Abstract
Rationale: Most airway diseases, including chronic obstructive pulmonary disease (COPD), are associated with excessive coughing. The extent to which this may be a consequence of increased activation of vagal afferents by pathology in the airways (e.g., inflammatory mediators, excessive mucus) or an altered neuronal phenotype is unknown. Understanding whether respiratory diseases are associated with dysfunction of airway sensory nerves has the potential to identify novel therapeutic targets.
Objectives: To assess the changes in cough responses to a range of inhaled irritants in COPD and model these in animals to investigate the underlying mechanisms.
Methods: Cough responses to inhaled stimuli in patients with COPD, healthy smokers, refractory chronic cough, asthma, and healthy volunteers were assessed and compared with vagus/airway nerve and cough responses in a cigarette smoke (CS) exposure guinea pig model.
Measurements and Main Results: Patients with COPD had heightened cough responses to capsaicin but reduced responses to prostaglandin E2 compared with healthy volunteers. Furthermore, the different patient groups all exhibited different patterns of modulation of cough responses. Consistent with these findings, capsaicin caused a greater number of coughs in CS-exposed guinea pigs than in control animals; similar increased responses were observed in ex vivo vagus nerve and neuron cell bodies in the vagal ganglia. However, responses to prostaglandin E2 were decreased by CS exposure.
Conclusions: CS exposure is capable of inducing responses consistent with phenotypic switching in airway sensory nerves comparable with the cough responses observed in patients with COPD. Moreover, the differing profiles of cough responses support the concept of disease-specific neurophenotypes in airway disease.
Clinical trial registered with www.clinicaltrials.gov (NCT 01297790).
Keywords: cough, vagus nerve, guinea pig, asthma, COPD
At a Glance Commentary
Scientific Knowledge on the Subject
Excessive cough is a major symptom of most respiratory diseases, but the mechanisms responsible are not known.
What This Study Adds to the Field
Translational animal models and a human vagus nerve assay provide mechanistic information and predict changes in the nature of the tussive responses observed in patients with chronic obstructive pulmonary disease compared with healthy volunteers. The divergent profile of tussive responses among patients with different respiratory conditions supports the hypothesis of distinct disease-specific neurophenotypes.
Chronic coughing is reported by about 12% of the adult population (1), and is a common feature of many pulmonary diseases. Cigarette smoke (CS) exposure is an important risk factor associated with cough in epidemiologic studies (2) and objective measurement of coughing has demonstrated that healthy smokers and patients with chronic obstructive pulmonary disease (COPD), whether current or ex-smokers, cough more frequently than healthy control subjects (3). Up to 40% of the variation in cough frequency in COPD can be explained by factors capable of activating the airway sensory nerves responsible for evoking cough, including smoking habits, airway inflammation, and the need to expectorate sputum. It is less clear, however, because of a paucity of studies, whether alterations in the function of airway nerves play a role in driving cough and other symptoms.
Cough responses are thought to be primarily mediated by two categories of vagal sensory afferent neurones whose cell bodies reside in the jugular and nodose ganglia; in the guinea pig the jugular ganglion projects capsaicin-sensitive (TRPV1-expressing) C-fibers to the airway, which typically respond to chemical stimuli (4–6). Cough-evoking nodose neurons are of the Aδ phenotype that typically respond to mechanical stimuli, changes in osmolality, and low pH (7).
Cough challenges using inhaled irritant agents have been used for decades in preclinical experiments and clinical studies to investigate the neuronal pathways controlling cough and how these may be modulated by disease states and therapeutic interventions. Capsaicin is the most widely used challenge agent in clinical studies evoking cough via TRPV1 activation. Citric acid is also an effective tussive agent most probably via activation of TRPV1, TRPA1, and perhaps also via acid-sensing ion channels (8–10). When inhaled, prostaglandin E2 (PGE2) characteristically causes bronchodilation, but sensations of airway irritation and transient coughing have also been reported (11). However, clinical studies have generally assessed the cough reflex using only a single challenge agent, which limits the conclusions that can be drawn about the mechanisms that may underlie any observations made.
