Abstract
Rationale: Pregnant women with latent tuberculosis infection (LTBI) are at high risk for development of TB, especially if infected with HIV.
Objectives: To assess the performance of LTBI tests in pregnant and postpartum women infected with HIV, investigate the immunology behind discordance in pregnancy, and explore the implications for the development of postpartum TB.
Methods: We screened pregnant women in their second/third trimester and at delivery for LTBI using the tuberculin skin test (TST) and IFN-γ release assay (IGRA) (QuantiFERON Gold). A subset of antepartum women had longitudinal testing, with repeat testing at delivery and postpartum and additional cytokines measured from the IGRA supernatant. The kappa statistic and Wilcoxon rank sum test were used to determine agreement and comparison of cytokine concentrations, respectively.
Measurements and Main Results: Of 252 enrolled, 71 (28%) women had a positive IGRA but only 27 (10%) had a positive TST (P < 0.005). There was 75% agreement (kappa, 0.25). When stratified by pregnancy versus delivery, 20% had IGRA+/TST− discordance at each time point. A positive IGRA was associated with known TB contact (odds ratio, 3.6; confidence interval, 1.2–11.1; P = 0.02). Compared with IGRA+/TST+, women with IGRA+/TST− discordance had significantly less IFN-γ (1.85 vs. 3.48 IU/ml; P = 0.02) and IL-2 (46.17 vs. 84.03 pg/ml; P = 0.01). Five developed postpartum TB, of which three had IGRA+/TST− discordance during pregnancy.
Conclusions: Choice of LTBI test in pregnant women infected with HIV affects results. Pregnant women with IGRA+/TST− discordance had less IFN-γ and IL-2 than those with concordant-positive results and may represent an especially high-risk subset for the development of active TB postpartum.
Keywords: IFN-γ release assay, tuberculin skin test, pregnancy, HIV, tuberculosis
At a Glance Commentary
Scientific Knowledge on the Subject
Tuberculosis (TB) is a major killer of women of reproductive age infected with HIV. Few countries, however, follow World Health Organization recommendations to give isoniazid preventive therapy to all pregnant women infected with HIV. Instead, most treat only women who test positive for latent TB. This approach is problematic because the dynamic effects of pregnancy on latent TB tests in women infected with HIV are poorly understood.
What This Study Adds to the Field
Choice of latent TB test affects results in pregnant women infected with HIV: 20% have IFN-γ release assay–positive/tuberculin skin test–negative discordance. This discordance is associated with lower IFN-γ and IL-2 response. The magnitude of changes in IFN-γ and IL-2 response during pregnancy may impact the development of postpartum TB.
Active tuberculosis (TB) is most common in women between 15 and 45 years old, the childbearing years (1, 2). In India, the country with the highest burden of TB, 75% of cases in women are diagnosed in this age group (2). Postpartum TB incidence is also high (3–7). Because TB is a major cause of maternal mortality, especially among women infected with HIV (8, 9), understanding how pregnancy affects diagnostics and the disease course would improve TB prevention efforts in this high-risk population.
Pregnancy instigates complex changes in cell-mediated immunity (10). As pregnancy progresses, cell-mediated immune function and T-helper cell type 1 (Th1) response decline, increasing the risk or severity of several infections, including influenza and listeria (10, 11). Th1 response recovers 6–12 weeks postpartum (4, 5). Understanding of the details of these changes, particularly as they relate to TB, remains incomplete.
Our previous study of pregnant Indian women uninfected with HIV identified significant discordance between the two most commonly used latent TB infection (LTBI) tests, the tuberculin skin test (TST) and the IFN-γ release assay (IGRA) (12), which both rely on cell-mediated immunity. The differential effects of pregnancy on the tests provide a window into the immunology of pregnancy and the host’s response to TB. Understanding discordance may provide insight into the underlying complex changes that increase the risk of TB for postpartum women.
In this study, we aimed to (1) determine if TST/IGRA discordance exists in pregnant women infected with HIV in a high-burden setting, (2) assess how the performance of these tests changes with stage of pregnancy; and (3) investigate immunologic contributors to IGRA+/TST− discordance in pregnancy. We further discuss the implications for predicting postpartum TB development. Some of the results of these studies have been previously reported in the form of abstracts (13, 14).
Methods
From 2011 to 2014, we enrolled pregnant women infected with HIV into a diagnostic LTBI study at Sassoon General Hospital, a public teaching hospital affiliated with Byramjee Jeejeebhoy Government Medical College in Pune, India. Each morning, women presenting to the antenatal clinic in their late second/third trimester or to the delivery ward were approached for enrollment. The first 100 women who consented for the cross-sectional study at each site underwent TST and IGRA testing once at enrollment during pregnancy or at delivery. An additional 50 antepartum women consented to a longitudinal study to evaluate the impact of stage of pregnancy on testing. This cohort underwent repeat testing at delivery and 3 months postpartum. Women in both the cross-sectional and longitudinal studies received telephone calls every 3 months until 1 year postpartum to determine the incidence of postpartum active TB. We included women greater than or equal to 18 years old. We excluded those with a history of allergic reaction to the TST, current active TB, or an immunosuppressive condition other than HIV. The longitudinal cohort planned to deliver at Sassoon Hospital and agreed to an in-person follow-up at 3 months postpartum.
Trained counselors administered sociodemographic questionnaires, including the Household Food Insecurity Access Scale (15). Trained nurses obtained medical and obstetric histories, including HIV history, TB risk factors, and the World Health Organization (WHO)-recommended TB symptom screen (16). Women with positive screens were referred to a physician for evaluation. If active TB was excluded, the woman was eligible for enrollment.
