Abstract
In this large, sham-controlled, randomized trial, we examined the efficacy of the combination of standard treatment and paraspinous lidocaine injection compared with standard therapy alone in subjects with chronic low back pain. There is little research-based evidence for the routine clinical use of paraspinous lidocaine injection for low back pain. A total of 378 subjects with nonspecific chronic low back pain were randomized to 3 groups: paraspinous lidocaine injection, analgesics, and exercises (group 1, LID-INJ); sham paraspinous lidocaine injection, analgesics, and exercises (group 2, SH-INJ); and analgesics and exercises (group 3, STD-TTR). A blinded rater assessed the study outcomes at 3 time points: baseline, after treatment, and after 3 months of follow-up. There were increased frequency of pain responses and better low back functional scores in the LID-INJ group compared with the SH-INJ and STD-TTR groups. These effects remained at the 3-month follow-up but differed between all 3 groups. There were significant changes in pain threshold immediately after treatment, supporting the effects of this intervention in reducing central sensitization. Paraspinous lidocaine injection therapy is not associated with a higher risk of adverse effects compared with conventional treatment and sham injection. Its effects on hyperalgesia might correlate with changes in central sensitization.
Keywords: Randomized clinical trial, paraspinous lidocaine injection, nonspecific chronic low back pain, evidence-based medicine, central sensitization
Chronic low back pain is a leading cause of disability28 and a major cause of health and socioeconomic problems in Western societies.29 It is defined as low back pain that persists for ≥3 months. Whereas patients with chronic low back pain constitute a minority of low back pain cases, they are responsible for 70% to 80% of its annual costs, estimated at $50 billion.9 Thus, in addition to being a major health problem in modern society, it is a significant socioeconomic challenge.10
Although there are several treatments for chronic nonspecific low back pain,1 few have demonstrated efficacy, most of which have limited effects. There are 2 main categories of treatment: pharmacological and nonpharmacological. Although nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for short-term symptomatic relief24 there are insufficient data to suggest that they provide long-term pain relief.
Trigger point injections of lidocaine have been widely used in clinical settings for various chronic pain syndromes8; however, there are few data to support their use in nonspecific chronic low back pain. Proper but limited evidence comes from trials that have used this technique to treat fibromyalgia,26 pelvic,15 and myofascial8 pain. Indeed, to our knowledge there are no randomized clinical trials testing lidocaine injections in patients with low back pain.
The principal goal of paraspinous lidocaine injection in patients with chronic low back pain is to induce spinal segmental desensitization. Recent studies have shown that plastic changes in the central and peripheral nervous systems mediate the genesis and maintenance of magnified chronic pain.
Thus, therapeutic approaches that modulate the nervous system, rather than merely interfere with inflammatory pathways, might be more effective in managing chronic pain. Similar to poststroke patients, in whom maladaptive plastic changes at the cortical level impair functional outcomes, mechanical nociceptive stimuli at the spinal segmental level can promote local spinal cord changes, as in cortical maladaptive plasticity. These changes sensitize facilitating pain of combined origin: musculoskeletal and neuropathic.2,19
On the basis of the mechanism of action of paraspinous lidocaine injection and its potential therapeutic effects, an evaluation of this intervention for nonspecific chronic low back pain in a properly powered and designed, controlled clinical trial is warranted. We conducted a randomized, single-blind, parallel (with an allocation ratio of 1:1:1), controlled trial to determine the analgesic and functional effects of paraspinous lidocaine injection in patients with chronic nonspecific low back pain, hypothesizing that lidocaine injections would effect greater reductions in pain compared with control treatments.
Methods
Study Population and Inclusion Criteria
This trial was conducted in the Department of Rehabilitation, Hospital das Clinicas, University, of Sao Paulo Medical School, one of the largest rehabilitation centers in Latin America. The trial was initiated in January of 2007 and closed to enrollment in January of 2013. We included 381 patients with a diagnosis of chronic nonspecific low back pain who were referred from various clinics in São Paulo that were linked to this rehabilitation center. Thus, patients were referred primarily by physiatrists, general practitioners, neurologists, orthopedic surgeons, and physiotherapists.
Patients were included if they had a diagnosis of nonspecific low back pain (defined as pain below the 12th rib and above the gluteal folds, with no other diagnosis for at least 6 months) per the following inclusion and exclusion criteria: 1) age between 20 and 60 years; 2) clinical symptoms of vertebral pain that is unresponsive to symptomatic treatment with anti-inflammatory drugs for 3 months6; 3) moderate to severe pain, with a visual analog scale (VAS) score >428; 4) diagnosis of chronic nonspecific low back pain (as defined previously); 5) absence of severe psychiatric disease that requires psychiatric care28; 6) absence of neurological disorders (lumbosciatic pain); 7) absence of concurrent fibromyalgia, per the 1990 diagnostic criteria of the American Academy of Rheumatology31; 8) absence of concurrent rheumatic disease; 9) no history of allergy to lidocaine (used for blocks); 10) no history of surgery on the lumbar spine; 11) subjects seeking disability insurance from government due to pain were not included; and 12) informed consent to participate in the study and availability to visit the clinic for treatment and evaluations.
