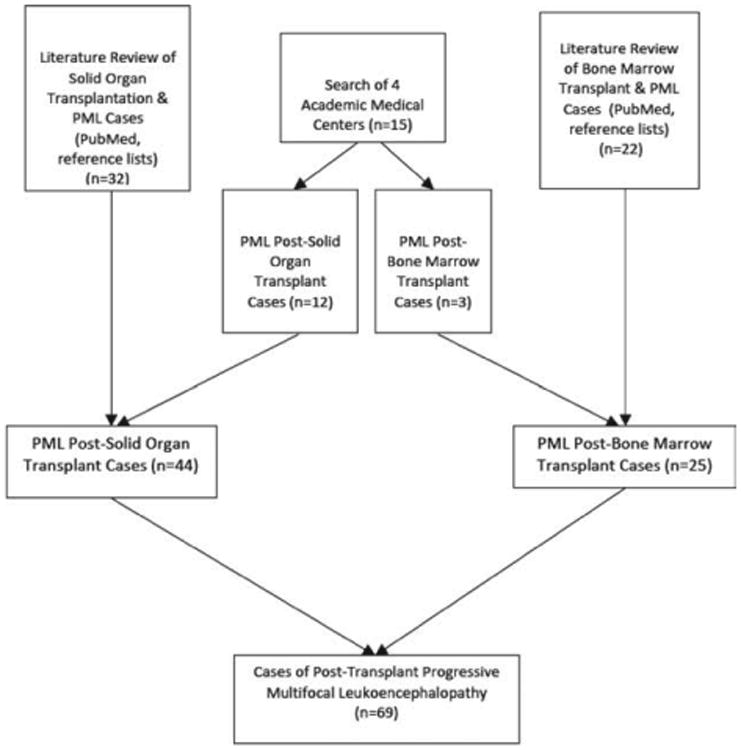
Flow diagram of patient selection and literature review for posttransplantation cases of PML. PML = progressive multifocal leukoencephalopathy.
Figure 2.
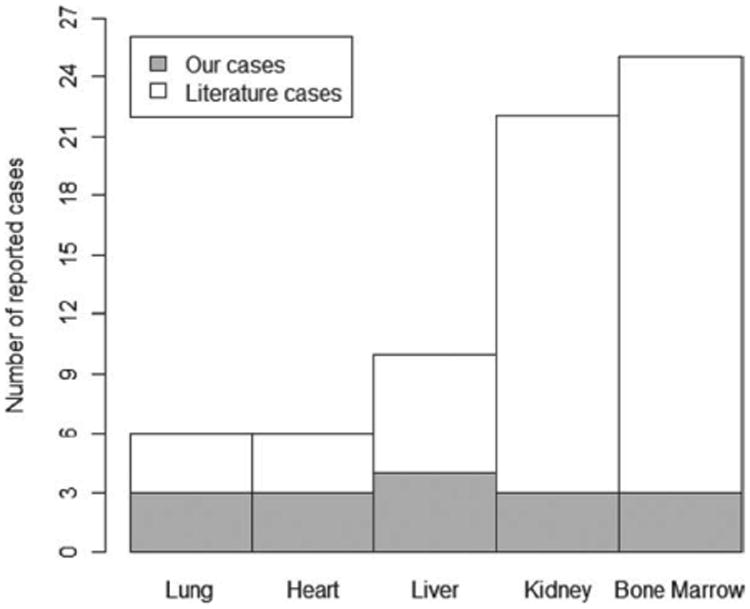
Histogram demonstrating frequency of PML cases reported by type of organ transplantation (n = 69). PML = progressive multifocal leukoencephalopathy.
Table 2. Clinical Characteristics of Reported Cases of PML in Solid Organ Transplantation Recipients (January 1971–July 2010).
| Author Last Name (Year of Publication) | Sex/Age at Transplantation | Underlying Disease Prompting Transplantation | Method of Diagnosis | Survival from Symptom Onset (mo)a | Attempted Treatment | |
|---|---|---|---|---|---|---|
| Lung (3) | ||||||
| 1 | Ouwens (2000)28 | 43/M | Bronchiectasis | CSF PCR | 20 | Y |
| 2 | Shitrit (2003)29 | 58/M | Idiopathic pulmonary fibrosis | Biopsy | NP | Y |
| 3 | Waggoner (2009)30 | 38/F | Idiopathic pulmonary fibrosis | CSF PCR | 5.25 | Y |
| Heart (4) | ||||||
| 4 | Hall (1988)31 | 59/M | Ischemic cardiomyopathy | Autopsy | 0.75 | N |
| 5 | Flomenbaum (1991)32 | 49/M | Ischemic heart disease, Coronary artery disease | Autopsy | <1 | N |
| 6 | Lewis (1993)33 | 59/M | Ischemic heart disease | Biopsy | 15 | N |
| 7 | McCalmont (2007)34 | 68/M | Idiopathic cardiomyopathy | Autopsy | 0.5 | N |
| Liver (6) | ||||||
| 8 | Worthmann (1994)35 | 53/F | Hepatitis C, cirrhosis | Autopsy | Asymptomatic | N |
| 9 | Bronster (1995)36 | 51/M | Cryptogenic cirrhosis | Biopsy | 2.25 | Y |
| 10 | Boulton-Jones (2001)37 | 60/F | Secondary biliary cirrhosis | CSF PCR | 22 | Y |
| 11 | Koralnik (2004)38 | 66/M | Nephrolithiasis | Biopsy | 4 | N |
| 12 | Lima (2005)39 | 39/F | Hepatitis C, cirrhosis | CSF PCR | 1.75 | Y |
| 13 | Alibert (2006)40 | 59/F | Hepatitis C, cirrhosis | CSF PCR | <6 | Y |
| Kidney (19) | ||||||
| 14 | Manz (1971)4 | 33/F | Minimal glomerular change disease | Autopsy | 3.5 | N |
| 15 | ZuRhein (1974)41 | 34/M | Chronic glomerulonephritis | Autopsy | 2 | N |
| 16 | Legrain (1974)42 | 19/F | Chronic pyelonephritis | Autopsy | 6 | N |
| 17 | McCormick (1976)43 | 34/M | Chronic proliferative glomerulonephritis | Autopsy | 2.75 | N |
| 18 | Selhorst (1978)44 | 50/F | Not provided | Biopsy | 27+ | Y |
| 19 | Egan (1980)46 | 53/M | Chronic glomerulonephritis | Autopsy | 2.5 | N |
| 20 | Ho (1980)47 | 65/F | Radiation nephritis | Autopsy | 6 | N |
| 21 | Reznik (1981)A48 | 51/M | Hypertensive nephropathy | Autopsy | 13 | N |
| 22 | Reznik (1981)B48 | 45/F | Extramembranous glomerulonephrosis | Autopsy | 38 | N |
| 23 | Holzhausen (1984)49 | 44/M | Glomerulonephritis | Autopsy | 2 | N |
| 24 | Saxton (1984)50 | 28/M | Not stated | Biopsy | 13.5+ | Y |
| 25 | Garcia (1985)51 | 28/M | Chronic active glomerulonephritis | Biopsy | ∼5+ | Y |
| 26 | Embrey (1988)52 | 18/M | Obstructive nephropathy, solitary kidney | Biopsy | NP | Y |
| 27 | Táborský (1998)53 | 66/M | Chronic glomerulonephritis | CSF PCR | 5.25 | Y |
| 28 | Berner (1999)53 | 69/M | Chronic pyelonephritis | CSF PCR | 4 | N |
| 29 | Phillips (2004)54 | 16/M | ANCA-associated glomerulonephritis | CSF PCR | <1.25 | N |
| 30 | Crowder (2005)55 | 47/M | Alport syndrome | Biopsy | 37+ | Y |
| 31 | Manfro (2009)56 | 52/F | Chronic pyelonephritis | Biopsy | 4+ | Y |
| 32 | Epker (2009)57 | 50/M | Proliferative glomerulonephritis, diabetes mellitus | CSF PCR | 6+ | Y |
n = 32.
