Abstract
Rationale: Primary graft dysfunction (PGD) is a significant cause of early morbidity and mortality after lung transplant and is characterized by severe hypoxemia and infiltrates in the allograft. The pathogenesis of PGD involves ischemia-reperfusion injury. However, subclinical increases in pulmonary venous pressure due to left ventricular diastolic dysfunction may contribute by exacerbating capillary leak.
Objectives: To determine whether a higher ratio of early mitral inflow velocity (E) to early diastolic mitral annular velocity (é), indicative of worse left ventricular diastolic function, is associated with a higher risk of PGD.
Methods: We performed a retrospective cohort study of patients in the Lung Transplant Outcomes Group who underwent bilateral lung transplant at our institution between 2004 and 2014 for interstitial lung disease, chronic obstructive pulmonary disease, or pulmonary arterial hypertension. Transthoracic echocardiograms obtained during evaluation for transplant listing were analyzed for E/é and other measures of diastolic function. PGD was defined as PaO2/FiO2 less than or equal to 200 with allograft infiltrates at 48 or 72 hours after reperfusion. The association between E/é and PGD was assessed with multivariable logistic regression.
Measurements and Main Results: After adjustment for recipient age, body mass index, mean pulmonary arterial pressure, and pretransplant diagnosis, higher E/é and E/é greater than 8 were associated with an increased risk of PGD (E/é odds ratio, 1.93; 95% confidence interval, 1.02–3.64; P = 0.04; E/é >8 odds ratio, 5.29; 95% confidence interval, 1.40–20.01; P = 0.01).
Conclusions: Differences in left ventricular diastolic function may contribute to the development of PGD. Future trials are needed to determine whether optimization of left ventricular diastolic function reduces the risk of PGD.
Keywords: left ventricular function, primary graft dysfunction, lung transplant, diastolic heart failure, pulmonary hypertension
At a Glance Commentary
Scientific Knowledge on the Subject
Primary graft dysfunction is a form of acute lung injury that occurs within 72 hours of lung transplant and remains a significant source of morbidity and mortality after transplant. The pathogenesis of primary graft dysfunction involves ischemia-reperfusion injury. However, subclinical increases in pulmonary venous pressure due to left ventricular diastolic dysfunction may contribute by exacerbating capillary leak.
What This Study Adds to the Field
Our study identifies left ventricular diastolic function as an independent risk factor for the development of primary graft dysfunction. These findings suggest that optimization of left ventricular diastolic function could reduce the risk of primary graft dysfunction after transplant.
The most important cause of early morbidity and mortality after lung transplant is primary graft dysfunction (PGD), a form of acute lung injury characterized by severe hypoxemia and infiltrates in the lung allograft within 72 hours of transplant (1). The syndrome occurs in 10–30% of lung transplant recipients and is thought to be partially mediated by capillary leak induced by ischemia-reperfusion injury (IRI). Grade 3 PGD is the most severe form and significantly lengthens the duration of mechanical ventilation, increases the risk of chronic lung allograft dysfunction, and quadruples the risk of death in the early post-transplant period (2–9).
While the diagnosis of PGD requires the exclusion of cardiac etiologies as the main source of pulmonary edema, it is unclear whether abnormal diastolic function and higher left ventricular (LV) filling pressures can worsen capillary leak from IRI. Elevated hydrostatic pressure worsens fluid leak in other forms of noncardiogenic edema, including acute respiratory distress syndrome (10–12). Furthermore, many of the advanced lung diseases treated with lung transplant are associated with a high prevalence of diastolic dysfunction, ranging from 20% to 70% (13–19). Multiple mechanisms may mediate the development of abnormal diastolic function in chronic lung diseases (16, 19–27). Chronically increased right ventricular (RV) afterload, RV dysfunction, and lung hyperinflation decrease LV preload, which can lead to LV atrophy and abnormal myocyte relaxation (16, 19, 21–27). Given the high prevalence of abnormal diastolic function in this population, as well as the association between higher LV filling pressures and other forms of acute lung injury, it is possible that higher LV end-diastolic pressure and pulmonary venous pressure after lung reperfusion could contribute to capillary leak and PGD.
Assessment of diastolic function has improved with the introduction of tissue Doppler imaging (TDI) on transthoracic echocardiograms (TTEs). Many of the traditional measurements of diastolic function, including early (E) and late (A) transmitral inflow velocity and deceleration time (DT), are dependent on loading conditions, which prevents accurate assessment of abnormal relaxation (28, 29). The ratio of E to mitral annular velocity (é) (E/é) is relatively immune to the effect of elevated filling pressures and correlates with the time constant for isovolumic relaxation, best reflecting delayed relaxation. Higher ratios of E/é correlate with more severe diastolic dysfunction and higher filling pressures. A cutoff of E/é greater than 8 has a high sensitivity for detecting diastolic dysfunction (29–31).
We aimed to determine the association of LV diastolic function at lung transplant evaluation with the risk of postoperative PGD. We hypothesized that higher E/é, suggestive of worse LV diastolic function, would be associated with a higher risk of grade 3 PGD. Some of the results of this study were reported previously in the form of an abstract (32).
Methods
We performed a retrospective cohort study of patients who underwent lung transplant at the Hospital of the University of Pennsylvania.
Study Population and Study Sample
The study population was enrolled from within the LTOG (Lung Transplant Outcomes Group), a multicenter prospective cohort of subjects aged 18–80 years from 11 U.S. transplant centers. The study sample included subjects in the LTOG who underwent initial bilateral lung transplant for interstitial lung disease (ILD), chronic obstructive pulmonary disease (COPD), or pulmonary arterial hypertension (PAH) at the Hospital of the University of Pennsylvania (one center of the LTOG) between June 2004 and June 2014. We included only bilateral lung transplant recipients to provide the cleanest phenotype of PGD (eliminating the potential contribution of a native lung). Subjects were excluded if they had concomitant LV systolic dysfunction with an LV ejection fraction of less than 50%, atrial fibrillation or flutter, or moderate to severe mitral regurgitation at the time of transplant evaluation, given the effect of these on the assessment of diastolic function (29). Subjects were also excluded if they had undergone combined heart-lung transplant or did not have available preoperative TTEs.