This study was approved by the Research Ethics Committee of the Clinics Hospital of University of São Paulo Medical School (CAPPesq 840/07). Patients were included after reading and signing an informed consent form. The trial was registered at the Brazilian National Registry (www.ensaiosclinicos.gov.br) and also at the World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02387567).
Interventions and Randomization
Participants were randomized to receive 1) paraspinous lidocaine injection (LID-INJ) and standard treatment, or 2) sham lidocaine injection (SH-INJ) and standard treatment, or 3) standard treatment only (STD-TTR). Randomization was performed using a computerized random number generator. We performed a simple randomization, on the basis of the large number of subjects. The randomization list was prepared by an investigator who was independent of patient care and also recruitment of subjects. This list was sealed in opaque envelopes and was revealed only after receipt of the consent form and a baseline assessment.
In the lidocaine injection group (LID-INJ), paraspinous lidocaine was injected weekly at the affected spinal segmental level with 3 mL 1%diffuse lidocaine infusion, performed by experienced physicians (M.I., S.T.I., L.G.O.T., L.C.O.T., I.D.R.), for 3 consecutive weeks. We used the standard technique of identifying the most painful spot by palpation of a ‘taut band.’ The taut band was identified using the thumb and the index finger. Needling depth was approximately 3 to 3.5 cm.
We used 3.7-cm 27-gauge disposable needles for the injection and for infiltration and needling of the involved muscles. In addition, standard treatment was prescribed as described in the next paragraph.
In the sham injection group (SH-INJ), weekly stimulation of the nonsensitized thoracic territorywas performed with the tip of a needle, without its introduction or the infusion of any local anesthetic. Standard treatment was prescribed.
For standard treatment only (STD-TTR), patients were instructed to perform exercises for the lumbar spine at home 3 times daily. Despite the various therapeutic options, we followed Chou and Huffman,7 who recommend, on the basis of their demonstrated benefits, the use of analgesics and NSAIDs, in association with maneuvers of self-care, such as keeping active, recommended to patients during the physician consultation and in information leaflets. Unlike acute cases, for which there is evidence for the use of superficial thermotherapy, there is no evidence for chronic cases. We opted for simple analgesics, such as acetaminophen, because of the risks of chronic use of at least 3 consecutive weeks of NSAIDs. The exercises consisted of stretching of hamstring, lumbar paraspinous, quadratus lumborum, iliopsoas, besides relaxation of gluteus medius and minimus and strengthening exercises of the gluteus maximus and medius muscles. The patients were to perform each exercise in front of the examiner at every follow-up assessment. At each visit, patients brought a diary with the number of exercises that were performed daily to ensure patient adherence. Patients were also prescribed simple analgesics (acetaminophen 2 g/d). Patients with an allergy to or restrictions for acetaminophen were prescribed dipyrone at an equivalent dose.
Assessments
The evaluations were performed by an independent and blinded appraiser (F.A., T.F. and R.B.N.) before treatment, after 1 week of the end of the 3 applications of injections (or at the same time point considering the standard treatment only), and also 3 months after the end of the applications (see Figure 1). Baseline assessments consisted of a demographic and baseline clinical assessment (sex, age, occupation, duration of pain [months], pain intensity, associated diseases, and usual occupation) and a physical examination (measurements of weight and height were taken to calculate body mass index).