Case was reported before the time of death (censored at time of reporting; eg, 7+ equals survival last reported at 7 months).
ANCA = antineutrophil cytoplasmic antibody; CSF = cerebrospinal fluid; NP = not provided; PCR = polymerase chain reaction.
PML following bone marrow transplantation was found in 3 patients in this series and 22 patients in the literature.58–77 An additional 7 bone marrow transplant recipients were reported in a case series on rituximab9 but were not included in this study because insufficient details were available. The method of diagnosis, age, sex, and reason for transplantation for bone marrow transplant recipients is reported in Tables 3 and 4. Method of diagnosis was brain biopsy (n = 11, 44%), CSF PCR (n = 6, 24%), autopsy (n = 3, 12%), and MRI brain (“suspected”, n = 2, 8%). Cases of bone marrow transplant recipients with PML have been published in the 1990s (n = 7) and 2000s (n = 16).
Table 3. Comparison of Solid Organ and Bone Marrow Transplant Recipients with Posttransplantation PML.
| Solid Organ Transplantation | Bone Marrow Transplantation | ||||||
|---|---|---|---|---|---|---|---|
| Current Series (n = 12) | Literature(n = 32) | Comparison of Current Series and Literature p Values | Combined(n = 44) | Current Series (n = 3) and Literature (n = 22) | Total (n = 69) | Combined Solid Organ vs Combined BMT Comparison | |
| Female, n (%) (95% CI) | 6 (50) | 11 (34) | 0.5482a | 17 (39) | 12 (48) | 26 (42) | 0.6145a |
| Mean age at transplantation, yr (95% CI) | 51.7 (45.6–57.8) | 47 (41.6–52.3) | 0.2292b | 48.3 (44.1–52.4) | 42.9 (38.0–47.8) | 46.3 (43.1–49.5) | 0.0912b |
| Range, yr | 39–68 | 16–69 | 16–69 | 14–68 | 14–69 | ||
| Number diagnosed at autopsy (%) | 1 (10) | 12 (38) | 0.1291c | 13 (30) | 3 (12) | 16 | 0.1728c |
| Median time from transplantation to first symptoms of PML, mo (95% CI) | 33.6 (15.2–60.7) | 27 (15–41.5) | 0.7046d | 27 (17.5–41.0) | 11 (6.7–16.7) | 17 (13.3–25.7) | 0.0005d |
| Range, mo | 0.2–198.8 | 1.5–245 | 0.2–245 | 1–78 | 0.2–245 | ||
| Median time from symptom onset to diagnosis of PML, mo (95% CI) | 1.7 (0.4–6.5) | 1.9 (1–3.5) | 0.5968d | 1.8 (1–2.9) | 0.9 (0.5–2.0) | 1.6 (1.0–2.2) | 0.1513d |
| Range, mo | 0.2–12.5 | 0.3–13.0 | 0.2–13.0 | 0.3–8.8 | 0.2–13 | ||
| Median time from symptom onset to death, mo (95% CI) | 8.0 (2.3, ∞) | 6 (4–22) | 0.956e | 6.4 (4.0–15.6) | 19.5 (7.0, ∞) | 8.4 (6.0–19.6) | 0.0679e |
| Range, mof | 0.85–152.6+ | 0.5–38+ | 0.5–152.6+ | 1.3–43.4+ | 0.5–152.6+ | ||
n = 69.
Two-sample proportions test with continuity correction.
t Test with unequal variances.
Two-sample proportions test with Yates continuity correction.
Two-sample Wilcoxon rank-sum (Mann-Whitney) test (p = 0.438 in 2-sample equality-of-medians chi-square test for median time from transplantation to symptom onset and 0.687 in symptom onset to diagnosis, p = 0.001 for difference between solid organ and BMT patients for median time to symptom onset of PML from transplantation).
Log-rank test.
The numbers written correspond to the observed follow-up times. The “+” indicates that some patients were censored (not observed until the time to death). BMT = bone marrow transplantation; CI = confidence interval; PML = progressive multifocal leukoencephalopathy.
Table 4. Clinical Characteristics of Reported Cases of PML in Bone Marrow Transplantation Recipients (January 1971–July 2010).
| Bone Marrow | Author Last Name (Year of Publication) | Sex/Age at Transplantation | Underlying Disease Prompting Transplantation | Method of Diagnosis | Survival from Symptom Onset (mo) | Attempted Treatment |
|---|---|---|---|---|---|---|
| 33 | Mesquita (1992)58 | 47/M | Polymorphic centroblastic lymphoma | Autopsy | 2+ | N |
| 34 | O'shaughnessy (1994)59 | 44/F | Chronic myeloid leukemia | Biopsy | 4 | Y |
| 35 | Seong (1996)60 | 31/F | Chronic myeloid leukemia | Biopsy | NP | NP |
| 36 | Przepoirka (1997)61 | 46/F | Low-grade lymphoma | Biopsy | 12 | Y |
| 37 | Coppo (1999)62 | 38/F | Acute leukemia, hepatitis C | CSF PCR | 3 | Y |
| 38 | Re (1999)63 | 54/F | Mantle cell lymphoma | Biopsy | 11.25 | Y |
| 39 | Owen (1995)64 | 43/M | Chronic myeloid leukemia | MRI | 2 | Y |
| 40 | Goldberg (2002)65 | 34/F | Non-Hodgkin's lymphoma | CSF PCR | 7+ | Y |
| 41 | Goldberg (2002)65 | 42/F | Non-Hodgkin's lymphoma | CSF PCR | 30 | N |
| 42 | Osorio (2002)66 | 60/M | Mantle cell lymphoma | Biopsy | 5 | Y |
| 43 | Buckanovich (2002)67 | 29/F | Hodgkin's lymphoma | MRI | 6.5 | Y |
| 44 | Steurer (2003)68 | 32/M | Diffuse large cell lymphoma | Biopsy | 3 | Y |
| 45 | Gabriel (2007)69 | 45/M | Cutaneous T-cell lymphoma | Autopsy | NP | N |
| 46 | Focosi (2007)70 | 33/F | Acute lymphoblastic leukemia | Biopsy | 11 | Y |
| 47 | Karfan-Dabaja (2007)71 | 51/M | Follicular non-Hodgkin's lymphoma | Biopsy | 2 | Y |
| 48 | Karfan-Dabaja (2007)71 | 41/M | Hodgkin's lymphoma | CSF PCR | 1.25+ | Y |
| 49 | Yasuda (2008)72 | 14/M | Wiskott-Aldrich syndrome | CSF PCR and autopsy | 2.5+ | Y |
| 50 | Sheikh (2009)73 | 38/F | Acute myeloid leukemia | Autopsy | 1.5+ | NP |
| 51 | Chowdhary (2008)74 | 51/M | Non-Hodgkin's lymphoma, myelodysplasia | Biopsy | NP | NP |
| 52 | Pelosini (2008)75 | 30/F | Acute lymphoblastic leukemia | Biopsy | 18 | Y |
| 53 | Fianchi (2010)76 | 63/F | Multiple myeloma | Biopsy | 15 | Y |
| 54 | Tuccori (2010)77 | 45/M | Mantle cell lymphoma | CSF PCR | 7+ | Y |
n = 22.