The use of a range of challenge agents in the same individuals may provide better insights into how neuronal function may be altered by respiratory disease and whether patterns of neuronal dysfunction are specific to different conditions. Because the precise mechanisms that underlie airway neuronal dysfunction are extremely challenging/impossible to elucidate in clinical studies, careful translation between human disease and animal disease models is essential if effective therapeutic agents targeting airway nerves are to be developed. Therefore, we have studied evoked coughing to a range of irritant agents in patients with COPD compared with healthy control subjects. To understand the specificity of any changes in cough response we included groups of healthy smokers (without COPD), patients with asthma, and patients with refractory chronic cough. We then hypothesized that the changes in cough responses in COPD could be modeled in animals using CS exposure. Some of these results have previously been reported in abstract form (12, 13).
Methods
Detailed methods are provided in the online supplement.
Clinical Study
Subjects
A total of 102 patients were recruited; 21 were healthy volunteers, 20 were healthy smokers, 18 had COPD (ex-smokers), 22 had asthma, and 21 had refractory chronic cough. The study was approved by the Research Ethics Committee (08/H1003/48) and all patients gave written informed consent.
Study design
Cough responses to inhaled capsaicin, citric acid, PGE2, and bradykinin were assessed in random order, at intervals of approximately 7 days (see Figure E1 in the online supplement). Both the subjects and the investigator were blinded to the challenge agents. Cough-specific quality of life and 24-hour ambulatory cough recordings were collected 1 week after the last challenge.
Procedures
Single breaths of doubling concentrations of each tussive agent were inhaled at 1-minute intervals from a nebulizer pot (De Vilbiss Health Care Inc., Somerset, PA) with a built-in flow regulator valve and fixed baffle assembly, controlled by a dosimeter (Koko dosimeter; De Vilbiss Health Care Inc.). The number of coughs in the 15 seconds following each inhalation was counted and the concentrations of challenge agent evoking at least five coughs (C5) recorded; C2 and C1 were also captured. The challenge agents used were capsaicin (0.97–1,000 μM), citric acid (0.03–4.0 M) (both Stockport Pharmaceuticals Ltd, Stockport, UK), PGE2 (1.22–2,500 μg/ml; Pharmacia Ltd, Kent, UK), and bradykinin (0.024–25 μg/ml; Cinalfa Basic; Bachem, Bubendorf, Switzerland).
Ambulatory cough monitoring
Twenty-four hour acoustic recordings were made using the VitaloJAK cough monitor (Vitalograph Ltd, Buckinghamshire, UK) and the number of cough sounds per hour quantified by a semiautomated method using validated custom-written software (14).
Preclinical Studies
Animals
Male guinea pigs (Dunkin-Hartley, Harlan, UK) weighing 350–400 g were housed in temperature-controlled (21°C) facilities for at least 1 week before any procedures. Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act of 1986 and the ARRIVE guidelines (15).
Human tissue
Whole lungs, unsuitable for transplantation, were acquired and consent for use obtained by the International Institute for the Advancement of Medicine. Approvals for use in scientific research and ethics were obtained from the Royal Brompton and Harefield Trust (Ref: 09/H0708/72).
Staining and identification of airway sensory neurons in guinea pigs
The neuronal tracer dye DiICl18 (3) (DiI; 1,1′-dioctacetyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate; Invitrogen, Thermo Fischer Scientific, Waltham, MA) was administered intranasally 12–14 days before collection of vagal ganglia, to identify those neuron cell bodies with airway termini (16).
CS exposures in guinea pigs
Guinea pigs were exposed to CS from research cigarettes (3R4F, with filters removed; University of Kentucky, Lexington, KY) for 1 hour, twice daily for 8 days, with 4 hours between each exposure period, as previously described (17). Air-exposed comparator animals were treated identically, except the cigarette was not lit.
Cough counting in guinea pigs
In all experiments the operator was blinded to the treatment groups. Twenty-four hours after the last CS-exposure, conscious, unrestrained guinea pigs were individually placed into transparent whole-body plethysmography chambers, and cough evoked on exposure to either capsaicin or PGE2 was counted as previously described (18).