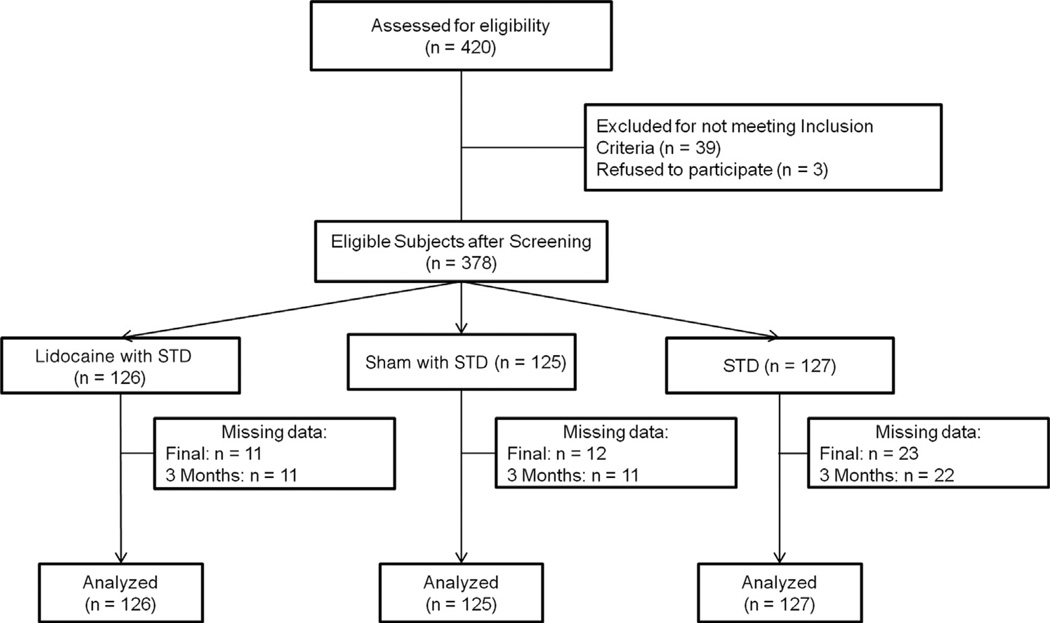
Figure 1.
Consolidated Standards of Reporting Trials 2010 patient flow diagram. Abbreviation: STD, standard treatment.
Primary Outcome Measure
The primary outcome measure was the VAS score for pain. The VAS comprised a 10-cm ruler numbered from 0 to 10, with 0 corresponding to no pain and 10 corresponding to maximum pain. Patients were asked to rate their average pain in the preceding 24 hours.
Secondary Outcome Measures
We also measured low back pain using the Brazilian Roland-Morris tool, which consisted of a specific questionnaire to assess function in patients with low back pain and has been validated in Brazil. Scores range from 0 to 24, wherein higher scores reflect greater disability due to low back pain.20
On the basis of the rationale that lidocaine injection for chronic low back pain alters central sensitization, we measured the pain pressure threshold (PPT) as an indication of central sensitization.13 The tolerance threshold pressure in the gluteus maximus, medius, and minimus; piriformis; quadratus lumborum; iliopsoas; lumbar spinous ligaments above T12 to L1 to S2 to S3; and to the “pinch-and-roll” maneuver on subcutaneous cellular tissue of L1 to S2 was measured using a pressure algometer.11 The subjects reported the amount of pressure that could be tolerated in kgf/cm2, in which lower values reflect greater pain. The method has been validated and is reproducible.11
Finally, we measured any unfavorable symptom, regardless of its relationship to treatment, during the treatment period and considered it an adverse effect.
Statistical Analysis
Sample size calculation was performed using data from a pilot study with 35 patients, considering 5% significance and 80% power. Using these pilot data and attrition rate (estimated to be 10%), 378 subjects would be needed (or 126 subjects per group).
Statistical analyses were performed using STATA 12 (StataCorp LP, College Station, TX) separately for each clinical outcome assessment (VAS, Roland-Morris, PPT). Initially, demographic and baseline clinical data were analyzed to assess differences between groups (Table 1). Thus, the quantitative characteristics of the patients were described in the 3 treatment groups with summary measures (mean, SD, median, minimum, and maximum) and compared between groups using analysis of variance (ANOVA) and pairwise comparison to evaluate changes over time. Categorical characteristics were also evaluated in the 3 groups, described using absolute and relative frequencies, and checked for association using a χ2 or likelihood ratio test. We used descriptive statistics and a histogram to verify that the data were normally distributed.
Table 1.
Demographic Characteristics According to Treatment Group
| Characteristic |
LID-INJ, Mean/SD |
SH-INJ, Mean/SD |
STD-TTR, Mean/SD |
| Age | 48.26/8.49 | 47.91/8.52 | 48.01/9.48 |
| Weight | 75.21/15.34 | 74.20/15.28 | 71.76/13.43 |
| Height | 1.63/.092 | 1.63/.084 | 1.64/.0885 |
| BMI | 28.04/5.43 | 27.98/5.92 | 26.74/4.77 |
| Pain duration, mo | 81.57/69.73 | 94.07/88.03 | 86.70/73.10 |
| VAS for pain at baseline | 7.1/1.5 | 7.0/1.5 | 7.0/1.6 |
| n/% | n/% | n/% | |
| Sex | |||
| Male | 39/30.95 | 37/29.60 | 46/36.22 |
| Female | 87/69.05 | 88/70.40 | 81/63.78 |
| Total | 126/100 | 125/100 | 127/100 |
| Race | |||
| Mixed | 43/34.13 | 37/29.60 | 40/31.50 |
| Caucasian | 66/52.28 | 70/56 | 72/56.69 |
| African American | 16/12.70 | 18/14.40 | 14/11.02 |
| Asian | 1/.79 | - | 1/.79 |
| Total | 126/100 | 125/100 | 127/100 |
Abbreviation: BMI, body mass index.