Patients at our center undergo routine testing during evaluation for lung transplant. Patients underwent right heart catheterization at rest. Pulmonary function and 6-minute walk distance tests were performed according to American Thoracic Society/European Respiratory Society guidelines (33, 34).
Echocardiogram
Standard two-dimensional and Doppler TTEs were performed with the patient in the supine and left lateral decubitus positions. The digital images were analyzed on TomTec computer workstations (TomTec Imaging Systems, Unterschleissheim, Germany) by two trained and certified cardiovascular research technicians in the Center for Quantitative Echocardiography at the University of Pennsylvania. The readers were blinded to clinical outcome. Intrarater and interrater reliability was assessed on 20% of the TTEs.
Left atrial, RV, and LV areas and volumes were measured at end diastole and end systole. Right atrial pressure on TTEs was categorized as 3, 8, and 15 mm Hg per the American Society of Echocardiography guidelines (35). RV function on TTE was assessed with tricuspid annular plane systolic excursion, RV fractional area change, and qualitative means. LV diastolic function was assessed on the basis of the following echocardiographic parameters collected according to the American Society of Echocardiography guidelines (29): E/A, DT, left atrial volume, and E/é. LV diastolic dysfunction was also identified as lateral é less than 10 cm/s or E/é greater than 8 (29–31).
Outcome Definition
PGD was graded by two readers using the PaO2/FiO2 ratio and the presence of allograft infiltrates as recommended in the International Society for Heart and Lung Transplantation guidelines with adjudication (1). PGD was defined as grade 3 PGD (PaO2/FiO2 ratio ≤200 with allograft infiltrates) at 48 or 72 hours after reperfusion. Subjects who died within 72 hours of transplant and fulfilled PGD criteria were defined as having grade 3 PGD. Mortality data were extracted from outpatient and inpatient charts (M.K.P.). All graders of outcomes were blinded to other clinical data and TTE data.
Covariates
Potential risk factors for grade 3 PGD or diastolic function previously identified in the literature or hypothesized to have clinical and/or biologic plausibility were included for analysis a priori (6, 8, 36–43). Recipient demographic, laboratory, spirometry, walk distance, echocardiography, and hemodynamic variables were evaluated for inclusion in the multivariable analysis.
Statistical Analysis
Continuous variables were summarized using the mean and SD or median and interquartile range (IQR). Categorical variables were summarized by frequency and percentage. Differences in TTE and hemodynamic characteristics between those with and without PGD were assessed with Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test, as appropriate. Bivariate analysis was used to evaluate the association of demographic, laboratory, pulmonary function, TTE, and hemodynamic parameters with grade 3 PGD at 48 or 72 hours. Recipient demographic, laboratory, spirometry, walk distance, echocardiography, and hemodynamic variables with a P value greater than 0.20 were included in the multivariable model. Among the variables evaluated for inclusion in the multivariable model were recipient age; race; body mass index (BMI); pulmonary diagnosis; FEV1; total lung capacity; history of hypertension; supplemental oxygen use with exertion; E/A; E/é; é; DT; left atrial diastolic volume; RV fractional area change; tricuspid annular plane systolic excursion; right atrial pressure categorized as 3, 8, and 15 mm Hg; mean pulmonary arterial pressure (mPAP); pulmonary vascular resistance; and cardiac index. If variables were collinear, the variable with the strongest correlation with PGD was included in multivariable analysis.
Multivariate logistic regression models were used to determine the association of diastolic function with grade 3 PGD. The effect of independent variables on the odds of PGD was displayed as a fraction of the SD to allow for comparison between variables. The association between diastolic function and 1-year mortality was assessed with multivariable logistic regression after adjusting for known PGD confounders and secondarily by Cox proportional hazards modeling with censoring at 1 year. Reliability was assessed using intraclass correlation coefficients (ICCs). A P value less than 0.05 indicated statistical significance. Statistical analyses were performed using STATA software version 12.0 (StataCorp LP, College Station, TX). Power calculations showed that our study would have greater than 90% power to detect 1 SD difference in E/é between those with and without grade 3 PGD with our sample size, assuming an estimated PGD incidence of 20%.
All participants provided written informed consent upon enrollment in the LTOG. The study protocol was approved by the Hospital of the University of Pennsylvania Institutional Review Board (IRB 819927).