Collection of isolated vagus nerve and vagal jugular and nodose ganglia from guinea pigs
Twenty-four hours after the last CS exposure, guinea pigs were killed by overdose of pentobarbitone (200 mg/kg intraperitoneally); vagus nerve trunks and ganglia were dissected as described previously (4, 19). Human vagus nerve trunks were placed into Krebs-Heinseleit solution, which was gassed with 95% O2/5% CO2 at room temperature until use.
Recording of isolated vagus nerve depolarization
Segments (∼15 mm) of guinea pig or human vagus nerve were mounted in a “grease-gap” recording system (18–20). The nerve segments were perfused constantly with Krebs-Heinseleit solution (at 37°C bubbled with 95% O2/5% CO2) and following each 2-minute challenge stimulus (capsaicin, 1 μM; or PGE2, 10 μM), depolarization was recorded on a Lectromed 2 (Digitimer) chart recorder with DAM50 differential amplifier (WPI Instruments, Hitchin, UK).
Imaging of calcium movement in isolated airway vagal ganglia neurons from guinea pigs
Enzymatic isolation, culture, and imaging of cytosolic calcium level changes in jugular and nodose neurons were recorded as detailed previously (4, 19). In all experiments, an initial response to a 15-second application of 50 mM potassium solution was obtained. Following a wash to return calcium levels to baseline, a subsequent response to a 30-second application of a single concentration of a single challenge stimulus (capsaicin, 1 μM; or PGE2, 10 μM) was performed.
Data Analyses
Clinical study
All data were assessed for normality before analysis. C5, C2, C1, and cough frequency were all log transformed (to normalize the distributions) before analysis. If all concentrations were inhaled without reaching C5, it was assumed for the purpose of analysis that the C5 was double the highest concentration inhaled. With 20 subjects per group, the study had 90% power to detect differences of ±1 doubling concentration for C5 (0.3 for logC5) among study groups, assuming a between-subject standard deviation of 0.3 for logC5 and the standard level of significance (P = 0.05). One-way analysis of variance (SPSS version 16; IBM, Armonk, NY) was used to compare logC5 values from healthy control subjects with patient groups for each agent; the same analysis was applied to logC1/C2. Multinomial logistic regression analysis was used to assess the ability of the combined challenge responses to predict the diagnostic groups.
Preclinical studies
Data were summarized as mean and standard errors of the mean, and comparisons among groups made using the Mann-Whitney U test.
Results
Clinical Study
Subjects
Study participant groups were generally of a similar age, although patients with COPD and refractory cough were approximately 10 years older than other groups; there was no significant difference in the sex distribution among the groups (Table 1). Patients with COPD exhibited typical airflow obstruction on spirometry and significant smoking histories, double that of the healthy smokers (Table 1). Twelve patients with COPD were taking an inhaled corticosteroid, 13 inhaled long-acting bronchodilators, and 11 inhaled anticholinergics. Patients with asthma had evidence of milder airflow obstruction with a median FEV1 within normal limits but FEV1/FVC ratio just within the abnormal range. Two patients were taking inhaled short-acting β-agonist only, 20 were taking inhaled corticosteroids, and 10 also took an inhaled long-acting bronchodilator.
Table 1.