For the main outcome, we analyzed the VAS score as the response rate (defined as at least a 30% change in VAS score) and compared differences in the frequency of response between treatment groups using Fisher exact test.
For the Brazilian Roland-Morris assessment, we treated this outcome as a continuous outcome and thus ran mixed ANOVA models, using Roland-Morris scores (differences compared with baseline) as the dependent variable and subject identifier, time of assessment, and group of treatment as independent variables. Because we considered the differences between after treatment versus baseline we did not calculate the interaction term between time and group.
To analyze differences in algometry pressure values at various points, we first considered only the points that were ipsilateral to the pain; for patients with bilateral pain, we calculated mean pressure values at each point. The pressure at each point was reported in the 3 treatment groups and in respective time points with summary measures, and 2-way ANOVA with repeated measures was performed, assuming the correlation matrix between the evaluation time points to have an autoregressive order of 1. The tests were performed with a 2-tailed significance level of 5%. Fig 2 and Tables 1, 2 and 3 report data of all randomized subjects and the method of last observation carried forward was used when necessary for missing data.
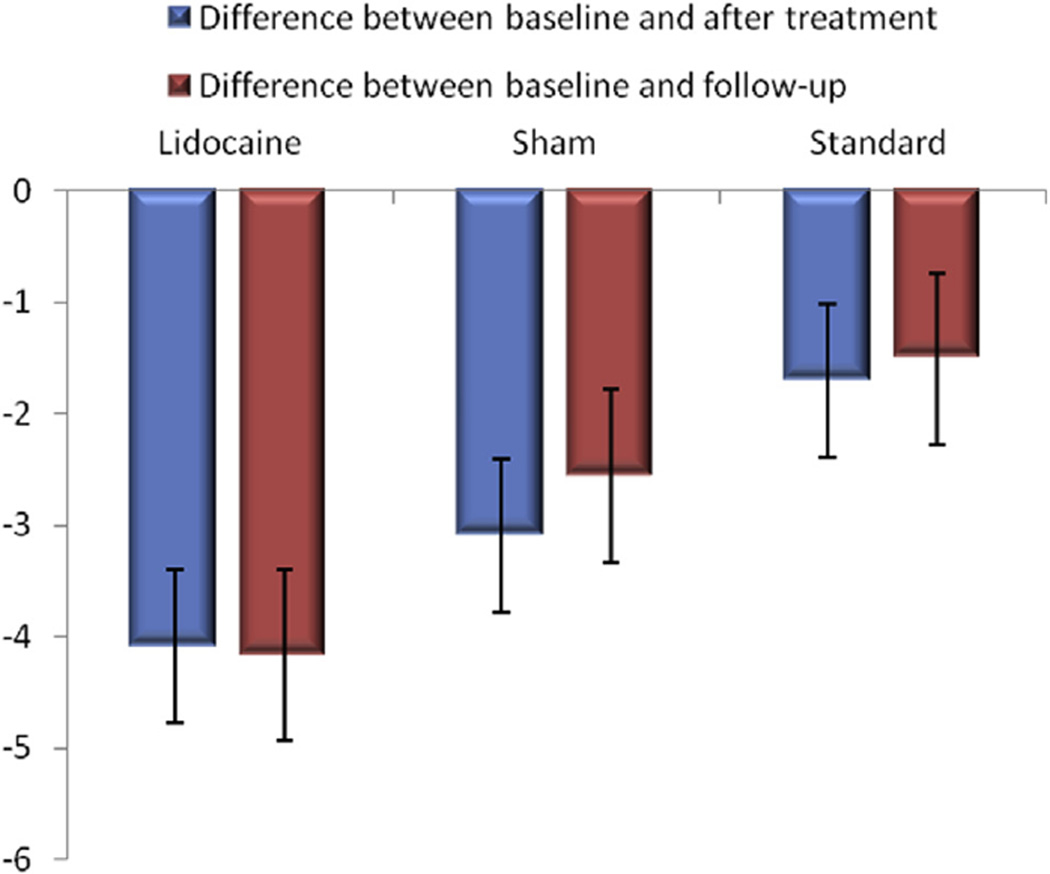
Figure 2.
Differences between baseline and after treatment and between baseline and follow-up according to treatment group.
Table 2.
Pain Response Rate and VAS Pain Across Treated Groups
| Intervention | ||||
|---|---|---|---|---|
| LID-INJ | SH-INJ | STD-TTR | ||
| Variable | (N = 126) | (N = 125) | (N = 127) | P* |
| Pain response rate, % (n) | ||||
| End of treatment | 71.4 (90) | 55.6 (70) | 53.9 (68) | .004 |
| Follow-up period (3 months) |
56.3 (71) | 49.6 (62) | 40.1 (51) | .036 |
| Mean VAS for pain (SD) | ||||
| Baseline | 7.1 (1.5) | 7.0 (1.5) | 7.0 (1.6) | |
| End of treatment | 3.9 (2.5) | 4.9 (2.5) | 4.6 (2.7) | |
| Follow-up period (3 months) |
4.4 (2.7) | 4.9 (2.4) | 4.9 (2.7) | |
NOTE. Response was defined as 30% decrease in the VAS score after intervention.