CSF = cerebrospinal fluid; MRI = magnetic resonance imaging; NP = not provided; PCR = polymerase chain reaction; PML = progressive multifocal leukoencephalopathy.
In total, the median time to development of PML symptoms following transplantation was 17 months but ranged widely from <1 month to more than 20 years. There were 12 patients who presented with PML more than 5 years posttransplantation (12/69, 17.4%; 95% CI, 9.7–28.8%) of whom 6 patients (6/69, 8.7%; 95% CI, 3.6–18.6%) presented after more than 10 years posttransplantation and 2 patients presented after 20 years (2.9%; 95% CI, 5.0–11.0%). The median time to PML symptoms following transplantation was longer in reported cases of solid organ transplant recipients compared to bone marrow transplant recipients (17 vs 11 months, p = 0.0005 [2-sample Wilcoxon-Mann-Whitney rank-sum test] and p = 0.001 [2-sample equality-of-medians Mood-Brown test]) although mean age at transplantation and the proportion of females did not differ (p > 0.05; see Table 2). The median time from symptom onset to death also showed a trend toward significance with bone marrow transplant recipients surviving longer (19.5 vs 6.4 months, p = 0.0679) (Fig 3). Consistent with the existing literature, the most common presenting symptoms of PML were cognitive deficits (n = 32 out of 68 with information on symptoms available; 47.1%), weakness (n = 30; 41.9%), visual symptoms (16; 23.5%), cerebellar symptoms (n = 13; 19.1%), dysarthria (n = 13, 18.8%), personality change (n = 10, 14.7%), aphasia (n = 10, 14.7%), and seizures (n = 7, 10.3%). Dizziness, hallucinations, falls, lethargy, fever, and headaches were each noted in less than 5% of patients.
Figure 3.
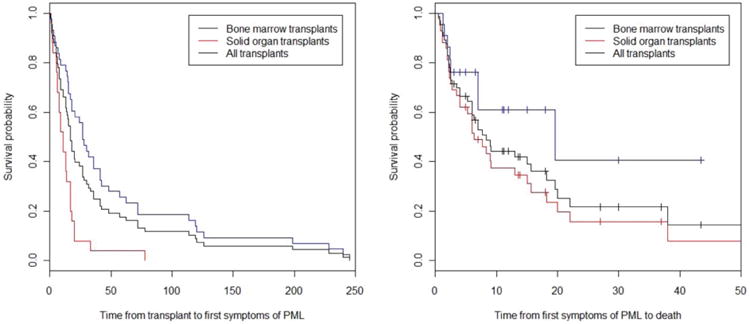
Survival curves. (A) Time to first symptoms of PML in transplant recipients (months) (n = 69; 44 solid organ recipients and 25 bone marrow recipients). (B) Time to death from first symptom onset among patients who develop PML posttransplantation (months). PML = progressive multifocal leukoencephalopathy.
Using data from all patients who were diagnosed with PML in life and had available information on time of symptomatic onset (n = 41), the hazard rate of developing PML among transplant recipients was greatest immediately posttransplantation (Fig 4). This risk decreased over time. Given the few transplant recipients who were observed to develop PML beyond year 6 posttransplantation (<20%), an estimate of the hazard of developing PML posttransplantation after year 6 should be made with caution.
Figure 4.
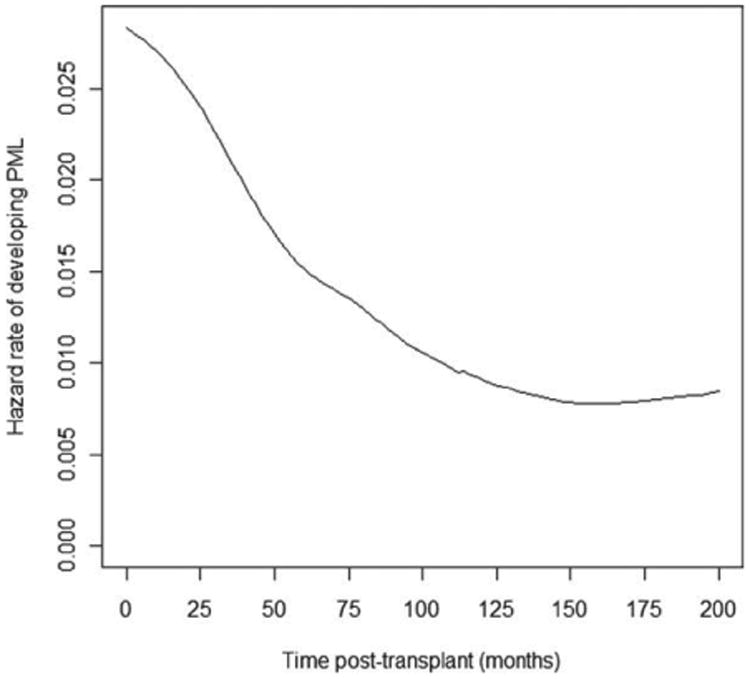
The hazard rate of developing PML as a function of time since transplantation in the subpopulation of transplant recipients ever developing PML. PML = progressive multifocal leukoencephalopathy.
Overall, when combining all cases of PML, the immunosuppressive drug exposures were reported in some form (ever exposed or currently exposed) in 68 of 69 cases (99%) with 2 early cases of post–kidney transplant recipients having an undetailed history of immunosuppressive drug exposure. Overall, 42 different immunosuppressive drugs were used in the collected cases with prednisone, cyclosporine, and azathioprine being most commonly prescribed (Fig 5). Bone marrow transplant recipients in particular had exposures to chemotherapeutic drugs including monoclonal antibodies and alkylating, antimicrotubule, or antimetabolite agents. Most patients were exposed to at least 2 immunosuppressive medications following transplantation (range, 1–9 medications).
Figure 5.
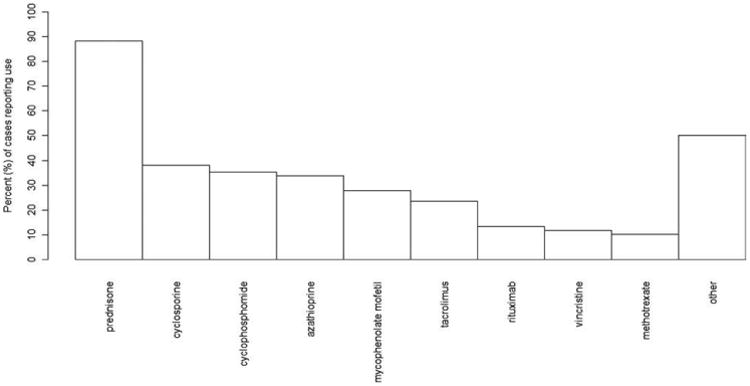
Immunosuppressive medication exposures among transplant recipients who developed PML (n = 68). PML = progressive multifocal leukoencephalopathy.
Treatment of PML in the posttransplantation period was inconsistent (Table 4). There is no controlled clinical experience with the different treatment options used to treat PML in posttransplantation patients. Treatments were reported in 41 patients including 10 from the current study (Table 5). 17 cases have stabilized or improved. Any 1 treatment has been tried less than 10 times in the available literature for posttransplantation PML. A small fraction of patients have survived with PML and reports of symptomatic improvement were the exception. Many (n = 20) had immunosuppressant reductions and alterations following PML. In the current study, 3 patients are still alive following attempted treatment, last reported alive at 13, 44, and 155 months following PML symptom onset. All 3 cases were diagnosed by positive JC virus detection in the CSF.