Characteristics of Participants
| Healthy Control Subjects | Healthy Smokers | COPD | Asthma | Chronic Cough | P Value | |
|---|---|---|---|---|---|---|
| Number (M:F) | 21 (10:11) | 20 (12:8) | 18 (9:9) | 22 (11:11) | 21 (9:12) | 0.865* |
| Age, yr | 53 (45.0–64.5) | 56 (42.0–61.0) | 64.5† (60.8–71.0) | 54 (45.0–61.3) | 63† (58–67.5) | <0.001‡ |
| FEV1 % predicted | 102.8 (96.4–108.1) | 98.7 (92.7–118.8) | 73.5† (49.6–95.9) | 96.3 (79.1–106.8) | 104.4 (94.0–115.2) | 0.001‡ |
| FVC % predicted | 108.0 (101.0–114.3) | 107.7 (96.3–121.2) | 102.8 (89.2–126.0) | 110.4 (91.3–120.5) | 113.0 (104.8–123.0) | 0.803‡ |
| FEV1/FVC ratio | 0.80 (0.76–0.82) | 0.77 (0.73–0.82) | 0.57† (0.43–0.68) | 0.70 (0.65–0.77) | 0.74 (0.70–0.80) | <0.001‡ |
| Smoking history, pack-years | 0.0 (0.0–0.0) | 18.5† (12.9–32.3) | 37.5† (30.0–50.0) | 0.0 (0.0–0.0) | 0.0 (0.0–6.0) | <0.001‡ |
| 24-h cough frequency, GM (95% CI), coughs/h | 0.99 (0.53–1.83) | 2.72† (1.85–4.02) | 4.02† (3.04–5.33) | 1.84 (0.97–3.47) | 13.76† (9.16–20.68) | <0.001§ |
| Day cough frequency, GM (95% CI), coughs/h | 1.12 (0.57–2.21) | 3.46† (2.30–5.20) | 5.62† (4.19–7.52) | 2.78† (1.46–5.31) | 18.71† (12.18–28.74) | <0.001§ |
| Night cough frequency, coughs/h | 0.00 (0.00–1.63) | 0.62 (0.14–.65) | 1.13 (0.15–2.51) | 0.40 (0.00–0.71) | 3.96† (0.53–9.17) | 0.001‡ |
| Day cough severity VAS, mm | 2 (1–8) | 17† (5–33) | 22† (3–31) | 10† (4–24) | 39† (20–52) | <0.001‡ |
| Night cough severity VAS, mm | 1 (0–5) | 6† (2–24) | 6 (0–28) | 3 (0–20) | 12† (10–15) | 0.014‡ |
| LCQ | 21.0 (20.9–21.0) | 19.4† (18.5–20.7) | 20.1† (15.8–20.7) | 19.3† (17.5–20.5) | 11.9† (10.1–14.9) | <0.001‡ |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; GM = geometric mean; LCQ = Leicester Cough Questionnaire; VAS = Visual Analog Scale.
Data are medians (interquartile ranges) unless otherwise stated. P values <0.05 are shown in bold.
Groups are compared using the chi-square test.
P < 0.05 compared with healthy control subjects.
Groups are compared using the Kruskal-Wallis test.
Groups are compared using one-way analysis of variance.
Clinical measures of cough
Cough frequency in patients with COPD was significantly higher than control subjects and healthy smokers, and comparable with that seen in our previous studies (see Figure E2) (3, 21, 22). Patients with asthma exhibited wide variation in cough frequency and only day cough frequency was greater than healthy control subjects. Again rates were similar to those previously reported in a previous larger study (23). Only patients with refractory chronic cough had higher cough rates than subjects with COPD. Across subject groups reported cough severity Visual Analog Scale displayed very similar patterns to cough frequency, whereas cough quality of life scores were similar in healthy smokers, patients with COPD, and patients with asthma despite the differences in cough frequency. Patients with chronic cough reported the greatest impacts on quality of life.
Responses to tussive agents
The mean cough responses to increasing doses of inhaled tussive agents are shown in Figure E3 and the concentrations evoking at least C5 in Figure 1. Bradykinin was a very poor tussive agent at the available concentrations; only two patients with chronic cough and one patient with asthma achieved a C5, therefore further patient groups were not challenged and these data not formally reported or analyzed. For all tussive agents the influence of sex on differences in cough responses between disease groups was assessed, but none were found to be significant.