Results
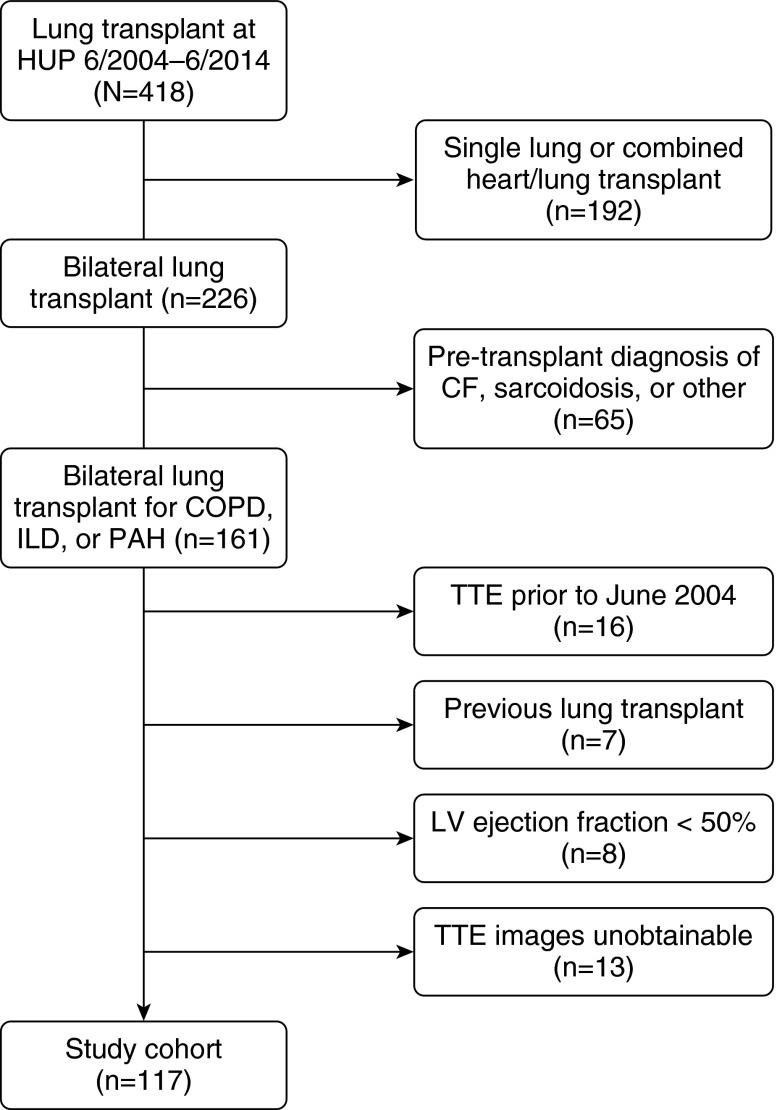
During the study period, 161 subjects underwent bilateral lung transplant for COPD, ILD, or PAH at our center. Of those, 16 had TTEs before June 2004, when our institution started performing TDI; 7 had previously received transplants; 8 had a qualitative LV ejection fraction less than 50%; and 13 did not have available images for review. Thus, our study sample ultimately included 117 subjects (73%) (Figure 1, Table 1; see also Table E1 in the online supplement). There were no differences in age, sex, race, or grade 3 PGD between the study sample and those excluded. Among subjects excluded for concomitant LV systolic dysfunction, previous lung transplant, or unobtainable TTE images, COPD was more common and PGD risk was possibly lower (Table E1).
Figure 1.
Study cohort. CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; HUP = Hospital of the University of Pennsylvania; ILD = interstitial lung disease; LV = left ventricular; PAH = pulmonary arterial hypertension; TTE = transthoracic echocardiogram.
Table 1.
Characteristics of the Cohort (N = 117) Stratified by Primary Graft Dysfunction Status
| Covariate | PGD (n = 30) | Non-PGD (n = 87) | P Value |
|---|---|---|---|
| Donor characteristics | |||
| Smoke exposure, n (%) | 7 (28.0) (n = 25) | 12 (16.0) (n = 75) | 0.19 |
| Cause of death, n (%) | n = 27 | n = 75 | 0.06 |
| Head trauma | 12 (44.4) | 27 (36.0) | |
| Anoxia | 0 (0.0) | 16 (21.3) | |
| Stroke | 12 (44.4) | 28 (37.3) | |
| Other | 3 (11.1) | 4 (5.3) | |
| Recipient characteristics | |||
| Age, yr | 58.0 [52.2–60.5] | 56.5 [49.5–60.5] | 0.60 |
| Male sex, n (%) | 20 (66.7) | 56 (64.4) | 0.82 |
| Race/ethnicity, n (%) | 0.14 | ||
| Caucasian | 22 (73.3) | 75 (86.2) | |
| African American | 7 (23.3) | 8 (9.2) | |
| Other | 1 (3.3) | 4 (4.6) | |
| Body mass index, kg/m2 | 29.7 ± 5.0 | 26.6 ± 4.9 | 0.004 |
| History of hypertension, n (%) | 15 (50.0) | 26 (29.9) | 0.05 |
| Use of antihypertensive or diuretic, n (%) | 19 (63.3) | 30 (34.5) | 0.006 |
| History of diabetes, n (%) | 6 (20.0) | 12 (13.8) | 0.42 |
| History of coronary artery disease, n (%) | 11 (36.7) | 32 (36.8) | 0.99 |
| History of obstructive sleep apnea, n (%) | 4 (13.3) | 9 (10.3) | 0.65 |
| Supplemental O2 with exertion, L | 10 [6–100] | 5.5 [3–10] (n = 86) | 0.009 |
| Six-minute walk distance, ft | 965.8 ± 278.8 | 1046.4 ± 351.3 (n = 86) | 0.26 |
| Pretransplant diagnosis, n (%) | 0.001 | ||
| Chronic obstructive pulmonary disease | 6 (20.0) | 49 (56.3) | |
| Interstitial lung disease | 19 (63.3) | 35 (40.2) | |
| Pulmonary arterial hypertension | 5 (16.7) | 3 (3.5) | |
| Pulmonary function tests | |||
| FVC, % predicted | 49 [40–73] | 51 [42–63] (n = 86) | 0.83 |
| FEV1, % predicted | 46.5 [31–63] | 29.5 [17–50] (n = 86) | 0.003 |
| FEV1/FVC | 84 [56–86] | 38 [26–85] | 0.02 |
| TLC, % predicted | 75 [54–110] (n = 26) | 100 [58–125] (n = 69) | 0.10 |
| DlCO, % predicted | 30.5 [21–38.5] (n = 16) | 34 [27–47] (n = 49) | 0.18 |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; PGD = primary graft dysfunction; TLC = total lung capacity.
Data are given as mean ± SD, median [interquartile range], or n (%).
The intrareader ICCs for chamber volume measurements by TTE were 0.72–0.99; for Doppler measurements, they were 0.75–0.95; and for TDI, they were 0.73–0.97. The interreader ICCs were 0.85–0.99 for Doppler measurements and 0.94–0.99 for TDI. These data show very high intra- and interreader reliability of TTE measures. Those with missing TTE data were similar to those with complete data (data not shown).