Table 5. Clinical Outcomes and Survival Time of Patients Who Have Undergone Treatment for PML Posttransplantation.
| Case Number |
Last Name of Primary Author (Year Published) |
Survival following PML Symptom Onset |
Cytosine Arabinoside |
Mirtazapine | Mefloquine | Cidofovir | Reduced Immunosuppression |
Other | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1* | Current series | 152.5+ | 2mg/kg/d IV × 5days | – | – | – | – | Alive | |
| 4* | Current series | 1.2 | – | – | – | Yes | PEG, tracheostomy | Died d/t PML | |
| 5* | Current series | 18.2 | Yes | – | – | – | – | Died d/t PML | |
| 7* | Current series | 9.1 | – | Yes | Yes | Reduced tacro; D/C campath | – | Died, details unknown | |
| 8* | Current series | 0.9 | – | – | – | D/C sirolimus, dex | – | Died d/t MSOF and sepsis | |
| 9* | Current series | 7.7 | – | Yes | – | – | – | Died d/t PML | |
| 10* | Current series | 2.4 | – | – | Yes | – | – | Died d/t PML with pneumonia | |
| 11* | Current series | 2.2 | – | Yes | Yes | D/C tacro; change to cyc, and pred | Remeron | Died d/t PML | |
| 12* | Current series | 15.6 | – | – | – | Yes | – | Died - Unknown cause | |
| 15* | Current series | 15.8+ | – | – | – | Change to pred only | – | Alive | |
| 1 | Ouwens (2000)28 | 20 | – | – | – | Reduced mm, cyc | Pred pulses | Died d/t PML | |
| 2 | Shitrit (2003)29 | NP | Yes | – | – | Reduced mm, tacro, pred | Probenicid | Stabilized neurological symptoms | |
| 3 | Waggoner (2009)30 | 5.25 | – | Yes | – | Yes | – | – | Died d/t PML |
| 9 | Bronster (1995)36 | 2.25 | Yes | – | – | – | Yes | – | Died d/t PML |
| 10 | Boulton-Jones (2001)37 | 22 | Yes | – | – | – | Yes | – | Stabilized, did not report death |
| 12 | Lima (2005)39 | 1.75 | 2mg/kg/d IV × 5 days | – | – | – | – | – | Died d/t PML |
| 13 | Alibert (2006)40 | <6 | – | – | – | 5mg/kg/d | Reduced pred, tacro | Pegylated INF-alpha | Died d/t PML |
| 18 | Selhorst (1978)44 | 27+ | – | – | – | D/C aza | Tilorone | Stabilized, death not reported | |
| 24 | Saxton (1984)49 | 13.5+ | 0.5mg/kg tid IV × 5 days | – | – | D/C pred, cyclo | – | Stabilized, death not reported | |
| 25 | Garcia (1985)50 | 5+ | – | – | – | D/C pred, aza | – | Improved, death not reported | |
| 26 | Embrey (1988)51 | NP | 0.5mg/kg tid IV × 5 days, then q6wks | – | – | D/C cyclo | – | Improved, death not reported | |
| 27 | Táborský (1998)52 | 5.25 | – | – | – | D/C cyclo, reduced aza, pred | Ganciclovir | Died d/t PML | |
| 30 | Crowder (2005)55 | 37+ | – | – | – | D/C mm, tacro | – | Improved, death not reported | |
| 31 | Manfro (2009)56 | 4+ | – | – | – | D/C mm, reduced pred | – | Improved, death not reported | |
| 32 | Epker (2009)57 | 6+ | – | – | – | Yes | – | Improved, death not reported | |
| 34 | O'shaughnessy (1994)59 | ∼4 | Yes | – | – | – | – | Died d/t PML | |
| 36 | Przepoirka (1997)61 | 12+ | – | – | – | – | IL-2 0.5MU/m2/d IV continuous × 4wks | Improved, death not reported | |
| 37 | Coppo (1999)62 | ∼4 | – | – | – | – | – | Bone marrow infusion | Died d/t PML |
| 38 | Re (1999)63 | >11.25 | cytarabine IT 40mg qwk × 3 | – | – | – | – | subQ IL-2 (9 × 106) q other day | Stabilized, did not report death |
| 39 | Owen (1995)64 | >2 | 2mg/kg IV × 5 days, q2wks | – | – | – | – | Cytosine IT 50mg qwkly | Died d/t PML |
| 40 | Goldberg (2002) A65 | 7+ | – | – | – | Yes | – | continuous infusion IL-2 | Died d/t PML |
| 42 | Osrio (2002)66 | 5 | Yes | – | – | – | – | – | Died d/t PML |
| 43 | Buckanovich (2002)67 | 6.5 | – | – | – | – | – | IL-2 infusion 0.5 million units/m2/d | Improved, did not report death |
| 44 | Streuer (2003)68 | 3 | – | – | – | 5mg/kg q-wkly then q2wkly (6 doses total) | – | – | Died d/t PML |
| 46 | Focosi (2007)70 | 11 | Yes | Yes; po ×3 | IVIG, donor lymphocyte infusions, probenecid | Improved, did not report death | |||
| 47 | Karfan-Dabaja (2007) A71 | 2 | – | – | – | 5mg/kg qwkly ×2 | – | Ziprasidone 20mg po daily then BID | Died d/t PML |
| 48 | Karfan-Dabaja (2007) B71 | 1.25+ | – | – | – | – | D/C tacro | – | Died d/t PML |
| 49 | Yasuda (2008)72 | 2.5+ | – | – | – | – | – | Acyclovir, ribavirin, INF-alpha, autologous lymphocytes | Died d/t PML |
| 52 | Pelosini (2008)75 | 18 | Yes; IT | – | – | 300mg/wk | – | Donor lymphocyte infusions, risperidone × 10 days until 8 mg/d, AraC intrathecally, cidofovir 5mg/kg/wk × 3 doses | Improved, did not report death |
| 53 | Fianchi (2010)76 | 15 | – | 750mg then 500mg q12h | – | – | – | Citalopram 20mg/d × 5 days, IVIG | Improved, did not report death |
| 54 | Tuccori (2010)77 | 7+ | – | Yes | – | Yes | – | Risperidone | Died d/t PML |
aza = azathioprine, BID = 2 times per day; cyclo = cyclosporine, dex = dexamethasone, d/t = due to; D/C = discontinue; IL-2 = interleukin-2; INF = interferon; IT = intrathecal; IV = intravenous; IVIG = intravenous immunoglobulin; mm = mycophenolate mofetil, MSOF = multisystem organ failure, NP = not provided, PEG = percutaneous endoscopic gastrostomy; PML = progressive multifocal leukoencephalopathy; po = by mouth; pred = prednisone, q6wks = every 6 weeks; q12h = every 12 hours; subQ = subcutaneous; tacro = tacrolimus; tid = 3 times daily.
Cause of death was PML in 42 of 50 patients who have a reported cause of death (case fatality 84.0%; 95% CI, 70.3–92.4%). A total of 16 patients had no reported death or cause of death, and 3 patients were still alive at the time of analysis. Other causes of death in this multi-center cohort were graft-vs-host disease, multisystem organ failure with sepsis, and progressive liver failure (1 case each) and, in the literature, concurrent central nervous system (CNS) lymphoma and PML (n = 1) and suicide (n = 2). Overall mortality in patients with PML posttransplantation is 41.2% at 6 months following symptom onset (95% CI, 27.2–52.5%), 55.7% at 12 months (95% CI, 40.2–67.2%), and 63.9% at 18 months (95% CI, 47.2–75.4%). Patients have been observed under follow-up for 38 months in the literature and 153 months in the present cohort.