Laboratory Testing
Trained laboratory staff obtained 3 ml of blood per enrollee for IGRA (QuantiFERON Gold In-Tube test [QGIT]; Cellestis, Valencia, CA) testing; 1 ml each for the negative control (“nil”) tube, positive mitogen control tube, and Mycobacterium tuberculosis (MTB)-specific antigen tube. In the delivery group, blood was collected within 48 hours after delivery. The test was performed in accordance with manufacturer instructions (17) at a laboratory certified by the College of American Pathologists to perform QGIT testing. ELISA was repeated on samples with indeterminate QGIT results. If the repeat sample was indeterminate, it was recorded as such. If the sample was positive or negative, that result was recorded. Laboratory staff were blinded to the subject’s clinical data, including TST results.
After phlebotomy for QGIT, trained nurses (n = 3) injected 0.1 ml (5 TU) of tuberculin purified protein derivative (Span Diagnostic Ltd., Surat, India) intradermally into the volar surface of the forearm. Outpatients were asked to return in 48–72 hours for TST interpretation by trained nurses using the ballpoint pen and ruler technique (18). Nurses were blinded to QGIT results. A compensation of INR 100 (∼$2 USD) was given to cover travel costs per local institutional review board (IRB)-recommended norms. For women enrolled at delivery, attempts were made to interpret the TST within 48–72 hours, before hospital discharge. A positive TST was defined as induration greater than or equal to 5 mm (19).
Laboratory results from both the cross-sectional and the longitudinal groups were included for antepartum analysis. Results from the tests administered to the longitudinal group at delivery were not included in the cross-sectional analysis because TSTs they received during pregnancy may have impacted subsequent results.
Remaining QGIT supernatants from nil, TB antigen, and mitogen tubes were stored at −80°C. We measured IL-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF), and IL-17a per manufacturer’s instructions for the Human Th1/Th2/Th17 Cytokine BD Cytometric Bead Array (CBA) (20) on a three-color BD FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Results were processed using FCAP Array software version 3 (BD Biosciences, San Jose, CA).
This study was approved by the Johns Hopkins University IRB, the Weill Cornell Medical College IRB, the Byramjee Jeejeebhoy Government Medical College IRB, and the Byramjee Jeejeebhoy Government Medical College Ethics Committee in India. All subjects provided written informed consent.
Statistical Analysis
The kappa statistic was used to quantify agreement between QGIT and TST. With our sample size, a two-sided 95% confidence interval (CI) for the kappa statistic would extend less than or equal to 0.17 from the observed value of kappa, assuming the true value of kappa is in the range 0.50–0.70 and the approximate prevalence of latent TB is 20–30%. For comparison of categorical variables, Fisher exact test was used. The Wilcoxon rank sum test was used for comparison of continuous variables. All P values were two-sided with statistical significance evaluated at the 0.05 alpha level. Risk factors for TST or QGIT positivity and test discordance (e.g., TST+/QGIT− or TST−/QGIT+) were calculated using a logistic regression model. From this, odds ratios (OR) with 95% CI were determined. Variables that were statistically significant or had a trend toward significance (P < 0.2) in univariate analysis or were of clinical importance were included in the multivariate analysis. All data were entered into an onsite Microsoft Access database. Analyses were performed in Stata Version 12.0 (College Station, TX) and GraphPad PRISM Version 6 (La Jolla, CA).
Results
Cross-sectional Comparison
We enrolled 252 women; 149 antepartum and 103 women at delivery. Table 1 describes their baseline characteristics. Median CD4 count was over 400 cells/mm3 and 116 (46%) were on combination three-drug antiretroviral therapy (cART), 44 (38%) of whom initiated cART during pregnancy. Median gestational age for antepartum women was 26 weeks (interquartile range [IQR], 22–31) and most (84%) had antenatal visits before enrollment. Only three (1%) women reported prior history of TB but 15 (6%) reported close contact with someone with pulmonary TB. Five (33%) reported exposure to multidrug-resistant TB. No participants had received isoniazid preventive therapy (IPT).
Table 1.
Participant Characteristics and LTBI Test Results by Time Point of Screening
| Characteristic | Antepartum (n = 149) | Delivery (n = 103) | P Value |
|---|---|---|---|
| Household sociodemographics | |||
| Urban/periurban residence, n (%) | 129 (86) | 72 (69) | 0.005* |
| House with ≤2 rooms, n (%) | 130 (87) | 85 (82) | 0.78 |
| Median adults in house | 2 (2–4) | 2 (2–5) | 0.53 |
| Median children in house | 1 (1–2) | 2 (1–2) | 0.01* |
| Personal sociodemographics, n (%) | |||
| Employed for pay | 29 (19) | 18 (17) | 0.31 |
| Education ≤fourth grade | 30 (20) | 39 (37) | 0.03* |
| Biomass cooking fuel | 15 (10) | 25 (24) | 0.002* |
| Moderate to severe food insecurity | 14 (9) | 11 (10) | 0.68 |
| Obstetric history | |||
| Gestational age | 26 (22–31) | NA | — |
| First prenatal visit, n (%) | 24 (16) | NA | — |
| First pregnancy, n (%) | 43 (29) | 39 (38) | 0.11 |
| HIV history | |||
| Median CD4 | 468 (343–606) | 447 (319–622) | 0.42 |
| cART use, n (%) | 68 (45) | 48 (46) | 0.33 |
| cART before pregnancy | 26 (18) | 18 (18) | 0.99 |
| TB risk factors, n (%) | |||
| History of TB | 1 (0.6) | 2 (2) | 0.36 |
| History of IPT | 0 (0) | 0 (0) | >0.95 |
| Close contact with TB | 12 (8) | 3 (3) | 0.11 |
| Contact with MDR-TB | 5 (3) | 0 (0) | 0.25 |
| Positive TB symptom screen | 15 (10) | 11 (11) | 0.79 |
| Household contact with TB symptoms | 12 (8) | 5 (4) | 0.31 |
| Smoker in the house | 31 (20) | 23 (22) | 0.38 |
| LTBI results | |||
| TST | 0.78 | ||
| Positive ≥5 mm, n (%) | 18 (12) | 9 (8) | |
| TST+ median induration, mm (IQR) | 16 (13–21) | 13 (12–15) | 0.19 |
| Negative, n (%) | 125 (83) | 88 (85) | |
| Did not return, n (%) | 6 (4) | 6 (6) | |
| QGIT | 0.86 | ||
| Positive, n (%) | 42 (28) | 29 (28) | |
| QGIT+ median IFN-γ, IU/ml (IQR) | 3.22 (1.26–9.79) | 1.84 (0.84–4.63) | 0.11 |
| Negative, n (%) | 103 (69) | 70 (67) | |
| Indeterminate, n (%) | 4 (2) | 4 (3) |
Definition of abbreviations: cART = combination antiretroviral therapy; IPT = isoniazid preventive therapy; IQR = interquartile range; LTBI = latent tuberculosis infection; MDR-TB = multidrug-resistant tuberculosis; NA = not applicable; QGIT = QuantiFERON TB Gold In-Tube test; TB = tuberculosis; TST = tuberculin skin test.