The subjects’ mean age was 55.6 ± 7.2 years, and most were men (65.0%) and white (82.9%). The pretransplant diagnoses were COPD in 55 subjects (47.0%), ILD in 54 (46.2%), and PAH in 8 (6.8%). Forty-one (35.0%) had hypertension, 18 (15.4%) had diabetes, and 13 (11.1%) had obstructive sleep apnea. Forty-one (35.0%) had coronary artery disease. Forty-nine (41.9%) were on antihypertensive or diuretic therapy at the time of evaluation, and 15 (12.8%) were on pulmonary vasodilators. Pulmonary hypertension (PH) was present in 76 subjects (65.0%). Thirty (25.6%) developed grade 3 PGD. The median duration between evaluation TTE and right heart catheterization was 2 days (IQR, 1–48). The median duration between TTE and transplant was 189 days (IQR, 103–382).
Patients with grade 3 PGD had higher BMI, were more likely to have PAH (and thus higher FEV1) and ILD, and more commonly had systemic hypertension (Table 1). Patients with grade 3 PGD also had significantly higher mPAP and pulmonary vascular resistance, and they tended to have a lower cardiac index (Table 2).
Table 2.
Echocardiographic and Hemodynamic Characteristics, Stratified by Primary Graft Dysfunction Status
| PGD (n = 30) | Non-PGD (n = 87) | P Value | |
|---|---|---|---|
| Echocardiographic characteristics | |||
| E/é | 6.8 [4.4–9.5] (n = 26) | 5.8 [4.5–7.1] (n = 69) | 0.27 |
| E/é >8, n (%) | 10 (38.5) (n = 26) | 10 (14.5) (n = 69) | 0.006 |
| E/A ratio | 0.9 [0.7–1.1] (n = 28) | 1.0 [0.9–1.2] (n = 78) | 0.08 |
| Lateral é velocity, cm/s | 11.0 ± 0.7 (n = 27) | 11.8 ± 0.4 (n = 73) | 0.32 |
| Lateral é velocity <10 cm/s, n (%) | 9 (33.3) (n = 27) | 22 (30.1) (n = 73) | 0.76 |
| Deceleration time, ms | 156.5 ± 49.0 (n = 28) | 161.7 ± 44.8 (n = 78) | 0.61 |
| Left atrial diastolic volume, ml | 26.1 [15.8–35.2] (n = 26) | 21 [13.9–28] (n = 71) | 0.07 |
| Left ventricular ejection fraction, % | 65 [60–70] | 65 [55–65] | 0.56 |
| Right ventricular fractional area change, % | 37.2 ± 13.9 (n = 28) | 45.1 ± 12.9 (n = 75) | 0.009 |
| TAPSE, cm | 2.2 ± 0.6 (n = 21) | 2.1 ± 0.4 (n = 54) | 0.01 |
| Estimated right atrial pressure | n = 23 | n = 74 | 0.11 |
| 3 mm Hg | 15 (65.2) | 60 (81.1) | |
| 8 mm Hg | 5 (21.7) | 12 (16.2) | |
| 15 mm Hg | 3 (13.0) | 2 (2.7) | |
| Hemodynamic characteristics | |||
| Mean pulmonary arterial pressure, mm Hg | 36 [25–45] | 26 [20–33] | 0.001 |
| PCWP, mm Hg | 11 [8–15] | 12 [8–16] (n = 86) | 0.45 |
| Pulmonary vascular resistance, Wood units | 3.6 [2.4–8.5] | 2.5 [1.7–3.4] (n = 83) | <0.001 |
| Cardiac output, L/min | 5.4 ± 1.9 | 5.6 ± 1.4 (n = 84) | 0.64 |
| Cardiac index, L/min/m2 | 2.7 ± 0.7 | 3.0 ± 0.7 (n = 84) | 0.07 |
| Presence of pulmonary hypertension, n (%) | 24 (80.0) | 52 (59.8) | 0.05 |
Definition of abbreviations: A = late mitral inflow velocity; é = early mitral annular velocity; E = early mitral inflow velocity; PCWP = pulmonary capillary wedge pressure; PGD = primary graft dysfunction; TAPSE = tricuspid annular plane systolic excursion.
Data are given as mean ± SD, median [interquartile range], or n (%).
Table 3 shows unadjusted and adjusted odds ratios (ORs) for the association of measures of diastolic function with grade 3 PGD. After adjustment for age, BMI, mPAP, and pretransplant diagnosis, higher E/é was independently associated with an increased risk of grade 3 PGD (OR, 1.93; 95% confidence interval [CI], 1.02–3.64; P = 0.04). The presence of E/é greater than 8 was also independently associated with a higher risk of grade 3 PGD (OR, 5.29; 95% CI, 1.40–20.01; P = 0.01) after adjustment for age, BMI, mPAP, and diagnosis. Further adjustment for recipient hypertension or diabetes, and for albumin at time of evaluation and transplant, did not affect the results (data not shown). In addition, albumin at evaluation or before transplant did not modify the relationship between diastolic function and PGD (P for interaction > 0.19 for both), indicating that the relationship between diastolic function and PGD was not affected by albumin level. Cardiopulmonary bypass was used in the majority of procedures in the sample (88.9%) and did not significantly change the effect of diastolic dysfunction on PGD in our final multivariable model (OR for E/é, 1.92; 95% CI, 1.02–3.63 [after adjustment for cardiopulmonary bypass use]; vs. OR, 1.93; 95% CI, 1.02–3.64 [without adjustment]). Inclusion of ischemia time (mean, 320.47 ± 75.6 min) and sex did not significantly alter our findings (OR for E/é, 1.95; 95% CI, 1.02–3.73; P = 0.04 [after adjustment for ischemia time]; OR for E/é, 1.89; 95% CI, 1.00–3.61; P = 0.05 [after adjustment for sex]). Among those with PH in our study (with a much smaller sample size), E/é still appeared to be associated with PGD (adjusted OR, 2.32; 95% CI, 0.98–5.47; P = 0.06; n = 76). Secondary measures of diastolic function were not associated with the risk of PGD.
Table 3.