IRIS was identified in 1 patient in the multicohort study (1/15) (case 7). Her blood CD4 cell count was 0 cells/mm3 at the time of first presentation with neurological symptoms. Due to the development of PML, the dosages of her tacrolimus and prednisone were decreased. Cidofovir and mirtazapine were given as treatment for PML. One month later, the severity of her neurological symptoms increased. MRI of the brain demonstrated multifocal areas of high signal intensity lesions in the white matter involving the frontal and parietal lobes, thalami, brain stem, and cerebellum, best seen on fluid-attenuated inversion recovery (FLAIR) sequences. There was contrast enhancement within the PML lesions. Her CD4 cell count was 88 cells/mm3 at the time of worsened symptoms. Repeat CSF analysis identified JC virus. She was given mefloquine (500mg loading dose then 250mg weekly); however, her neurological status continued to deteriorate until her death 3 months later. There were no identified cases of PML-IRIS among transplant recipients in the reported literature.
Immunological data on the patients in this series was inconsistent. CD4 cell counts were reported in 10 cases (3 solid organ, 7 bone marrow) with a collected mean of 319/μl, and median 167/μl.
Discussion
Transplant recipients are at a small but significant risk of developing PML. This risk occurs throughout the course of the posttransplantation period. The risk of PML is 1.24 per 1,000 posttransplantation person years for heart and lung transplant recipients at 1 institution with 98% or higher follow up (95% CI, 0.025–3.61 per 1,000 posttransplantation years) and does not appear to have changed over time. This incidence is comparable to the risk of PML with natalizumab treatment (approximately 1 per 1,000 people treated for 1 year7 or 1 in 1,000 patients treated for 18 months)78 and exceeds the risk reported among HIV patients treated with HAART (0.6 cases per 1,000 people treated for 1 year; 95% CI, 0.4–1.0 cases per 1,000). The incidence of PML post–heart and/or lung transplantation is also likely higher than in rheumatological disorders such as systemic lupus erythematosus.79
Until recently, PML cases following transplantation were often diagnosed at autopsy, leading to inconsistent information on the clinical characteristics and survival in this group. We present a cohort of PML in a group of transplant recipients in which the diagnosis was made in life in 14 of 15 cases. The type of organ transplantation, underlying diagnoses necessitating organ replacement, and immunosuppressive regimens employed at these centers are representative of transplant populations at other major academic medical institutions in high income settings.5 The survival rates at the 3 U.S. centers can be compared to national averages of posttransplantation survival through the United Organ Sharing Network5 and meet or exceed survival rates of transplant populations at other centers over the time periods studied. This cohort thus provides a useful perspective on the rate of PML that can be expected at other centers.
The most important underlying risk factor for PML in patients undergoing solid organ transplantation is immunosuppressive medication, which is necessary to prevent graft rejection. Because most patients are exposed to a combination of immunosuppressive therapies over time, it is unclear whether any 1 drug is primarily associated with the reactivation of JC virus. In this study, 69 recipients had exposure to 42 different immunosuppressive agents, including chemotherapeutic drugs. The duration and doses varied over time although nearly all patients were exposed to at least 2 medications. Most regimens included prednisone, which is standard for posttransplantation patients and cannot, by itself, be taken to represent a specific drug exposure risk for the development of PML. Prior hematologic malignancies, absolute level of immunosuppression, in combination with a history of chemotherapy, may partially explain the lower median time to symptom onset in bone marrow vs solid organ transplant recipients. Another possible explanation is reporting bias in the literature. Bone marrow transplant recipients often had myeloablative chemotherapy prior to transplantation, which may have increased their risk of developing PML in the early posttransplantation period. Solid organ transplant recipients are, however, likely to die sooner with a diagnosis of PML with a lower median survival of 6.4 months overall (95% CI, 4.0–15.6).
As higher rates of posttransplantation survival are achieved, it is presumed that the total number of cases of PML will similarly increase. Because we were unable to find an end to the risk period for the development of PML posttransplantation (ie, there is no time beyond which patients are “free” of risk for developing PML), it is possible that the higher number of posttransplantation years alive will carry a higher risk of PML in this population. PML symptom onset occurred from 1 to 63 months in the present cohort and beyond 20 years following transplantation in the available literature. An estimation of the hazard rate of PML in the subpopulation of transplant recipients who do develop disease provides insight into the time period within which a person is expected to develop PML, if ever. Our plot suggests that if a transplant recipient will ever develop PML, he or she has the greatest risk of disease onset immediately posttransplantation. This risk decreases smoothly thereafter and eventually stabilizes. Our data do not suggest a discernible period after which there is a dramatic reduction in risk, but rather a very gradual decrease in risk over time. PML must therefore be suspected at all times during the posttransplantation period. Again, reporting bias may influence these results.
The case fatality among patients who develop PML posttransplantation is high. A conservative estimate, given here, is based on 42 PML-related deaths out of 50 patients who have a reported cause of death (case fatality 84.0%; 95% CI, 70.3–92.4%). By comparison, 8 of the first 28 confirmed cases of PML with natalizumab have been reported as fatal (29%).7 The 1-year survival in posttransplantation PML is 56%, equivalent to that reported among HIV patients treated with HAART (39– 56%).10,80 Among survivors, some patients had their immunosuppressive drug regimen significantly reduced or withdrawn at the time of diagnosis of PML but it remains unclear what treatment, if any, leads to improved outcomes in PML posttransplantation. Treatments that were given late in the disease course were of dubious benefit. The limited experience with PML treatment in this setting suggests that no 1 treatment is definitely effective. Primary graft rejection is of significant concern with immunosuppressive drug reduction, but here it is consistent with survival in some cases of PML.
This study has limitations. The case series presented is a retrospective cohort study. As in all studies of PML posttransplantation, the graft, donor, and recipient JC virus status prior to transplantation are unknown. The centers in this study also do not collect information on the presence of BK virus in the donor or recipient of the graft. This information, if available, would almost certainly be helpful to assess risk profiles for recipients and determine whether donor or recipient viral exposures are important factors in the eventual development of posttransplantation PML.
Three cases of PML were suspected but could not be confirmed by brain biopsy or autopsy due to patient and family wishes and predated the routine use of PCR for diagnosis. The survival rate of solid organ transplant recipients has also changed over time such that an increase in PML cases in recent decades cannot be attributed to changes in immunosuppressive drug selection or transplantation care. Rather, improved survival following transplantation may increase the risk of PML in this population because their risk period has increased in length. Given the small sample size, the changing immunosuppressive drug regimen in each patient, and the uncertain mechanism of JC virus dissemination to the brain in the immunosuppressed, we were unable to correlate development of PML with any 1 specific drug. The varied exposures to immunosuppression in these cases suggest that PML may not be linked to a single drug with a single mechanism of action in the immunosuppressed.
In some cases, the underlying diagnosis prompting transplantation, including cryptogenic cirrhosis, hepatitis C, chronic lymphocytic leukemia, systemic amyloidosis, and Hodgkin's and non-Hodgkin's lymphoma have been associated with development of PML even in the absence of transplantation and immunosuppressive drugs.8 Cases of PML have been recognized in hematological malignancy untreated by transplantation, as in the original report by Åström and colleagues,3 however, the range of transplantation graft organs, spectrum of underlying disorders, and targets of the immunosuppressive drugs found here suggest that it is transplantation and immunosuppression in general and not the underlying disease prompting them that are the most important determinants for development of PML. Among solid organ recipients, there are very few disorders that are linked with PML in the absence of immunosuppression, supporting this hypothesis.