Statistically significant.
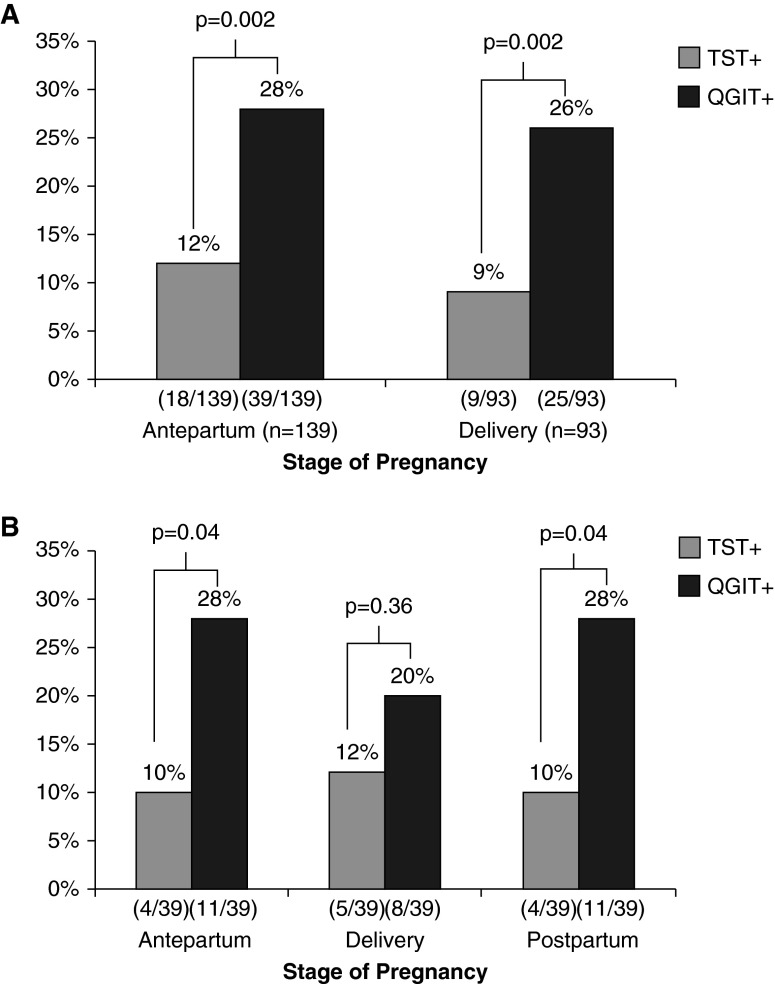
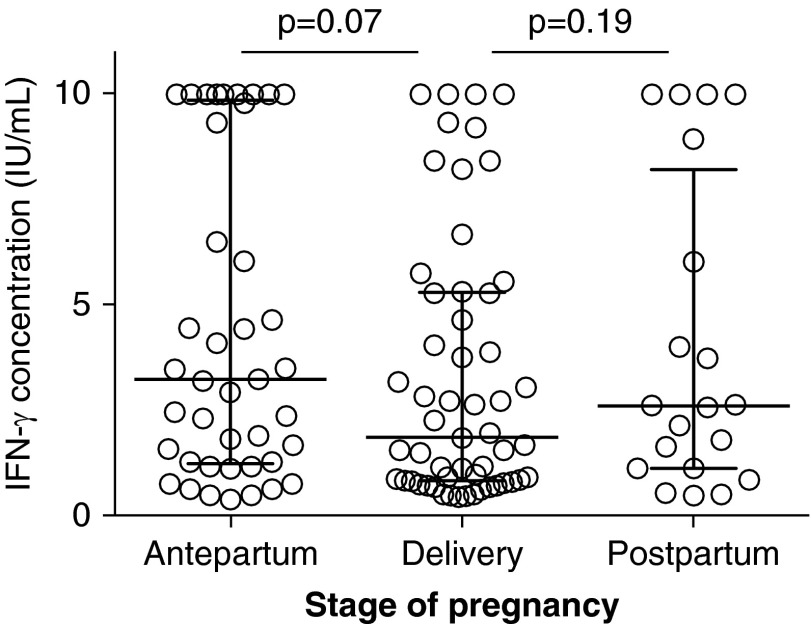
Overall, 71 (28%) women had a positive QGIT but only 27 (10%) had a positive TST (P < 0.005). Of 232 (92%) women with both a valid QGIT and TST result, the proportion positive by each test was significantly different in both the antepartum (28% QGIT+ vs. 12% TST+; P = 0.002) and delivery (26% QGIT+ vs. 9% TST+; P = 0.002) groups (Figure 1A). For those with a positive TST, the median induration was smaller in the delivery group than antepartum (13 vs. 16 mm), although this did not reach statistical significance (P = 0.19). Similarly, IFN-γ concentration of those with positive QGIT was lower at delivery than antepartum (1.80 IU/ml, IQR 0.8–5.2 vs. 3.20 IU/ml, IQR 1.2–9.6; P = 0.07) (Figure 2).