Fisher exact test.
Table 3.
PPT Differences (From Baseline) Per Segment Across Treatment Groups
| Segment | Main Factor Treatment, P |
Time/Treatment Interaction, P |
LID-INJ, P | LID-INJ§ | SH-INJ, P | SH-INJ§ | STD-TTR, P | STD-TTR§ |
|---|---|---|---|---|---|---|---|---|
| T12 to L1 | .0000 | .1219 | .0025† | .93 | .5719 | .3 | .0933 | .49 |
| L1 to L2 | .0000 | .2117 | .0127* | .86 | .4715 | .4 | .1054 | .37 |
| L2 to L3 | .0000 | .2634 | .0078† | .82 | .4477 | .34 | .1187 | .51 |
| L3 to L4 | .0001 | .2072 | .0191* | .78 | .7233 | .23 | .2380 | .42 |
| L4 to L5 | .0000 | .0411* | .0010† | .92 | .6693 | .16 | .2963 | .29 |
| L5 to S1 | .0000 | .0967 | .0047† | .97 | .6838 | .26 | .1204 | .43 |
| S1 to S2 | .0000 | .0050† | .0004‡ | 1.05 | .9148 | .05 | .1526 | .44 |
| S2 to S3 | .0000 | .0130* | .0008‡ | .94 | .8372 | −.08 | .3623 | .3 |
NOTE. P values from ANOVA models and post hoc comparisons.
P ≤ .05.
P ≤ .005.
P ≤ .001.
Mean difference in PPT pre-post kgf/cm2.
Results
A total of 378 subjects participated in the study; 45 patients did not complete the protocol because of issues that were unrelated to the study protocol, such as lack of transportation, inability to schedule appointments, and personal problems (the number of dropouts per group was as follows: 11 in the LID-INJ; 12 in the SH-INJ, and 22 in the STD-TTR; Fig 1). The missing data were analyzed with the intention-to-treat method using the last measurement carried forward. These details are also shown in our Consolidated Standards of Reporting Trials flow chart (Fig 1). The demographic characteristics of the sample are presented in Table 1.
Primary Outcome: Pain Response
There was a significant difference in response rate (decrease of at least 30% in VAS score compared with baseline) between groups (Fisher exact test, P = .004). In the LID-INJ group, 71.4% (90 of 126) of patients were responders, significantly more than subjects in the SH-INJ group (54.4%, 68 of 125, P = .006), and STD–TTR-treated patients(53.5%, 68 of 127, P = .004; Table 2).
On the basis of these results, the number needed to treat27 at the end of treatment was 5.6 (comparing LID-INJ with STD-TTR); thus, for approximately every 6 patients, 1 would achieve at least a 30% reduction in pain after paraspinous lidocaine injection that would not have occurred with standard treatment alone. Similar results were obtained in the comparison with SH-INJ (number needed to treat = 5.9).
Response rates in the follow-up differed significantly between groups (Fisher exact test, P = .036). However, overall response rates were smaller compared with immediately after treatment, especially for the LID-INJ and SH-INJ groups (LID-INJ, 56.3%; SH-INJ, 49.6%; STD-TTR, 40.2%; Table 2).
Secondary Assessment: Brazilian Roland-Morris
With regard to the Roland-Morris assessment (Brazilian version), we analyzed whether changes immediately after and at the follow-up assessment differed between treatment groups in a mixed model. We found a significant group effect when analyzing differences from baseline between groups (P < .001) but no effect of time (P = .40), indicating that the differences between groups immediately after treatment and at follow-up were significant but similar between immediately after treatment and at follow-up.
Comparing LID-INJ versus SH-INJ and LID-INJ versus STD-TTR, LID-INJ was associated with significantly better functional scores compared with SH-INJ (P < .001) and STD-TTR (P < .001; Fig 2).
Secondary Assessment: PPT
This assessment confirmed the differences between treatment groups. Table 3 presents the results per ligament segment using repeated measures ANOVA and Bonferroni correction with time (baseline, after intervention, and follow-up) and the interaction between treatment group and time. We noted a clear effect of paraspinous lidocaine injection on PPT on each ligament segment (P < .05 for all segments between after vs before treatment for LID-INJ only). In contrast, SH-INJ and STD-TTR were unable to reach statistical significance. These results also support our hypothesis that paraspinous lidocaine injection reduces central sensitization compared with other treatments.