Logistic Regression Models for the Effect of Measures of Diastolic Function on Primary Graft Dysfunction Risk
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| E/é, per 1 SD increase* | |||
| Unadjusted | 1.50 | 0.95–2.37 | 0.08 |
| Adjusted† | 1.93 | 1.02–3.64 | 0.04 |
| E/lateral é ratio >8 | |||
| Unadjusted | 3.69 | 1.31–10.39 | 0.01 |
| Adjusted† | 5.29 | 1.40–20.01 | 0.01 |
| Lateral é < 10 cm/s | |||
| Unadjusted | 1.15 | 0.45–2.98 | 0.76 |
| Adjusted† | 1.84 | 0.55–6.15 | 0.32 |
| Deceleration time, per 1 SD increase* | |||
| Unadjusted | 0.89 | 0.57–1.38 | 0.60 |
| Adjusted† | 1.06 | 0.58–1.94 | 0.84 |
| E/A ratio, per 1 SD increase* | |||
| Unadjusted | 0.69 | 0.42–1.13 | 0.14 |
| Adjusted† | 0.65 | 0.35–1.21 | 0.18 |
| LA diastolic volume, per 1 ml increase | |||
| Unadjusted | 1.04 | 1.00–1.09 | 0.08 |
| Adjusted† | 0.98 | 0.92–1.04 | 0.57 |
Definition of abbreviations: A = late mitral inflow velocity; CI = confidence interval; E = early mitral inflow velocity; é = early mitral annular velocity; LA = left atrial.
SD of E/é, 3.01; E/A, 0.33; deceleration time, 45.77.
Adjusted for recipient age, body mass index, pulmonary diagnosis, and mean pulmonary arterial pressure.
Nineteen subjects died within 1 year of transplant. Higher E/é was associated with a borderline significantly increased risk of 1-year mortality after adjustment for recipient age, BMI, mPAP, and pretransplant diagnosis (OR per 1 SD increase, 1.68; 95% CI, 0.94–3.00; P = 0.08). Using Cox proportional hazards modeling, higher E/é was associated with a significantly increased risk of death (with censoring at 1 yr) (hazard ratio per 1 SD increase, 1.17; 95% CI, 1.02–1.34; P = 0.03).
Discussion
In our retrospective cohort study of patients with ILD, COPD, and PAH, worse LV diastolic function during evaluation for lung transplant was associated with a significantly increased risk of grade 3 PGD and worse 1-year post-transplant survival. Abnormal LV diastolic function increased the risk of PGD independently of previously studied recipient risk factors for PGD (e.g., higher BMI, pulmonary diagnosis, and higher mPAP). To our knowledge, this study is the first to demonstrate an independent effect of LV diastolic function on the development of PGD. Researchers in previous studies have not identified an association between diastolic function and PGD, possibly because they used measures of diastolic function that were dependent on loading conditions (44). However, E/é is independent of loading conditions, is strongly correlated with invasive conductance catheter assessment of LV stiffness and relaxation, and may more clearly capture diastolic function (29, 30).
Chronically elevated RV afterload, RV dysfunction, and lung hyperinflation result in relative underfilling of the left ventricle in advanced lung disease. Animal models and human case series have demonstrated that, in response to a chronically reduced LV preload, there is a decrease in the LV myocyte cross-sectional area, a prolongation of LV action potential, and a decrease in the calcium-ATPase pump involved in myocyte relaxation, resulting in impaired LV relaxation and diminished compliance (16, 19, 21–24, 45). In addition, the left ventricle’s ability to relax may be further impaired perioperatively by the release of proinflammatory cytokines in PGD, including IL-6, which leads to a reduction in sarcoplasmic reticulum Ca2+-ATPase (46–48). The left ventricle appears to undergo reverse remodeling following restoration of preload over time; however, this adaptation is not immediate, and abnormal filling may persist up to 1 year after transplant (16, 23, 49–54). Therefore, it is possible that acute and chronic impairment in LV diastolic function may contribute to pulmonary edema and PGD.
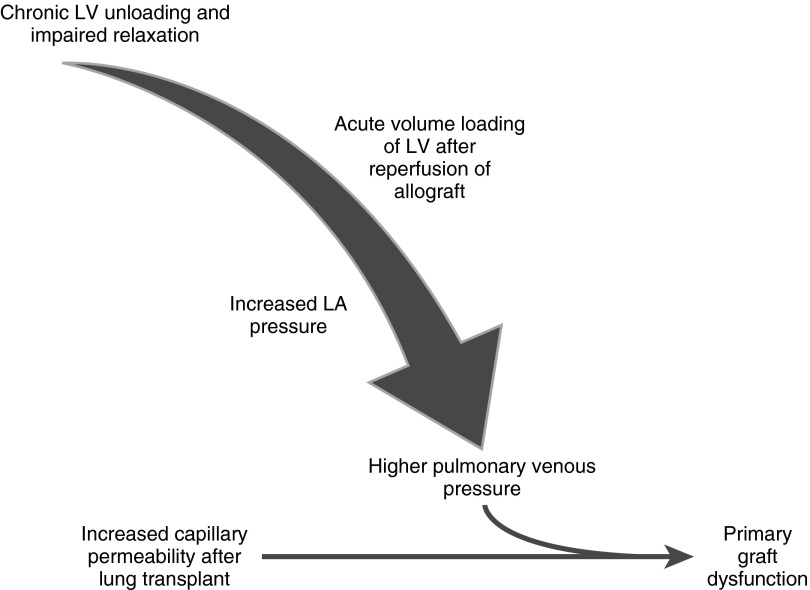
Restoration of LV preload to a stiff left ventricle following allograft reperfusion may increase pulmonary venous pressure and exacerbate IRI-induced noncardiogenic edema (Figure 2). Increased hydrostatic pressure has been shown to promote fluid and solute flux across damaged pulmonary microvascular membrane in acute lung injury (10–12). Sibbald and colleagues demonstrated that higher pulmonary capillary wedge pressure (PCWP) resulted in a greater increase in extravascular lung water content in patients with acute respiratory distress syndrome than in those with cardiogenic pulmonary edema (10). Therefore, elevated filling pressures due to impaired diastolic function may increase extravasation of fluid across endothelium damaged by IRI and contribute to PGD. In earlier studies of PGD, researchers used a PCWP cutoff ranging from 12 to 18 mm Hg to exclude cardiogenic edema. The measurement of the PCWP not only is inaccurate in a postoperative patient receiving mechanical ventilation but also is potentially hazardous in the post–lung transplant patient with solely pulmonary arterial blood supply (1, 55, 56). Furthermore, the capillary pressure at which pulmonary edema occurs in the setting of increased alveolocapillary permeability is unknown, but it is likely lower than in the setting of intact endothelium. Regardless of this threshold, our results suggest that LV diastolic function contributes to the development of PGD. While previous work suggested that plasma albumin is inversely related to pulmonary vascular permeability in patients at risk for acute lung injury (57, 58), the association between diastolic function and PGD was not affected by albumin level in our study.