Finally, by reviewing the available literature and comparing it to the expected incidence of cases, it is clear that most cases of PML posttransplantation are not identified through publication. If the above incidence is generalizable (1.24 per 1,000 posttransplantation years), the number of published cases is a small fraction of expected cases. In the United States, heart, lung, and heart-lung transplant recipients total 70,016 and a 5-year survival rate from 50% to 70% can be expected.5 This would be expected to yield at least 200 cases of posttransplantation PML in heart and lung recipients instead of the 10 reported from the United States including this series. Although our incidence calculation is based on a small number of cases, it is likely that PML posttransplantation is severely underreported and perhaps underrecognized. Our collected series of cases almost certainly represents a reporting bias as well. We can assume that only some posttransplantation PML cases have been reported in the literature and the characteristics of the reported cases may differ in important but unknown ways from the true population of PML posttransplantation patients. Existing databases may be unable to answer remaining questions. Over a similar timeframe (2000–2008), the Normative Health Informatics database in the United States reported no cases of medical chart–confirmed PML among 6,649 solid organ recipients and 1 case among 2,129 bone marrow transplant recipients.81 The absence of PML-IRIS cases seen in all reported cases is almost certainly due to underreporting and the lack of a standard definition of PML-IRIS in transplant recipients rather than an absolute lack of PML-IRIS in this group.
This suggests that there is a benefit for national registration of rare but life-threatening neurological diseases that represent an underlying infectious etiology but emerge in identifiably vulnerable groups. A national registry for PML would provide a more effective means by which to identify important risk factors for disease development, clarification on the most important at-risk groups, and the relative burden of PML posed to transplant recipients. This could lead to modification of risk factors, and eventually, mechanisms for the recording of tried and failed treatments. Such collective reporting, even by means of passive surveillance and consensus on case definitions of PML and PML-IRIS in transplant recipients, would provide useful new data to neurologists who may be repeating previously failed treatment in new patients or lose vigilance for the disorder when the risk remains important. A national registry that links donor and recipient serostatus on JC virus and BK virus in transplant patients would heighten the value of such an endeavor and recognize the new trend of neurological infectious diseases that are potentially transmitted iatrogenically, many years prior to disease manifestations.
At present, PML poses an especially difficult situation for the solid organ transplant recipient. Immune reconstitution—clinically prompted by immunosuppressant reduction—remains the only recognized mechanism by which patients may improve with PML but creates the possibility of graft rejection. In patients undergoing heart and lung transplantations especially, the risk of organ rejection includes loss of life and suitable replacement organs are usually not immediately available. A stronger appreciation of the risk of PML posttransplantation and a concerted effort toward controlled studies and early treatment will become increasingly important in this population. Urgent workup of suspected cases of PML by CSF PCR and where suspicion is high, brain biopsy, may be life-saving. At least in some cases, reduction or change in immunosuppression and perhaps treatment attempts are compatible with long-term recipient survival.
Acknowledgments
We thank Dr K. Vomáčková, MD, Czech Republic, and Dr B. Knoll, MD, Division of Infectious Diseases, Harvard University, for their English translation of Czech and German articles included in this manuscript. Dr Mateen is supported by the 2010 American Academy of Neurology Practice Research Fellowship Grant. Dr van de Beek is supported by grants from the Organisation of Scientific Research (Rubicon) and the Netherlands Organisation for Health Research and Development (Veni and Vidi).
Footnotes
Potential conflict of interest: A.N. has received and has grants pending from the NIH; has received consulting fees or honoraria from Biogen and Elan; is a member of a scientific advisory board for Diogenix; has patents on the development of neuroprotective agents; and has given expert testimony for several law firms. D.B.C. has consulted for Biogen Idec, Genentech, Millennium, Genzyme, Bristol Myers Squibb, and Pfizer; has given expert testimony for Biogen Idec; has received payment for lectures including service on speakers bureaus for Glaxo Smith Kline and Millennium; has received payment for development of educational presentations for Millennium; and has received travel/accommodations/meeting expenses from Biogen Idec. D.M.H. has grants pending from Bayer Pharmaceuticals; and has received payment for manuscript preparation from the American College of Physicians. D.v.d.B. received grants from the Netherlands Organisation of Scientific Research (Rubicon and TOP grants), The Netherlands Organisation for Health Research and Development (Veni, Vidi, and Health Care Efficiency Research grants), and the Netherland Heart Foundation. F.J.M. has received a grant for salary support from the American Academy of Neurology.
References
- 1.Johnson RT. Acute disseminated encephalomyelitis and progressive multifocal leukoencephalopathy. In: Shapira AH, editor. Neurology and clinical neuroscience. Philadelphia: Elsevier; 2007. pp. 1060–1062. [Google Scholar]
- 2.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 3.Åström KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy: a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Manz HJ, Dinsdale HB, Morrin PA. Progressive multifocal leukoencephalopathy after renal transplantation. Ann Intern Med. 1971;75:77–81. doi: 10.7326/0003-4819-75-1-77. [DOI] [PubMed] [Google Scholar]
- 5.United Network for Organ Sharing. [Last accessed March 22 2011]; Available at: http://www.unos.org/
- 6.Khanna N, Elzi L, Mueller NJ, et al. Incidence and outcome of progressive multifocal leukoencephalopathy over 20 years of the Swiss HIV cohort study. Clin Infect Dis. 2009;48:1459–1466. doi: 10.1086/598335. [DOI] [PubMed] [Google Scholar]
- 7.Clifford DB, Deluca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 8.Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psych. 2010;81:247–254. doi: 10.1136/jnnp.2009.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the research on adverse drug events and reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73:1551–1558. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelburne SA, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active retroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rosner BA. Fundamentals of biostatistics. 6th. Pacific Grove, CA: Duxbury Press; 2006. [Google Scholar]
- 13.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2005;15:1–11. [Google Scholar]
- 14.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13. [Google Scholar]