Adverse Events and Safety
Overall, patients tolerated the paraspinous lidocaine injection well. The frequency of adverse effects did not differ significantly between treatment groups (P = .29) and we report in the next paragraph the main adverse effects according to group of treatment.
In the LID-INJ group, we observed 1 case of vagal syncope, which subsided after 40 minutes of bed rest. There were 2 cases of local hematoma, 2 cases of pain at the injection site, and 1 case of worsening pain. One patient developed high blood pressure, because he had halted his antihypertensive medication.
In the STD-TTR group, 2 patients had referred epigastric pain due to paracetamol use; 1 complained of bitterness in the mouth after treatment; and 1 patient complained of headache, seizure, and tremor.
In the SH-INJ group, 2 patients presented with intolerance to paracetamol; the dosage was reduced in 1 patient, and the medication for the other was changed to dipyrone.
Discussion
Weekly paraspinous lidocaine injections, in combination with standard treatment, resulted in significantly greater frequencies of pain response and better low back functional scores compared with sham injection with standard treatment and standard treatment alone. These effects subsided at the 3-month follow-up assessment but remained significant between treatment groups. There were significant changes in pain threshold immediately after treatment, also supporting the efficacy of this intervention in reducing central sensitization.
Although paraspinous lidocaine injection is commonly used in clinical settings, to our knowledge, no study has examined its effects in the treatment of chronic nonspecific low back pain. Thus, our study provides important evidence to support the clinical use of this intervention, particularly in light of the many available treatments for pain control that are usually associated with modest effects in improving pain, function, and quality of life. Also, considering our sample size and inclusion criteria, our trial provides a reasonable external validity for our findings.
Although for acute low back pain the use of simple painkillers or NSAIDs in association with self-care and educational measures is recommended,23 the efficacy of pharmacological interventions in chronic low back pain is limited.3 When these agents are insufficient, a combination of nonpharmacological procedures with proven benefits, such as intensive interdisciplinary rehabilitation, therapeutic exercises, acupuncture, massage therapy, spinal manipulation, yoga, cognitive behavioral therapy, and progressive relaxation, is recommended.23 We have shown that the combination of paraspinous lidocaine injection with standard therapy is superior compared with standard therapy alone (standard pharmacological and nonpharmacological therapies [analgesic and exercises]). During the 3-week study period, we observed a significant reduction in pain intensity and improved functional capacity with regard to low back pain in all study groups—despite complaints of pain for over 3 months. In addition, response rate varies significantly across different studies of chronic low back pain and some factors such as patient characteristics, number of sessions, and intervention dosage may influence the response rate.5,14,17,18
The mechanisms of pain reduction after paraspinous lidocaine injection are unknown. Because chronic pain can lead to peripheral and central sensitization25 and on the basis of the superior effects of this treatment in reducing hyperalgesia, as indexed by changes in pain threshold, it is likely that this modality alters central sensitization. A trial that examined the analgesic and antihyperalgesic effects of lidocaine injection in patients with fibromyalgia syndrome reported that versus saline injection, the main effect of this treatment was decreasing pain thresholds.26 In fact, any intervention that can change excitability of the peripheral or central nervous system can ultimately have an effect in mechanisms of central sensitization. In this context, lidocaine injection can enhance these effects on the basis of its local effect of peripheral nerve excitability. On the basis of this potential mechanism of action, it is conceivable that more sessions of lidocaine injection will be associated with greater effects.
Clinical Implications
Although the number needed to treat that was associated with paraspinous block was moderate (approximately 6 in various comparisons), because of the safe profile and low cost of this technique, it is an important therapy that should be considered. Paraspinous lidocaine injection does not require the use of imaging techniques to guide its implementation. Further, it has been used for other conditions such as myofascial pain, fibromyalgia, and pelvic pain, and the trials have shown positive results.
For instance, in a study on pelvic pain, the mean pain score decreased by 44.7% after obturator externus injection of lidocaine compared with before injection. In this trial, 82% of patients (19 of 23) had good or excellent ratings in satisfaction score during the 2 weeks after treatment. There were no complications from lidocaine muscle injection.16
Despite our initial results, the benefit of this procedure should be examined further, because its operating cost is low and because its effects develop within 3 weeks and are long-lasting (up to 3 months after completion). In general, patients tolerated the paraspinous lidocaine injection well. We observed 1 case of vagal syndrome with syncope, which subsided after 40 minutes of bed rest. There were 2 cases of local hematoma, 2 cases of pain at the injection site, and 1 case of worsening pain. Like Chou and Huffman,7 we believe that in chronic cases, the association of therapeutic interventions is the most appropriate strategy.