Figure 1.
Prevalence of latent tuberculosis infection by stage of pregnancy in women infected with HIV. (A) Cross-sectionally, there was insignificantly lower percent positivity of TST and QGIT at delivery versus during pregnancy but significant discordance between TST and QGIT. (B) In the longitudinal cohort, there was a decrease in QGIT-positive results at delivery with rebound postpartum, although these were not significant changes. The TST, however, mirrored the changes in the QGIT with increased positivity at delivery and a decrease postpartum, suggesting that decreased TST performance is more related to HIV than pregnancy. QGIT = QuantiFERON TB Gold In-Tube test; TST = tuberculin skin test.
Figure 2.
Although percent of QuantiFERON TB Gold In-Tube test positive remained relatively constant, there was a trend for decreased median concentration of IFN-γ between antenatal and delivery (P = 0.07), and between delivery and postpartum (P = 0.19).
Among the 12 (4%) women who did not return for TST reading, seven (58%) were QGIT positive (three antepartum, four delivery). All eight (3%) women who had indeterminate QGIT results had low IFN-γ mitogen responses and were TST negative.
In multivariate analysis controlling for CD4 and cART use, a positive QGIT was significantly associated with contact with someone with active TB (OR, 3.6; CI, 1.2–11.1; P = 0.02). Controlling for the same factors, a positive TST was not associated with any known TB risk factors.
TST and QGIT Concordance and Discordance
Overall, the TST and QGIT had 75% agreement (κ = 0.25, fair agreement). Seventeen (7%) women had concordant positive tests, 158 (68%) had concordant negative tests, and 47 (20%) had QGIT+/TST− discordance. Only 10 (4%) had QGIT−/TST+ discordance.
In the antepartum group, agreement between QGIT and TST was 74% for antepartum women (κ = 0.25, fair agreement). Eleven (8%) had concordant positive tests. Similarly, in the delivery group, there was 76% agreement (κ = 0.24, fair agreement). Only six (6%) had concordant positive tests. In each group, 20% had QGIT+/TST− discordance. Changing the cutoff for a positive test for either the TST or QGIT did not significantly affect concordance (data not shown).
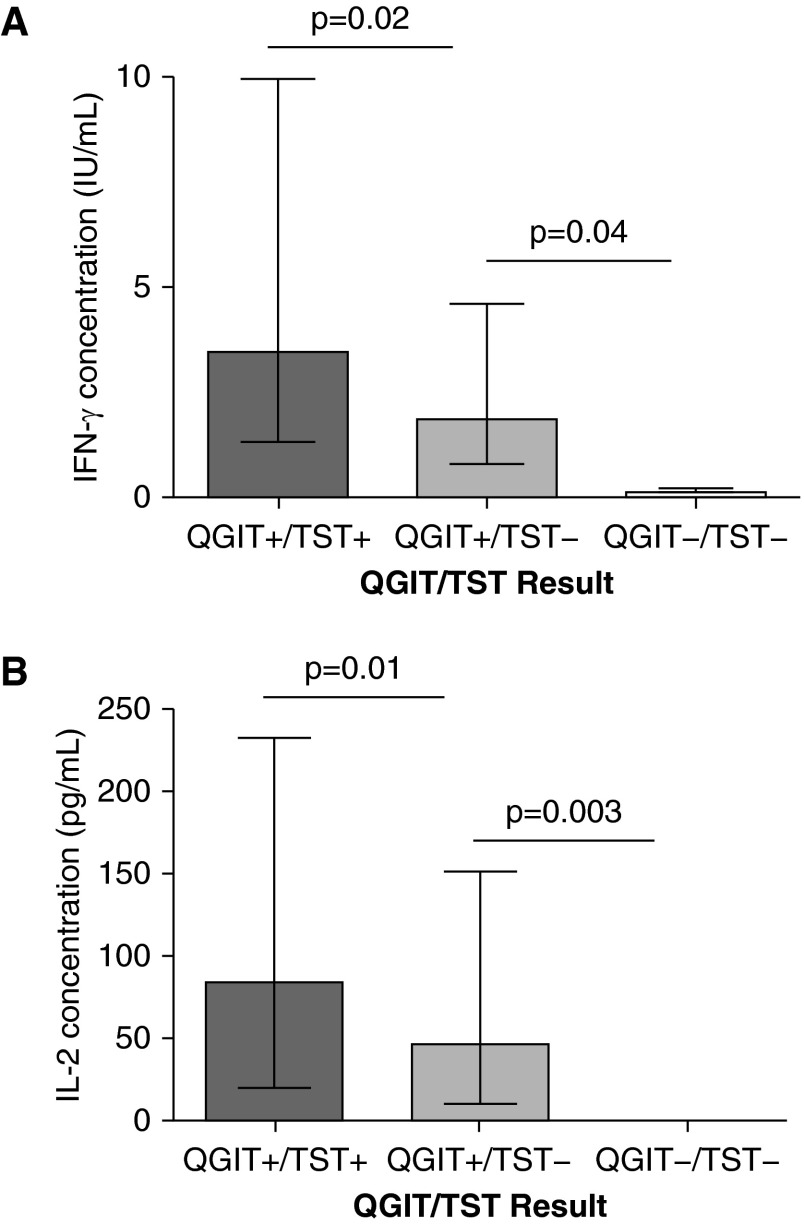
Compared with the antepartum group, the delivery group had lower median IFN-γ concentrations (measured by QGIT) from the mitogen tubes (5.20 IU/ml, IQR 1.9–9.8 at delivery vs. 10.0 IU/ml, IQR 3.9–10.0 antepartum; P = 0.0001). In MTB-antigen stimulated tubes, women with QGIT+/TST− discordance also had significantly lower IFN-γ than women who had concordant positive results (1.85 IU/ml, IQR 0.8–4.5 vs. 3.48 IU/ml, IQR 1.6–10.0; P = 0.02) (Figure 3A). When stratified by pregnancy stage, IFN-γ remained significantly lower in antepartum women with QGIT+/TST− versus QGIT+/TST+ (2.10 IU/ml, IQR 1.1–4.4 vs. 6.0 IU/ml, IQR 3.3–10.0; P = 0.04).