Figure 2.
Proposed mechanism of diastolic dysfunction in primary graft dysfunction. LA = left atrial; LV = left ventricular.
Our data demonstrate the importance of the LV in the early post-transplant period and may suggest preventative strategies for PGD. Several centers have found that postoperative venoarterial extracorporeal membrane oxygenation (VA-ECMO) can reduce the risk of PGD in certain high-risk groups, including those with PH (59, 60). VA-ECMO may allow for controlled LV filling and recovery, preventing acute increases in pulmonary venous pressure in the early period of IRI. Tudorache and colleagues weaned patients off VA-ECMO when the left atrial pressure no longer increased in response to a decrease in VA-ECMO flow rate, suggesting that the left ventricle was able to accommodate the normalized preload (61). Okada and colleagues used β-blockers to treat recurrent episodes of mPAP elevation and pulmonary edema following bilateral lung transplantation (62). β-blockade not only reduces excessive RV contraction but may also affect LV diastolic function. Prior heart-lung transplantation studies have been inconsistent in regard to whether PGD rates are decreased, which would be expected with replacement of a native left ventricle with abnormal diastolic function.
Our study has several limitations. Our study included subjects at high risk of diastolic dysfunction, including patients with ILD, COPD, and PAH and only those with bilateral transplants as a proof of concept. Further studies should include subjects with other transplant indications as well as recipients of single lungs. The duration between TTE and transplant was approximately 6 months. Nonsystematic variability in diastolic function in the interim (or at transplant) should have biased our results to the null; therefore, the association between diastolic function and PGD may be even stronger than shown. While we adjusted for known confounders of both diastolic function and PGD in our multivariate model, residual confounding by differences in perioperative management strategies is possible. Missing TTE data are inevitable in a population with severe lung disease, owing to difficult windows. However, those with missing TTE data were similar to those with complete data, making selection bias less likely (63).
In summary, we have demonstrated that, after adjustment for confounders, worse LV diastolic function before lung transplant is associated with the development of grade 3 PGD and may exacerbate capillary leak caused by IRI. Identification of this risk factor on TTEs obtained at the time of evaluation may provide the opportunity for optimization of diastolic function before and during lung transplant. This relationship needs to be confirmed in future multicenter studies exploring the link between severity of PH and diastolic function and evaluating the role of perioperative interventions to allow for LV recovery.
Acknowledgments
Acknowledgment
The authors thank Ted Plappert, CVT, for his assistance with echocardiographic analysis.
Footnotes
Supported by National Institutes of Health grants T32 HL-007891 (M.K.P.), K24 HL-103844 (S.M.K.), K23 HL-095661 (B.K.), R01 HL-118018 (B.K.), R01 HL-087115 (J.D.C.), R01 HL-081619 (J.D.C.), R01 HL-096845 (J.D.C.), R01 HL-114626 (J.D.C.), K24 HL-115354 (J.D.C.), and K23 HL-121406 (J.M.D.) and an Actelion ENTELLIGENCE grant (J.M.D.).
Author Contributions: M.K.P. designed the study; performed data collection, analysis, and interpretation; wrote the first manuscript draft; and approved the final draft of the manuscript. B.K. and J.N.K. assisted with study design, data collection and interpretation, and manuscript revision and approved the final draft of the manuscript. R.S. contributed to data analysis and interpretation, reviewed and revised the manuscript, and approved the final draft of the manuscript. J.M.D., R.J.S., J.C.L., and J.D.C. assisted with data interpretation, reviewed and revised the manuscript, and approved the final draft of the manuscript. S.M.K. assisted with study design, contributed to data collection and interpretation, reviewed and revised the manuscript, and approved the final draft of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1522OC on January 8, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT working group on primary lung graft dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, Robinson N, Localio AR, Wille K, Lama V, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231–1239. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, Kotloff RM. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 5.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 6.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah RJ, Diamond JM, Cantu E, Lee JC, Lederer DJ, Lama VN, Orens J, Weinacker A, Wilkes DS, Bhorade S, et al. Latent class analysis identifies distinct phenotypes of primary graft dysfunction after lung transplantation. Chest. 2013;144:616–622. doi: 10.1378/chest.12-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME, Radosevich DM, Herrington CS, Dahlberg PS. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131:73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, Dahlberg PS. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Sibbald WJ, Short AK, Warshawski FJ, Cunningham DG, Cheung H. Thermal dye measurements of extravascular lung water in critically ill patients: intravascular Starling forces and extravascular lung water in the adult respiratory distress syndrome. Chest. 1985;87:585–592. doi: 10.1378/chest.87.5.585. [DOI] [PubMed] [Google Scholar]
- 11.Guyton AC. Interstitial fluid pressure: II. Pressure-volume curves of interstitial space. Circ Res. 1965;16:452–460. doi: 10.1161/01.res.16.5.452. [DOI] [PubMed] [Google Scholar]