- 15.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 16.Stata Corp. Stata (v.11) College Station, TX: Stata Corp; 2009. [Google Scholar]
- 17.Agresti A. Categorical data analysis. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 18.Brown GW, Mood AM. Proceedings of the Second Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, CA: University of California Press; 1951. On median tests for linear hypotheses; pp. 159–166. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 21.Hess K, Gentleman R. Hazard function estimation in survival analysis. R package version 1.2.4. 2009 [Google Scholar]
- 22.Mueller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 23.Mateen FJ, Dierkhising RA, Rabinstein AA, et al. Neurological complications following adult lung transplantation. Am J Transplant. 2010;10:908–914. doi: 10.1111/j.1600-6143.2009.02998.x. [DOI] [PubMed] [Google Scholar]
- 24.van de Beek D, Patel R, Daly RC, et al. Central nervous system infections in heart transplant recipients. Arch Neurol. 2007;64:1715–1720. doi: 10.1001/archneur.64.12.noc70065. [DOI] [PubMed] [Google Scholar]
- 25.Aksamit AJ, Jr, de Groen PC. Cyclosporine-related leukoencephalopathy and PML in a liver transplant recipient. Transplantation. 1995;60:874–876. [PubMed] [Google Scholar]
- 26.Yehia B, Davison A, Sisson S. Transplant troubles. Am J Med. 2009;122:629–631. doi: 10.1016/j.amjmed.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.van de Beek D, Kremers W, Daly RC, et al. Effect of neurologic complications on outcome after heart transplant. Arch Neurol. 2008;65:226–231. doi: 10.1001/archneurol.2007.52. [DOI] [PubMed] [Google Scholar]
- 28.Ouwens JP, Haaxma-Reiche H, Verschuuren EA, et al. Visual symptoms after lung transplantation: a case of progressive multifocal leukoencephalopathy. Transpl Infect Dis. 2000;2:29–32. doi: 10.1034/j.1399-3062.2000.020106.x. [DOI] [PubMed] [Google Scholar]
- 29.Shitrit D, Nirit L, Shiran SI, et al. Progressive multifocal leukoencephalopathy in a lung transplant recipient. J Heart Lung Transplant. 2003;22:946–950. doi: 10.1016/s1053-2498(02)00804-5. [DOI] [PubMed] [Google Scholar]
- 30.Waggoner J, Martinu T, Palmer SM. Progressive multifocal leukoencephalopathy following heightened immunosuppression after lung transplant. J Heart Lung Transplant. 2009;28:395–398. doi: 10.1016/j.healun.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall WA, Martinez J, Dummer JS. Progressive multifocal leukoencephalopathy after cardiac transplantation. Neurology. 1988;38:995–996. doi: 10.1212/wnl.38.6.995-a. [DOI] [PubMed] [Google Scholar]
- 32.Flomenbaum MA, Jarcho JA, Schoen FJ. Progressive multifocal leukoencephalopathy fifty-seven months after heart transplantation. J Heart Lung Transplant. 1991;10:888–893. [PubMed] [Google Scholar]
- 33.Lewis AR, Kline LB, Pinkard NB. Visual loss due to progressive multifocal leukoencephalopathy in a heart transplant patient. J Clin Neuroophthalmol. 1993;13:237–241. [PubMed] [Google Scholar]
- 34.McCalmont V, Bennett K. Progressive multifocal leukoencephalopathy; a case study. Prog Transplant. 2007;17:157–160. doi: 10.1177/152692480701700212. [DOI] [PubMed] [Google Scholar]
- 35.Worthmann F, Türker T, Müller AR, et al. Progressive multifocal leukoencephalopathy after orthotopic liver transplantation. Transplantation. 1993;57:1268–1270. doi: 10.1097/00007890-199404270-00023. [DOI] [PubMed] [Google Scholar]
- 36.Bronster DJ, Lidov MW, Wolfe D, Schwartz ME, Miller CM. Progressive multifocal leukoencephalopathy after orthotopic liver transplantation. Liver Transpl Surg. 1995;1:371–372. doi: 10.1002/lt.500010606. [DOI] [PubMed] [Google Scholar]
- 37.Boulton-Jones JR, Fraser-Moodie C, Ryder SD. Long term survival from progressive multifocal leucoencephalopathy after liver transplantation. J Hepatol. 2001;35:828–831. doi: 10.1016/s0168-8278(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 38.Koralnik IJ, Schellingerhout D, Frosch MP. Case 14–2004: a 66-year-old man with progressive neurologic deficits. N Engl J Med. 2004;350:1882–1893. doi: 10.1056/NEJMcpc030038. [DOI] [PubMed] [Google Scholar]
- 39.Lima MA, Hanto DW, Curry MP, Wong MT, et al. Atypical radiological presentation of progressive multifocal leukoencephalopathy following liver transplantation. J Neurovirol. 2005;11:46–50. doi: 10.1080/13550280590900742. [DOI] [PubMed] [Google Scholar]
- 40.Alibert S, Gérolami R, Tammam D, et al. Progressive multifocal leukoencephalopathy secondary to JC virus infection after liver transplantation and treatment of recurrent hepatitis C. Gastroenterol Clin Biol. 2006;30:473–475. doi: 10.1016/s0399-8320(06)73206-7. French. [DOI] [PubMed] [Google Scholar]
- 41.ZuRhein GM, Varakis J. Letter: Progressive multifocal leukoencephalopathy in a renal allograft recipient. N Engl J Med. 1974;291:798. doi: 10.1056/nejm197410102911524. [DOI] [PubMed] [Google Scholar]
- 42.Legrain M, Graveleau J, Brion S, et al. Progressive multifocal leukoencephalopathy following kidney transplantation. J Neurol Sci. 1974;23:49–62. doi: 10.1016/0022-510x(74)90141-5. French. [DOI] [PubMed] [Google Scholar]
- 43.McCormick WF, Schochet SS, Sarles HE, Calverley JR. Progressive multifocal leukoencephalopathy in renal transplant recipients. Arch Intern Med. 1976;136:829–834. [PubMed] [Google Scholar]
- 44.Selhorst JB, Ducy KF, Thomas JM, Regelson W. PML: remission and immunologic reversals PP.26. Neurology. 1978;28:337. [Google Scholar]
- 45.Egan JD, Ring BL, Reding MJ, Wells IC. Reticulum cell sarcoma and progressive multifocal leukoencephalopathy following renal transplantation. Transplantation. 1980;29:84–86. doi: 10.1097/00007890-198001000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Ho K, Garancis JC, Paegle RD, et al. Progressive multifocal leukoencephalopathy and malignant lymphoma of the brain in a patient with immunosuppressive therapy. Acta Neuropathol (Berl) 1980;52:81–83. doi: 10.1007/BF00687233. [DOI] [PubMed] [Google Scholar]
- 47.Reznik M, Halleux J, Urbain E, et al. Progressive multifocal leukoencephalopathy after renal transplantation Report of two cases. Acta Neurol Belg. 1981;81:205–214. French. [PubMed] [Google Scholar]
- 48.Holzhausen V, Mampel E, Koall W, et al. Progressive multifocal leukoencephalopathy as a complication of immunosuppression in kidney transplantation. Z Gesamte Inn Med. 1977;32:578–581. German. [PubMed] [Google Scholar]
- 49.Saxton CR, Gailiunas P, Jr, Helderman JH, et al. Progressive multifocal leukoencephalopathy in a renal transplant recipient. Am J Med. 1984;77:333–337. doi: 10.1016/0002-9343(84)90715-0. [DOI] [PubMed] [Google Scholar]
- 50.Garcia JH, Pearson J, Bonnin J, Gupta KL. Medical pathology conference: deteriorating neurologic function in a 28-year-old renal transplant recipient. Ala J Med Sci. 1985;22:208–214. [PubMed] [Google Scholar]
- 51.Embrey JR, Silva FG, Helderman H, et al. Long-term survival and late development of bladder cancer in renal transplant patient with progressive multifocal leukoencephalopathy. J Urol. 1988;139:580–581. doi: 10.1016/s0022-5347(17)42533-x. [DOI] [PubMed] [Google Scholar]
- 52.Táborský P, Hrabánek J, Jirásek A, Matl I. Progressive multifocal leukoencephalopathy in a female patient after kidney transplantation. Vnitr Lek. 1998;44:108–110. Czech. [PubMed] [Google Scholar]