Figure 3.
Women with discordant QGIT+/TST− had lower IFN-γ (A) and IL-2 (B) production as compared with concordant positive QGIT+/TST+, suggesting a difference in population and not just a difference in false-positive and -negative latent tuberculosis infection test results. QGIT = QuantiFERON TB Gold In-Tube test; TST = tuberculin skin test.
Similar to IFN-γ, IL-2 (measured by CBA) was only produced after MTB-antigen stimulation. Overall, those with QGIT+/TST− discordance had lower IL-2 concentrations compared with those with QGIT+/TST+ (46.17 pg/ml, IQR 14.2–121.8 vs. 84.03 pg/ml, IQR 20.8–212.2; P = 0.01) (Figure 3B). When stratified by pregnancy stage, a similar decrease in IL-2 was seen in discordant QGIT+/TST− compared with concordant positive in pregnancy (28.15 pg/ml, IQR 3.6–59.9 vs. 143.33 pg/ml, IQR 50.4–219.1; P = 0.15) with a marginal difference at delivery (90.10 pg/ml, IQR 22.6–190.0 in QGIT+/TST− vs.170.20 pg/ml, IQR 58.1–398.1 in QGIT+/TST+; P = 0.63).
In multivariate analysis adjusting for CD4 count, cART use, and history of TB contact, having a higher IFN-γ concentration had an inverse association with QGIT+/TST− discordance (OR, 0.86; CI, 0.67–0.96; P = 0.01).
Longitudinal Comparison
Fifty women were enrolled into the longitudinal cohort; 39 (83%) completed all three visits at antepartum, delivery, and 3 months postpartum. Figure 1B shows that women in this cohort also displayed QGIT+/TST− discordance. Similar to the cross-sectional groups, the median concentration of IFN-γ in QGIT+ decreased between antepartum and delivery but increased by 3 months postpartum (2.50 IU/ml, IQR 1.1–6.0 to 1.06 IU/ml, IQR 0.7–1.9, to 2.13 IU/ml, IQR 1.1–3.1; P = 0.35). Unlike the cross-sectional analysis, the median TST induration among TST+ increased marginally between antepartum and delivery and continued to increase postpartum (17 to 19.5 to 28 mm; P = 0.41).
Median IL-2 decreased marginally between antepartum and delivery and 3 months postpartum (79.66 pg/ml, IQR 24.9–171.5 to 65.79 pg/ml, IQR 21.2–274.6, to 30.99 pg/ml, IQR 11.7–93.7; P = 0.48). There were no significant differences in levels of IL-4, IL-10, TNF, or IL-17a between unstimulated and stimulated samples or based on LTBI status.
Women Who Developed Active TB
Five (2%) of 252 enrolled women developed active TB (three from longitudinal, two from cross-sectional), all within 1 year postpartum. The median time to development of TB was 97 days postpartum (IQR, 51–270). This yielded an incidence estimate of 2 cases per 100 person years. The median CD4 count at enrollment for these five women was 683 cells/mm3 and four (80%) had initiated cART during pregnancy. All five women had a positive QGIT at enrollment and one had a positive TST. Based on this, the sensitivity of the QGIT for development of postpartum TB was 100%, but the specificity was 27% and the positive predictive value was only 7%.
Women who developed TB had larger decreases in IFN-γ and IL-2 between antepartum and delivery compared with those who did not. Of the three women who developed active TB from the longitudinal study, the mean IFN-γ in mitogen-stimulated samples decreased 1.6-fold (10–6.06 IU/ml) between antepartum and delivery, and 2.9-fold (8.4–2.9 IU/ml) in MTB antigen-stimulated samples. In comparison, the eight women in the longitudinal study with a positive QGIT that did not develop active TB (three who initiated cART in pregnancy) had mean IFN-γ from mitogen samples increase 1.2-fold (6.17–7.34 IU/ml) between pregnancy and delivery but decrease 2.4-fold in MTB-stimulated samples (3.95–1.64 IU/ml). Similarly, MTB antigen-stimulated samples from women who developed active TB experienced an eightfold drop in mean IL-2 (189.79–23.14 pg/ml), but women who did not develop active TB had only a 1.9-fold drop (149.0–75.60 pg/ml) between pregnancy and delivery.
Discussion
Our study has several key findings. We demonstrate that choice of LTBI test for pregnant and postpartum women infected with HIV matters. To our knowledge, this is the first study comparing the performance of these two tests in pregnant women infected with HIV. We found that QGIT positivity was almost three times higher than the more widely used TST at every time point tested. Although there is no gold standard for the diagnosis of LTBI, the prevalence of LTBI as defined by a positive QGIT in our study was more consistent than TST with the prevalence of LTBI in the general population uninfected with HIV in India (∼35–40%) (21). Moreover, we show that a positive QGIT is associated with having contact with someone with pulmonary TB, similar to our prior observations among pregnant women uninfected with HIV in India and that of others (12, 22–24). Although other studies in pregnant women uninfected with HIV in the United States (22, 25) did not show the same QGIT+/TST− discordance, the patients were substantially less likely to have been exposed to TB. Only 5–13% of women in these studies were IGRA+ (vs. 10–23% TST+) and the main discordance seen was IGRA−/TST+ (7–16%). Testing was also conducted earlier in pregnancy, when immune suppression is milder (25). A recent yet unpublished study of pregnant Kenyan women infected with HIV in their second/third trimester, by contrast, reported 25% QGIT+/TST− discordance (26).