Study Limitations
This study has some limitations that need to be entertained. First, the study cannot be considered fully double-blind because patients randomized to the standard treatment condition were aware that they did not receive local anesthetic injections. Therefore it is possible that a placebo-related phenomenon is increased in the conditions that have injection (sham or active) because of the “therapeutic ritual” of the intervention by itself. In addition, because of the challenges with placebo injection, the method we used (ie, no substance was injected, subjects were only “needled” above the level of the back pain) may not have resulted in a perfect placebo condition. Therefore, it is possible that some of the results of this study may be explained by a placebo response. The role of expectation and anticipation and the clinical benefit these 2 conditions play has been well documented when conscious or subconscious physiological functions are involved,4 therefore, the observed placebo effect might be the result of an induced association between the sham injection with the expectation of pain relief. However, considering the large responses in the other groups also, it is conceivable that true treatment effect is observed. In fact, other trials in chronic pain subjects reported response rates in the placebo group (in addition to the standard treatment) that were similar to those in our study. In these studies the placebo response varied from 40% to 49%.12,22,30 Therefore, it is conceivable that the placebo effects may in fact enhance the effects of the standard treatment suggesting the standard treatment effects may also be optimized and have an effect beyond that of no treatment.
Another important limitation is subject adherence to the standard treatment: in this trial, a combination of exercises and simple analgesics. Although we asked the patients to record the exercises routine in a diary, we did not collect these data to analyze adherence to the exercise regimen. Therefore, it is possible that the standard treatment, especially the exercise component, may not have been optimized, thus limiting the effects of the standard therapy. The final important limitation is the number of dropouts. Forty-five subjects dropped out of this trial (approximately 12% of the sample). Although this number may be considered relatively large, it is in the lower margin compared with other clinical trials21,32 and also we used intention-to-treat analysis to account for dropouts.
Conclusions
In this sham-controlled clinical trial, paraspinous lidocaine injection was superior to sham injection and standard treatment alone. Paraspinous lidocaine injection is an effective and safe treatment option when administered by an experienced physician. Pain thresholds, measured using algometry, can be considered an effective clinical tool to monitor the response to treatment in patients with chronic low back pain, especially when assessing the effects on maladaptive plasticity that is associated with chronic pain.
Perspective.
There are few data to support paraspinous lidocaine injection use in patients with nonspecific chronic low back pain. Our results show that this therapy when combined with standard therapy significantly increases the number of responders versus standard treatment alone. Its effects on hyperalgesia might correlate with a change in central sensitization.
Acknowledgments
Authors are grateful to Denise A.S. Pinheiro and Joyce M. Alvarenga Reis for their help with data collection and overall coordination of the trial. Authors are also grateful to Alejandra Malavera for her help with formatting and editing of the manuscript.
This study was supported by the Physical and Rehabilitation Medicine Institute, Clinics Hospital of University of São Paulo Medical School. Dr. Morales-Quezada received funding support from an Institutional National Research Service Award from the National Center for Complementary and Integrative Health grant T32AT000051, the Ryoichi Sasakawa Fellowship Fund, and by the Program in Placebo Studies at Beth Israel Deaconess Medical Center.
Footnotes
Clinical Trial Registration: NCT02387567.
The authors have no conflicts of interest to declare.
References
- 1.Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion AF, Reis S, Staal JB, Ursin H, Zanoli G. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade DC, Borges I, Bravo GL, Bolognini N, Fregni F. Therapeutic time window of noninvasive brain stimulation for pain treatment: inhibition of maladaptive plasticity with early intervention. Expert Rev Med Devices. 2013;10:339–352. doi: 10.1586/erd.12.90. [DOI] [PubMed] [Google Scholar]
- 3.Balague F, Ochoa Amaya G, Genevay S. Conservative treatment of chronic low back pain: What is new in 2008? [in French] Rev Med Suisse. 2009;5:560–562. 564. [PubMed] [Google Scholar]
- 4.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93:1207–1246. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghi B, Aurini L, White PF, Mordenti A, Lolli F, Borghi R, Martignani M, Greggi T. Long-lasting beneficial effects of periradicular injection of meloxicam for treating chronic low back pain and sciatica. Minerva Anestesiol. 2013;79:370–738. [PubMed] [Google Scholar]
- 6.Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Huffman LH. Medications for acute and chronic low back pain: A review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–514. doi: 10.7326/0003-4819-147-7-200710020-00008. [DOI] [PubMed] [Google Scholar]