- 12.Prewitt RM, McCarthy J, Wood LD. Treatment of acute low pressure pulmonary edema in dogs: relative effects of hydrostatic and oncotic pressure, nitroprusside, and positive end-expiratory pressure. J Clin Invest. 1981;67:409–418. doi: 10.1172/JCI110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussuges A, Pinet C, Molenat F, Burnet H, Ambrosi P, Badier M, Sainty JM, Orehek J. Left atrial and ventricular filling in chronic obstructive pulmonary disease: an echocardiographic and Doppler study. Am J Respir Crit Care Med. 2000;162:670–675. doi: 10.1164/ajrccm.162.2.9908056. [DOI] [PubMed] [Google Scholar]
- 14.Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133:1354–1359. doi: 10.1378/chest.07-2685. [DOI] [PubMed] [Google Scholar]
- 15.Gurudevan SV, Malouf PJ, Auger WR, Waltman TJ, Madani M, Raisinghani AB, DeMaria AN, Blanchard DG. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol. 2007;49:1334–1339. doi: 10.1016/j.jacc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Hardziyenka M, Campian ME, Verkerk AO, Surie S, van Ginneken AC, Hakim S, Linnenbank AC, de Bruin-Bon HA, Beekman L, van der Plas MN, et al. Electrophysiologic remodeling of the left ventricle in pressure overload-induced right ventricular failure. J Am Coll Cardiol. 2012;59:2193–2202. doi: 10.1016/j.jacc.2012.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Moustapha A, Kaushik V, Diaz S, Kang SH, Barasch E. Echocardiographic evaluation of left-ventricular diastolic function in patients with chronic pulmonary hypertension. Cardiology. 2001;95:96–100. doi: 10.1159/000047353. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos CE, Pitsiou G, Karamitsos TD, Karvounis HI, Kontakiotis T, Giannakoulas G, Efthimiadis GK, Argyropoulou P, Parharidis GE, Bouros D. Left ventricular diastolic dysfunction in idiopathic pulmonary fibrosis: a tissue Doppler echocardiographic study. Eur Respir J. 2008;31:701–706. doi: 10.1183/09031936.00102107. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest. 2012;141:1457–1465. doi: 10.1378/chest.11-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Sánchez M, Muñoz-Esquerre M, Huertas D, Gonzalez-Costello J, Ribas J, Manresa F, Dorca J, Santos S. High prevalence of left ventricle diastolic dysfunction in severe COPD associated with a low exercise capacity: a cross-sectional study. PLoS One. 2013;8:e68034. doi: 10.1371/journal.pone.0068034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorn GW, II, Robbins J, Ball N, Walsh RA. Myosin heavy chain regulation and myocyte contractile depression after LV hypertrophy in aortic-banded mice. Am J Physiol. 1994;267:H400–H405. doi: 10.1152/ajpheart.1994.267.1.H400. [DOI] [PubMed] [Google Scholar]
- 23.Hardziyenka M, Campian ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, Beekman L, Remme CA, Bresser P, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: evidence for atrophic remodeling. J Am Coll Cardiol. 2011;57:921–928. doi: 10.1016/j.jacc.2010.08.648. [DOI] [PubMed] [Google Scholar]
- 24.Manders E, Bogaard HJ, Handoko ML, van de Veerdonk MC, Keogh A, Westerhof N, Stienen GJ, Dos Remedios CG, Humbert M, Dorfmüller P, et al. Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2014;64:28–37. doi: 10.1016/j.jacc.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten SE. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007;131:1050–1057. doi: 10.1378/chest.06-2245. [DOI] [PubMed] [Google Scholar]
- 26.Smith BM, Prince MR, Hoffman EA, Bluemke DA, Liu CY, Rabinowitz D, Hueper K, Parikh MA, Gomes AS, Michos ED, et al. Impaired left ventricular filling in COPD and emphysema: is it the heart or the lungs? The Multi-Ethnic Study of Atherosclerosis COPD Study. Chest. 2013;144:1143–1151. doi: 10.1378/chest.13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138:32–38. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 28.Mottram PM, Marwick TH. Assessment of diastolic function: what the general cardiologist needs to know. Heart. 2005;91:681–695. doi: 10.1136/hrt.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 30.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 31.Oh JK, Park SJ, Nagueh SF. Established and novel clinical applications of diastolic function assessment by echocardiography. Circ Cardiovasc Imaging. 2011;4:444–455. doi: 10.1161/CIRCIMAGING.110.961623. [DOI] [PubMed] [Google Scholar]
- 32.Porteous MK, Ky B, Plappert T, Diamond JM, Shah RJ, Brown M, Christie JD, Kawut SM. Impact of diastolic dysfunction on primary graft dysfunction (PGD) after lung transplantation [abstract 15] J Heart Lung Transplant. 2015;34(4 Suppl):S14–S15. [Google Scholar]
- 33.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 34.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 35.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 36.Barr ML, Kawut SM, Whelan TP, Girgis R, Böttcher H, Sonett J, Vigneswaran W, Follette DM, Corris PA ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: recipient-related risk factors and markers. J Heart Lung Transplant. 2005;24:1468–1482. doi: 10.1016/j.healun.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 38.Kuntz CL, Hadjiliadis D, Ahya VN, Kotloff RM, Pochettino A, Lewis J, Christie JD. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23:819–830. doi: 10.1111/j.1399-0012.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 39.Lederer DJ, Kawut SM, Wickersham N, Winterbottom C, Bhorade S, Palmer SM, Lee J, Diamond JM, Wille KM, Weinacker A, et al. Lung Transplant Outcomes Group. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184:1055–1061. doi: 10.1164/rccm.201104-0728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JC, Christie JD, Keshavjee S. Primary graft dysfunction: definition, risk factors, short- and long-term outcomes. Semin Respir Crit Care Med. 2010;31:161–171. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 41.Samano MN, Fernandes LM, Baranauskas JC, Correia AT, Afonso JE, Jr, Teixeira RH, Caramori ML, Pêgo-Fernandes PM, Jatene FB. Risk factors and survival impact of primary graft dysfunction after lung transplantation in a single institution. Transplant Proc. 2012;44:2462–2468. doi: 10.1016/j.transproceed.2012.07.134. [DOI] [PubMed] [Google Scholar]