Our findings suggest that TST underestimates the burden of LTBI in pregnancy, and that QGIT has high sensitivity for predicting postpartum TB, similar to a report in Kenyan women (6). Countries that already perform targeted screening or are considering integrating LTBI screening into antenatal programs may benefit from using the IGRA in addition to or instead of TST. Although the TST is less expensive, it presents operational challenges, which result in unread TST results in 15% or more of pregnant and postpartum women (27).
By measuring IFN-γ concentrations longitudinally, we also discovered that stage of pregnancy seems to specifically affect the IFN-γ response to MTB antigens. Although there was no difference in the number of QGIT+ women by stage, the IFN-γ concentration among QGIT+ trended down between pregnancy and delivery in both the mitogen and the MTB antigen tubes. This finding was also seen in a study from the United States (22) and is consistent with the known immune changes of pregnancy that favor suppression of the proinflammatory Th1 immune response to allow a successful pregnancy (28). This suppression reaches a nadir late in pregnancy (29, 30), near delivery. We believe the decrease in IFN-γ response to MTB antigens between pregnancy and delivery is a novel finding and suggests that pregnant women have an impaired immune response to TB. Larger cohorts are needed to validate this finding, but reduced IFN-γ response to MTB antigens has also been seen in patients infected with HIV before cART initiation who developed post-cART TB immune reconstitution inflammatory syndrome (31). Rapid increase in immune recovery has also been associated with increased risk of TB immune reconstitution inflammatory syndrome (31, 32). Similarly, we hypothesize that immune control of LTBI weakens during pregnancy, allowing progression from latent to active TB, which only becomes symptomatic during rapid postpartum immune recovery. We only measured response to MTB antigens at one postpartum time point, but future studies are underway to intensively investigate immune changes in the immediate postpartum period.
Another key finding of our study is the 20% prevalence of QGIT+/TST− discordance among pregnant women infected with HIV in a TB endemic area. IGRA+/TST− discordance has been documented in other high-risk populations, including recent household TB contacts (33); the elderly (34); recent immigrants from TB-endemic countries (34, 35); and immunocompromised populations (35, 36), including those with rheumatoid arthritis (37) and HIV (38). The immunologic etiology behind this type of discordance, however, has not been thoroughly investigated.
In our study, women with QGIT+/TST− discordance had significantly decreased IFN-γ and IL-2 production in response to MTB antigens compared with those with concordant positive results, providing a possible immunologic explanation for discordance. The TST is an indirect measurement of the Th1 immune response; it requires IFN-γ, TNF-α, IL-2, and other Th1 cytokines to stimulate a delayed-type hypersensitivity reaction, which results in skin induration (31, 39). The IGRAs, however, only measure IFN-γ produced in the blood. Our results suggest that lower IFN-γ and IL-2 may prevent the immune system from triggering the delayed-type hypersensitivity reaction, resulting in a falsely negative TST. It is possible that QGIT+/TST− discordance could be related to operator-dependent errors with the TST. We do not think this is likely because our nurses were formally trained to perform TST. Any potential operational errors that are inherent to the TST, however, simply emphasize the risks of TST reliance. If discordance was caused by the different antigens or duration of antigen stimulation, we would expect to observe QGIT+/TST− more often. Studies in other populations with discordance are needed to confirm if these findings are generalizable or unique to pregnancy.
Finally, decreased IL-2 and IFN-γ may also be associated with increased risk of active TB. IL-2 is produced primarily by memory T cells, indicating remote exposure to TB; IFN-γ is secreted by effector cells and represents a more acute exposure (31, 40). Studies have shown patients with active TB have a lower IL-2/IFN-γ ratio than those with LTBI (41). No longitudinal studies of predictive value of low IL-2 and IFN-γ exist. In our study, five women developed active TB postpartum. Of the four women who had recorded TST and QGIT results during pregnancy, three of them had discordant results. Moreover, the three women with samples from pregnancy and delivery showed a 2.9-fold decrease in IFN-γ and eightfold decrease in IL-2 between antepartum and delivery. These findings are hypothesis generating, suggesting that reduced IL-2 and IFN-γ associated with discordance and the magnitude of decline in these cytokines during pregnancy may be predictors of progression to active TB. Longitudinal studies with larger sample sizes are needed to validate this finding.
Our study had the following limitations. Because the additional cytokine analyses were a secondary outcome, the blood samples were processed per the manufacturer’s instructions to measure IFN-γ. Longer incubation times may have been needed to measure maximal concentrations of other cytokines, including IL-2 (42). We were also limited in the number of additional cytokines tested and samples we could run with the CBA. We plan on more extensive studies, which will include other TB-relevant cytokines/chemokines (e.g., IP-10, type 1 IFN). We also did not collect information regarding comorbidities that could affect the Th1 immune response, such as helminth infections, hepatitis B, or diabetes. Given our sample size and the less than 5% prevalence of gestational diabetes and hepatitis B in this population (3, 43), we were unable to adjust for such conditions. Because these women had CD4 counts above 400, were pregnant, and did not have any opportunistic infections, we do not think our findings are a result of unadjusted concomitant comorbidities. By screening women for active TB using the WHO TB symptom screen and physical examination, it is possible that we missed women with subclinical or extrapulmonary TB, although all women had follow-up through 1 year postpartum.