- 8.Couto C, de Souza IC, Torres IL, Fregni F, Caumo W. Paraspinal stimulation combined with trigger point needling and needle rotation for the treatment of myofascial pain: A randomized sham-controlled clinical trial. Clin J Pain. 2014;30:214–223. doi: 10.1097/AJP.0b013e3182934b8d. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin D, Conrad D, Volinn E. Cost, controversy, crisis: Low back pain and the health of the public. Annu Rev Public Health. 1991;12:141–156. doi: 10.1146/annurev.pu.12.050191.001041. [DOI] [PubMed] [Google Scholar]
- 10.Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. Phantom pain and risk factors: A multivariate analysis. J Pain Symptom Manage. 2002;24:578–585. doi: 10.1016/s0885-3924(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 11.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30:115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 12.Frerick H, Keitel W, Kuhn U, Schmidt S, Bredehorst A, Kuhlmann M. Topical treatment of chronic low back pain with a capsicum plaster. Pain. 2003;106:59–64. doi: 10.1016/s0304-3959(03)00278-1. [DOI] [PubMed] [Google Scholar]
- 13.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, Cutait MM, Fregni F, Camanho GL. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Rheum. 2008;59:1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 14.Kalita J, Kohat AK, Misra UK, Bhoi SK. An open labeled randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. J Neurol Sci. 2014;342:127–132. doi: 10.1016/j.jns.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Kim DS, Jeong TY, Kim YK, Chang WH, Yoon JG, Lee SC. Usefulness of a myofascial trigger point injection for groin pain in patients with chronic prostatitis/chronic pelvic pain syndrome: A pilot study. Arch Phys Med Rehabil. 2013;94:930–936. doi: 10.1016/j.apmr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Kim DH, Yoon DM, Yoon KB. Clinical effectiveness of the obturator externus muscle injection in chronic pelvic pain patients. Pain Pract. 2015;15:40–46. doi: 10.1111/papr.12138. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Lee CS. A randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of the extended-release tramadol hydrochloride/acetaminophen fixed-dose combination tablet for the treatment of chronic low back pain. Clin Ther. 2013;35:1830–1840. doi: 10.1016/j.clinthera.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Machado LA, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Analgesic effects of treatments for nonspecific low back pain: A meta-analysis of placebo-controlled randomized trials. Rheumatology (Oxford) 2009;48:520–527. doi: 10.1093/rheumatology/ken470. [DOI] [PubMed] [Google Scholar]
- 19.Moseley GL, Flor H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabil Neural Repair. 2012;26:646–652. doi: 10.1177/1545968311433209. [DOI] [PubMed] [Google Scholar]
- 20.Nusbaum L, Natour J, Ferraz MB, Goldenberg J. Translation, adaptation and validation of the Roland-Morris questionnaire–Brazil Roland-Morris. Braz J Med Biol Res. 2001;34:203–210. doi: 10.1590/s0100-879x2001000200007. [DOI] [PubMed] [Google Scholar]
- 21.Palangio M, Morris E, Doyle RT, Dornseif BE, Valente TJ. Combination hydrocodone and ibuprofen versus combination oxycodone and acetaminophen in the treatment of moderate or severe acute low back pain. Clin Ther. 2002;24:87–99. doi: 10.1016/s0149-2918(02)85007-x. [DOI] [PubMed] [Google Scholar]
- 22.Pappagallo M, Breuer B, Lin HM, Moberly JB, Tai J, Noto C, Sanchez A, Manfredi PL. A pilot trial of intravenous pamidronate for chronic low back pain. Pain. 2014;155:108–117. doi: 10.1016/j.pain.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis S, Lahad A. Clinical guidelines for diagnosis and treatment of acute low back pain [in Hebrew] Harefuah. 2007;146:631–635. 644. [PubMed] [Google Scholar]
- 24.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: An updated Cochrane review. Spine. 2008;33:1766–1774. doi: 10.1097/BRS.0b013e31817e69d3. [DOI] [PubMed] [Google Scholar]
- 25.Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13:513–520. doi: 10.1007/s11926-011-0206-6. [DOI] [PubMed] [Google Scholar]
- 26.Staud R, Weyl EE, Bartley E, Price DD, Robinson ME. Analgesic and anti-hyperalgesic effects of muscle injections with lidocaine or saline in patients with fibromyalgia syndrome. Eur J Pain. 2014;18:803–812. doi: 10.1002/j.1532-2149.2013.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephan H, Groger P, Weyland A, Hoeft A, Sonntag H. The effect of sufentanil on cerebral blood flow, cerebral metabolism and the CO2 reactivity of the cerebral vessels in man [in German] Anaesthesist. 1991;40:153–160. [PubMed] [Google Scholar]
- 28.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waddell G, Burton AK. Concepts of rehabilitation for the management of low back pain. Best Pract Res Clin Rheumatol. 2005;19:655–670. doi: 10.1016/j.berh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Wen W, Sitar S, Lynch SY, He E, Ripa SR. A multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of single-entity, once-daily hydrocodone tablets in patients with uncontrolled moderate to severe chronic low back pain. Expert Opin Pharmacother. 2015;16:1593–1606. doi: 10.1517/14656566.2015.1060221. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 32.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]