- 42.Thabut G, Vinatier I, Stern JB, Lesèche G, Loirat P, Fournier M, Mal H. Primary graft failure following lung transplantation: predictive factors of mortality. Chest. 2002;121:1876–1882. doi: 10.1378/chest.121.6.1876. [DOI] [PubMed] [Google Scholar]
- 43.Ware LB, Lee JW, Wickersham N, Nguyen J, Matthay MA, Calfee CS California Transplant Donor Network. Donor smoking is associated with pulmonary edema, inflammation and epithelial dysfunction in ex vivo human donor lungs. Am J Transplant. 2014;14:2295–2302. doi: 10.1111/ajt.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadlapati A, Lynch JP, III, Saggar R, Ross D, Belperio JA, Weigt S, Ardehali A, Grogan T, Yang EH, Aboulhosn J. Preoperative cardiac variables of diastolic dysfunction and clinical outcomes in lung transplant recipients. J Transplant. 2013;2013:391620. doi: 10.1155/2013/391620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardziyenka M, Campian ME, Bouma BJ, Linnenbank AC, de Bruin-Bon HA, Kloek JJ, van der Wal AC, Baan J, Jr, de Beaumont EM, Reesink HJ, et al. Right-to-left ventricular diastolic delay in chronic thromboembolic pulmonary hypertension is associated with activation delay and action potential prolongation in right ventricle. Circ Arrhythm Electrophysiol. 2009;2:555–561. doi: 10.1161/CIRCEP.109.856021. [DOI] [PubMed] [Google Scholar]
- 46.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94:1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 47.Wu CK, Lee JK, Chiang FT, Yang CH, Huang SW, Hwang JJ, Lin JL, Tseng CD, Chen JJ, Tsai CT. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39:984–992. doi: 10.1097/CCM.0b013e31820a91b9. [DOI] [PubMed] [Google Scholar]
- 48.Moreno I, Vicente R, Ramos F, Vicente JL, Barberá M. Determination of interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc. 2007;39:2425–2426. doi: 10.1016/j.transproceed.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 49.Jörgensen K, Houltz E, Westfelt U, Nilsson F, Scherstén H, Ricksten SE. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest. 2003;124:1863–1870. doi: 10.1378/chest.124.5.1863. [DOI] [PubMed] [Google Scholar]
- 50.Menzel T, Wagner S, Kramm T, Mohr-Kahaly S, Mayer E, Braeuninger S, Meyer J. Pathophysiology of impaired right and left ventricular function in chronic embolic pulmonary hypertension: changes after pulmonary thromboendarterectomy. Chest. 2000;118:897–903. doi: 10.1378/chest.118.4.897. [DOI] [PubMed] [Google Scholar]
- 51.Xie GY, Lin CS, Preston HM, Taylor CG, Kearney K, Sapin PM, Smith MD. Assessment of left ventricular diastolic function after single lung transplantation in patients with severe pulmonary hypertension. Chest. 1998;114:477–481. doi: 10.1378/chest.114.2.477. [DOI] [PubMed] [Google Scholar]
- 52.Rensing BJ, McDougall JC, Breen JF, Vigneswaran WT, McGregor CG, Rumberger JA. Right and left ventricular remodeling after orthotopic single lung transplantation for end-stage emphysema. J Heart Lung Transplant. 1997;16:926–933. [PubMed] [Google Scholar]
- 53.Dittrich HC, Chow LC, Nicod PH. Early improvement in left ventricular diastolic function after relief of chronic right ventricular pressure overload. Circulation. 1989;80:823–830. doi: 10.1161/01.cir.80.4.823. [DOI] [PubMed] [Google Scholar]
- 54.Tischler MD, Sutton MS, Bittl JA, Parker JD. Effects of percutaneous mitral valvuloplasty on left ventricular mass and volume. Am J Cardiol. 1991;68:940–944. doi: 10.1016/0002-9149(91)90413-f. [DOI] [PubMed] [Google Scholar]
- 55.Al-Kharrat T, Zarich S, Amoateng-Adjepong Y, Manthous CA. Analysis of observer variability in measurement of pulmonary artery occlusion pressures. Am J Respir Crit Care Med. 1999;160:415–420. doi: 10.1164/ajrccm.160.2.9808082. [DOI] [PubMed] [Google Scholar]
- 56.Leatherman JW, Shapiro RS. Overestimation of pulmonary artery occlusion pressure in pulmonary hypertension due to partial occlusion. Crit Care Med. 2003;31:93–97. doi: 10.1097/00003246-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Aman J, van der Heijden M, van Lingen A, Girbes AR, van Nieuw Amerongen GP, van Hinsbergh VW, Groeneveld AB. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39:89–97. doi: 10.1097/CCM.0b013e3181feb46a. [DOI] [PubMed] [Google Scholar]
- 58.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, III, Hoth JJ, Mikkelsen ME, Gentile NT, et al. U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS) Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereszlenyi A, Lang G, Steltzer H, Hetz H, Kocher A, Neuhauser P, Wisser W, Klepetko W. Bilateral lung transplantation with intra- and postoperatively prolonged ECMO support in patients with pulmonary hypertension. Eur J Cardiothorac Surg. 2002;21:858–863. doi: 10.1016/s1010-7940(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 60.Ius F, Kuehn C, Tudorache I, Sommer W, Avsar M, Boethig D, Fuehner T, Gottlieb J, Hoeper M, Haverich A, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;144:1510–1516. doi: 10.1016/j.jtcvs.2012.07.095. [DOI] [PubMed] [Google Scholar]
- 61.Tudorache I, Sommer W, Kühn C, Wiesner O, Hadem J, Fühner T, Ius F, Avsar M, Schwerk N, Böthig D, et al. Lung transplantation for severe pulmonary hypertension--awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation. 2015;99:451–458. doi: 10.1097/TP.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 62.Okada Y, Hoshikawa Y, Ejima Y, Matsumura Y, Sado T, Shimada K, Aikawa H, Sugawara T, Matsuda Y, Takahashi T, et al. β-blocker prevented repeated pulmonary hypertension episodes after bilateral lung transplantation in a patient with primary pulmonary hypertension. J Thorac Cardiovasc Surg. 2004;128:793–794. doi: 10.1016/j.jtcvs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1201. [Google Scholar]