Pregnancy offers a unique opportunity to screen for and treat TB, because it is one of the few times young women in high-burden TB countries voluntarily access health care (44). Best practices, however, remain unknown, largely because of the poorly understood impact of the immune changes associated with pregnancy on TB screening and pathogenesis. The WHO recommends IPT for all people infected with HIV (45) but few countries have adopted this policy for pregnant women. Where targeted treatment is preferred, our study suggests that the QGIT may be superior to the TST in identifying pregnant populations infected with HIV that would benefit from IPT in TB-endemic countries, such as India. The results also suggest that delivery is not the optimal time for LTBI screening. Importantly, our results also provide a starting point for further investigation into the immune changes of pregnancy that specifically impact TB pathogenesis. Pregnancy also provides a useful model to examine the interaction between host immune changes and response to pathogens more generally, giving our findings potential significance beyond pregnant women. Dedicated immunologic studies in pregnancy are urgently needed to improve TB prevention and management strategies in pregnant and postpartum women and their children.
Acknowledgments
Acknowledgment
The authors thank the research team, particularly the counselors (S.P.A., S.S.M., S.R.W.) and nurses (K.C., S.J.P., A.N.G.), and the women who participated in this study. They also thank Cellestis and Bectin Dickinson for providing discounted QuantiFERON Gold In-Tube test kits and Cytometric Bead Array kits, respectively, for the study.
Footnotes
Supported by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health (U01 AI069497 for the Byramjee Jeejeebhoy Government Medical College HIV Clinical Trials Unit, A.G.), the Gilead Foundation, and the Ujala Foundation. J.S.M. was supported by a training grant from the National Institutes of Health (T32 AI007613) and the Weill Cornell Clinical and Translational Science Center (KL2 TR00458). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions: J.S.M. contributed to the design, data acquisition, and analysis. R.B., S.K., V.M., N.S., S.J., A.C., and V.K. contributed to the study design. U.B. and P.D. contributed to data acquisition. K.M.D. assisted with data analysis. N.G., D.W.F., and A.G. contributed to study design and data analysis. All authors assisted with drafting and finalizing the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201508-1595OC on January 14, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Deluca A, Chaisson RE, Martinson NA. Intensified case finding for tuberculosis in prevention of mother-to-child transmission programs: a simple and potentially vital addition for maternal and child health. J Acquir Immune Defic Syndr. 2009;50:196–199. doi: 10.1097/QAI.0b013e3181900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, Kakrani A, Philip S, Desai D, Sastry J, et al. Group BJMC-JHUS. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002-2005. Nephrol Dial Transplant. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 4.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185:779–784. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- 5.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonnalagadda SR, Brown E, Lohman-Payne B, Wamalwa D, Farquhar C, John-Stewart GC. Predictive value of interferon-gamma release assays for postpartum active tuberculosis in HIV-1-infected women. Int J Tuberc Lung Dis. 2013;17:1552–1557. doi: 10.5588/ijtld.13.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranbhai V, Moodley D, Chipato T, Stranix-Chibanda L, Nakabaiito C, Kamateeka M, Musoke P, Manji K, George K, Emel LM, et al. HPTN 046 Protocol Team. The association between the ratio of monocytes: lymphocytes and risk of tuberculosis among HIV-infected postpartum women. J Acquir Immune Defic Syndr. 2014;67:573–575. doi: 10.1097/QAI.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillay T, Khan M, Moodley J, Adhikari M, Padayatchi N, Naicker V, Pillay DG, Coovadia HM. The increasing burden of tuberculosis in pregnant women, newborns and infants under 6 months of age in Durban, KwaZulu-Natal. S Afr Med J. 2001;91:983–987. [PubMed] [Google Scholar]
- 9.Menéndez C, Romagosa C, Ismail MR, Carrilho C, Saute F, Osman N, Machungo F, Bardaji A, Quintó L, Mayor A, et al. An autopsy study of maternal mortality in Mozambique: the contribution of infectious diseases. PLoS Med. 2008;5:e44. doi: 10.1371/journal.pmed.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 12.Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, Nangude A, Chopade K, Suryavanshi N, Deshpande P, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLoS One. 2014;9:e92308. doi: 10.1371/journal.pone.0092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathad JS, Kanade S, Sangar V, Mave V, Suryavanshi N, Gupte N, Bhosale R, Gupta A. Screening HIV-positive pregnant women for TB in India: tuberculin skin test (TST) vs. QuantiFERON(R) Gold In-tube (QGIT) Int J Tuberc Lung Dis. 2013;17:S504. [Google Scholar]
- 14.Mathad JS, Bhosale R, Kanade S, Deshpande P, Kulkarni V, Nevrekar N, Mave V, Suryavanshi N, Gupte N, Gupta A.Effect of HIV on latent TB screening of pregnant women in Pune, India. Presented at the Conference on Retroviruses and Opportunistic Infections. March 3–6, 2014, Boston. Abstract 818 [Google Scholar]
- 15.Coates J, Bilinsky P. Household food insecurity access scale (HFIAS) for measurement of household food access (v.3) Washington, DC: Food and Nutrition Technical Assistance Project; 2007; [Google Scholar]
- 16.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, Grant AD, Churchyard GJ, Kimerling M, Shah S, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.QuantiFERON-TB Gold (In-Tube Method) [package insert]. Cellestis Inc., Valencia, CA. January 2009
- 18.Morán-Mendoza O, Tello-Zavala MC, Rivera-Camarillo M, Ríos-Meza Y. Comparison of different methods and times for reading the tuberculin skin test. Int J Tuberc Lung Dis. 2013;17:1273–1278. doi: 10.5588/ijtld.13.0147. [DOI] [PubMed] [Google Scholar]
- 19.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 20.Array CB.CBA) Human Th1/Th2/Th17 Cytokine Kit [package insert]. Franklin Lakes, NJ: Becton Dickinson; 2009