- 53.Berner B, Krieter DH, Wolfgang Rumpf K, et al. Progressive multifocal leukoencephalopathy in a renal transplant patient diagnosed by JCV-specific DNA amplification and an intrathecal humoral immune response to recombinant virus protein 1. Nephrol Dial Transplant. 1999;14:462–465. doi: 10.1093/ndt/14.2.462. [DOI] [PubMed] [Google Scholar]
- 54.Phillips T, Jacobs R, Ellis EN. Polyoma nephropathy and progressive multifocal leukoencephalopathy in a renal transplant recipient. J Child Neurol. 2004;19:301–304. doi: 10.1177/088307380401900412. [DOI] [PubMed] [Google Scholar]
- 55.Crowder CD, Gyure KA, Drachenberg CB, et al. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am J Transplant. 2005:1151–1158. doi: 10.1111/j.1600-6143.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 56.Manfro RC, Vedolin L, Cantarelli M, et al. Progressive multifocal leukoencephalopathy in a kidney transplant recipient after conversion to mycophenolic acid therapy. Transpl Infect Dis. 2009;11:189–190. doi: 10.1111/j.1399-3062.2009.00368.x. [DOI] [PubMed] [Google Scholar]
- 57.Epker JL, van Biezen P, van Daele PL, et al. Progressive multifocal leukoencephalopathy, a review and an extended report of five patients with different immune compromised states. Eur J Intern Med. 2009;20:261–267. doi: 10.1016/j.ejim.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 58.Mesquita R, Parravicini C, Björkholm M, et al. Macrophage association of polyoma virus in progressive multifocal leukoencephalopathy: an immunohistochemical and ultrastructural study. APMIS. 1992;100:993–1000. doi: 10.1111/j.1699-0463.1992.tb04031.x. [DOI] [PubMed] [Google Scholar]
- 59.O'shaughnessy D, Goldman JM, Roddie M, Schofield JB. Dizziness and confusion after bone marrow transplantation. BMJ. 1994;309:262–265. doi: 10.1136/bmj.309.6949.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seong D, Bruner JM, Lee KH, et al. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation in a patient with chronic myelogenous leukemia. Clin Infect Dis. 1996;23:402–403. doi: 10.1093/clinids/23.2.402. [DOI] [PubMed] [Google Scholar]
- 61.Przepiorka D, Jaeckle KA, Birdwell RR, et al. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20:983–987. doi: 10.1038/sj.bmt.1701010. [DOI] [PubMed] [Google Scholar]
- 62.Coppo P, Laporte JPh, Aoudjhane M, et al. Progressive multifocal leucoencephalopathy with peripheral demyelinating neuropathy after autologous bone marrow transplantation for acute myeloblastic leukemia (FAB5) Bone Marrow Transplant. 1999;23:401–403. doi: 10.1038/sj.bmt.1701555. [DOI] [PubMed] [Google Scholar]
- 63.Re D, Bamborschke S, Feiden W, et al. Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation and alpha-interferon immunotherapy. Bone Marrow Transplant. 1999;23:295–298. doi: 10.1038/sj.bmt.1701568. [DOI] [PubMed] [Google Scholar]
- 64.Owen RG, Patmore RD, Smit GM, Barnard DL. Cytomegalovirus-induced T-cell proliferation and the development of progressive multifocal leucoencephalopathy following bone marrow transplantation. Br J Haematol. 1995;89:196–198. doi: 10.1111/j.1365-2141.1995.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg SL, Pecora AL, Alter RS, et al. Unusual viral infections (progressive multifocal leukoencephalopathy and cytomegalovirus disease) after high-dose chemotherapy with autologous blood stem cell rescue and peritransplantation rituximab. Blood. 2002;99:1486–1488. doi: 10.1182/blood.v99.4.1486. [DOI] [PubMed] [Google Scholar]
- 66.Osorio S, de la Cámara R, Colbano N, et al. Progressive multifocal leukoencephalopathy after stem cell transplantation, unsuccessfully treated with cidofovir. Bone Marrow Transplant. 2002;30:963–966. doi: 10.1038/sj.bmt.1703704. [DOI] [PubMed] [Google Scholar]
- 67.Buckanovich RJ, Liu G, Stricker C, et al. Nonmyeloablative allogeneic stem cell transplantation for refractory Hodgkin's lymphoma complicated by interleukin-2 responsive progressive multifocal leukoencephalopathy. Ann Hematol. 2002;81:410–413. doi: 10.1007/s00277-002-0481-4. [DOI] [PubMed] [Google Scholar]
- 68.Steurer M, Clausen J, Gotwald T, et al. Progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation and posttransplantation rituximab. Transplantation. 2003;76:435–448. doi: 10.1097/01.TP.0000078897.11633.5F. [DOI] [PubMed] [Google Scholar]
- 69.Gabriel IH, Olavarria E, Jones RR, et al. Graft versus lymphoma effect after early relapse following reduced-intensity sibling allogeneic stem cell transplantation for relapsed cytotoxic variant of mycosis fungoides. Bone Marrow Transplant. 2007;40:401–403. doi: 10.1038/sj.bmt.1705741. [DOI] [PubMed] [Google Scholar]
- 70.Focosi D, Fazzi R, Montanaro D, et al. Progressive multifocal leukoencephalopathy in a haploidentical stem cell transplant recipient: a clinical, neuroradiological and virological response after treatment with risperidone. Antiviral Res. 2007;74:156–158. doi: 10.1016/j.antiviral.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Kharfan-Dabaja MA, Ayala E, Greene J, et al. Two cases of progressive multifocal leukoencephalopathy after allogeneic hematopoietic cell transplantation and a review of the literature. Bone Marrow Transplant. 2007;39:101–107. doi: 10.1038/sj.bmt.1705548. [DOI] [PubMed] [Google Scholar]
- 72.Yasuda Y, Yabe H, Inoue H, et al. Progressive multifocal leukoencephalopathy after allogeneic bone marrow transplantation for Wiskott-Aldrich syndrome. Pediatr Int. 2008;50:238–240. doi: 10.1111/j.1442-200X.2008.02549.x. [DOI] [PubMed] [Google Scholar]
- 73.Sheikh SI, Stemmer-Rachamimov A, Attar EC. Autopsy diagnosis of progressive multifocal leukoencephalopathy with JC virus-negative CSF after cord blood stem-cell transplantation. J Clin Oncol. 2009;27:e46–e47. doi: 10.1200/JCO.2009.22.4451. [DOI] [PubMed] [Google Scholar]
- 74.Chowdhary S, Chamberlain M. A progressive neurologic disorder with multiple CNS lesions: a neuroimaging clinicopathologic correlation. Progressive multifocal leukoencephalopathy (PML) J Neuroimaging. 2008;18:340–344. doi: 10.1111/j.1552-6569.2007.00106.x. [DOI] [PubMed] [Google Scholar]
- 75.Pelosini M, Focosi D, Rita F, et al. Progressive multifocal leukoencephalopathy; report of three cases in HIV-negative hematological patients and review of the literature. Ann Hematol. 2002;87:405–412. doi: 10.1007/s00277-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 76.Fianchi L, Colosimo C, De Luca A, et al. Atypical presentation of progressive multifocal leukoencephalopathy in a multiple myeloma patient after auto-SCT successfully treated with combination therapy. Bone Marrow Transplant. 2010;45:1668–1670. doi: 10.1038/bmt.2010.33. [DOI] [PubMed] [Google Scholar]
- 77.Tuccori M, Focosi D, Maggi F, et al. Progressive multifocal leukoencephalopathy: a report of three cases in HIV-negative patients with non-Hodgkin's lymphomas treated with rituximab. Ann Hematol. 2010;89:519–522. doi: 10.1007/s00277-009-0819-2. [DOI] [PubMed] [Google Scholar]
- 78.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 2009;60:3761–3765. doi: 10.1002/art.24966. [DOI] [PubMed] [Google Scholar]
- 80.De Luca A, Ammassari A, Cingolani A, et al. Disease progression and poor survival of AIDS-associated progressive multifocal leukoencephalopathy despite highly active antiretroviral therapy. AIDS. 1998;12:1937–1938. [PubMed] [Google Scholar]
- 81.Amend KL, Turnbull B, Foskett N, et al. Incidence of progressive multifocal leukoencephalopathy in patients without HIV. Neurology. 2010;75:1326–1332. doi: 10.1212/WNL.0b013e3181f73600. [DOI] [PubMed] [Google Scholar]