- 21.Revised National Tuberculosis Control Program. Central TB Division, Directorate General of Health Services. New Delhi, India: Ministry of Health and Family Welfare; 2011. India TB RNTCP Status Report. [Google Scholar]
- 22.Lighter-Fisher J, Surette AM. Performance of an interferon-gamma release assay to diagnose latent tuberculosis infection during pregnancy. Obstet Gynecol. 2012;119:1088–1095. doi: 10.1097/AOG.0b013e3182546aff. [DOI] [PubMed] [Google Scholar]
- 23.Luetkemeyer AF, Charlebois ED, Flores LL, Bangsberg DR, Deeks SG, Martin JN, Havlir DV. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med. 2007;175:737–742. doi: 10.1164/rccm.200608-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcells ME, Pérez CM, Chanqueo L, Lasso M, Villanueva M, Espinoza M, Villarroel L, García P. A comparative study of two different methods for the detection of latent tuberculosis in HIV-positive individuals in Chile. Int J Infect Dis. 2008;12:645–652. doi: 10.1016/j.ijid.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Worjoloh A, Kato-Maeda M, Osmond D, Freyre R, Aziz N, Cohan D. Interferon gamma release assay compared with the tuberculin skin test for latent tuberculosis detection in pregnancy. Obstet Gynecol. 2011;118:1363–1370. doi: 10.1097/AOG.0b013e31823834a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaCourse S, Cranmer LM, Matemo D, Kinuthia J, Horne DJ, John-Stewart G.Performance of latent TB infection diagnostics in HIV-infected pregnant women in western Kenya. Presented at the International AIDS Society Conference. July 19–22, 2015. Vancouver, Canada. Abstract 167 [Google Scholar]
- 27.Schulte JM, Bryan P, Dodds S, Potter M, Onorato IM, O'Sullivan MJ. Tuberculosis skin testing among HIV-infected pregnant women in Miami, 1995 to 1996. J Perinatol. 2002;22:159–162. doi: 10.1038/sj.jp.7210617. [DOI] [PubMed] [Google Scholar]
- 28.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 29.Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, Loubeau M, Ge Y, Garrido JL, Rodríguez-García M, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010;64:411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 30.Kruse N, Greif M, Moriabadi NF, Marx L, Toyka KV, Rieckmann P. Variations in cytokine mRNA expression during normal human pregnancy. Clin Exp Immunol. 2000;119:317–322. doi: 10.1046/j.1365-2249.2000.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, Huffam S, Oelrichs R, Sophea P, Saphonn V, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 32.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro-Rodrigues R, Kim S, Coelho da Silva FD, Uzelac A, Collins L, Palaci M, Alland D, Dietze R, Ellner JJ, Jones-López E, et al. Discordance of tuberculin skin test and interferon gamma release assay in recently exposed household contacts of pulmonary TB cases in Brazil. PLoS One. 2014;9:e96564. doi: 10.1371/journal.pone.0096564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinfurter P, Blumberg HM, Goldbaum G, Royce R, Pang J, Tapia J, Bethel J, Mazurek GH, Toney S, Albalak R Tuberculosis Epidemiological Studies Consortium. Predictors of discordant tuberculin skin test and QuantiFERON®-TB Gold In-Tube results in various high-risk groups. Int J Tuberc Lung Dis. 2011;15:1056–1061. doi: 10.5588/ijtld.10.0650. [DOI] [PubMed] [Google Scholar]
- 35.Saracino A, Scotto G, Fornabaio C, Martinelli D, Faleo G, Cibelli D, Tartaglia A, Di Tullio R, Fazio V, Prato R, et al. QuantiFERON-TB Gold In-Tube test (QFT-GIT) for the screening of latent tuberculosis in recent immigrants to Italy. New Microbiol. 2009;32:369–376. [PubMed] [Google Scholar]
- 36.Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, Park BH, Moon JW, Park MS, Kim YS, et al. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 2009;9:207. doi: 10.1186/1471-2334-9-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song GG, Bae SC, Lee YH. Interferon-gamma release assays versus tuberculin skin testing in patients with rheumatoid arthritis. Int J Rheum Dis. 2013;16:279–283. doi: 10.1111/1756-185X.12098. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W, Shao L, Zhang Y, Zhang S, Meng C, Xu Y, Huang L, Wang Y, Wang Y, Weng X, et al. High-sensitive and rapid detection of Mycobacterium tuberculosis infection by IFN-gamma release assay among HIV-infected individuals in BCG-vaccinated area. BMC Immunol. 2009;10:31. doi: 10.1186/1471-2172-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 40.Bittel P, Mayor D, Iseli P, Bodmer T, Suter-Riniker F. IGRA-positive patients and interferon-gamma/interleukin-2 signatures: can the Fluorospot assay provide further information? Infection. 2014;42:539–543. doi: 10.1007/s15010-014-0588-2. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Diao N, Lu C, Wu J, Gao Y, Chen J, Zhou Z, Huang H, Shao L, Jin J, et al. Evaluation of the diagnostic potential of IP-10 and IL-2 as biomarkers for the diagnosis of active and latent tuberculosis in a BCG-vaccinated population. PLoS One. 2012;7:e51338. doi: 10.1371/journal.pone.0051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiappini E, Della Bella C, Bonsignori F, Sollai S, Amedei A, Galli L, Niccolai E, Del Prete G, Singh M, D’Elios MM, et al. Potential role of M. tuberculosis specific IFN-γ and IL-2 ELISPOT assays in discriminating children with active or latent tuberculosis. PLoS One. 2012;7:e46041. doi: 10.1371/journal.pone.0046041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mave V, Kadam D, Kinikar A, Gupte N, Bhattacharya D, Bharadwaj R, McIntire K, Kulkarni V, Balasubramanian U, Suryavanshi N, et al. SWEN India and Byramjee-Jeejeebhoy Medical College Clinical Trials Unit Study team. Impact of maternal hepatitis B virus coinfection on mother-to-child transmission of HIV. HIV Med. 2014;15:347–354. doi: 10.1111/hiv.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojanuga DN, Gilbert C. Women’s access to health care in developing countries. Soc Sci Med. 1992;35:613–617. doi: 10.1016/0277-9536(92)90355-t. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. Guidelines for intensified tuberculosis case finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. Geneva: World Health Organization; 2011. [Google Scholar]