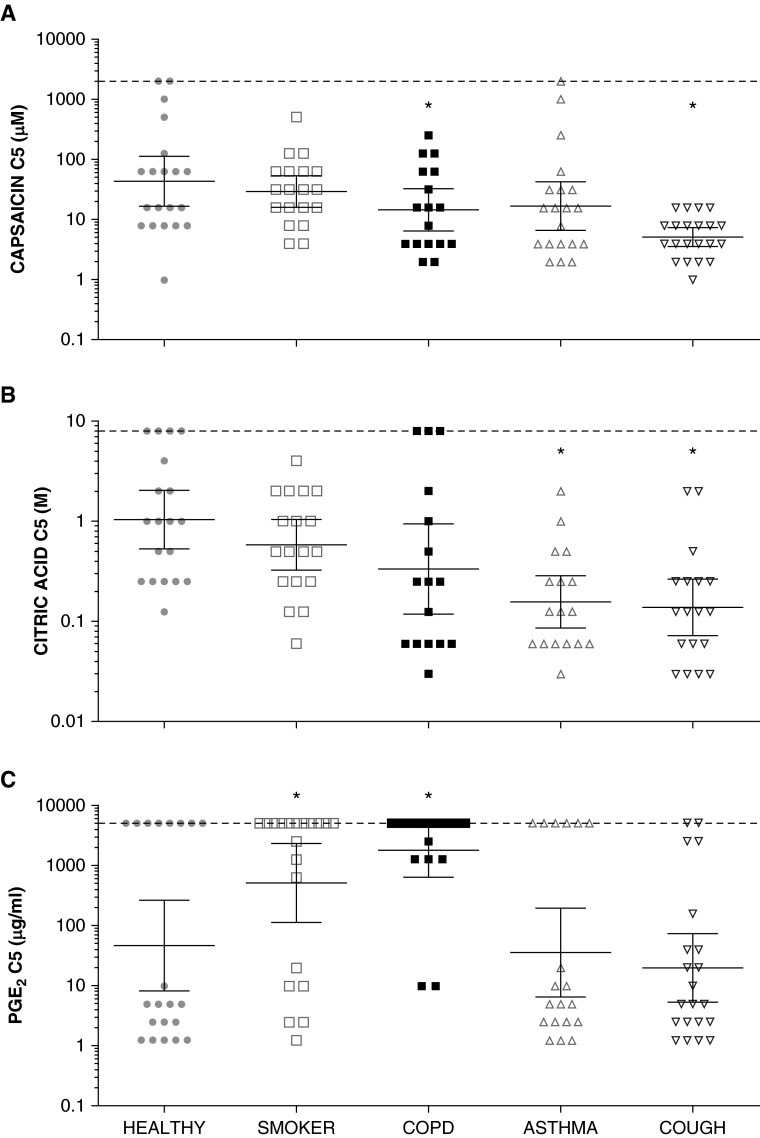
Figure 1.
(A–C) Cough reflex sensitivity to tussive agents in airway diseases compared with healthy control subjects. Concentrations of tussive agents causing at least five coughs for all patient groups (C5). The lower the C5, the greater the sensitivity of the cough reflex. Note the logarithmic scale on the y-axes. Horizontal lines and error bars represent mean values and 95% confidence intervals. Asterisks denote significant differences compared with healthy control group (P < 0.05, analysis of variance). COPD = chronic obstructive pulmonary disease; PGE2 = prostaglandin E2.
Cough responses to capsaicin
Capsaicin logC5 was significantly lower in subjects with COPD (P = 0.035) and those with refractory chronic cough (P < 0.001) when compared with healthy volunteers (Figure 1A, Table 2), but not in healthy smokers (P = 0.514). In patients with asthma logC5 tended to be lower than healthy control subjects but the difference did not reach significance (P = 0.10). LogC2 and logC1 responses to capsaicin exhibited similar patterns of response, but the differences between groups only achieved statistical significance for logC1 (Table 2).
Table 2.
Cough Responses to Tussive Agents in Airway Diseases Compared with Healthy Control Subjects
| Healthy Control Subjects | Healthy Smokers | COPD | Asthma | Chronic Cough | P Value | |
|---|---|---|---|---|---|---|
| Capsaicin, μM | ||||||
| logC5 | 3.75 (±2.06) | 3.37 (±1.24) | 2.52 (±1.54)* | 2.82 (±1.99) | 1.63 (±0.81)* | 0.001 |
| logC2 | 2.12 (±1.59) | 2.02 (±1.11) | 1.48 (±1.07) | 1.57 (±1.06) | 1.22 (±0.92) | 0.091 |
| logC1 | 2.01 (±1.38) | 1.81 (±1.31) | 1.25 (±0.90)* | 1.36 (±0.78)* | 1.11 (±0.90)* | 0.035 |
| Citric acid, M | ||||||
| logC5 | 0.37 (±1.40) | −0.54 (±1.16) | −1.01 (±1.99) | −1.33 (±1.77)* | −1.98 (±1.30)* | 0.002 |
| logC2 | −1.98 (±0.98) | −2.14 (±1.35) | −1.79 (±1.49) | −2.35 (±1.13) | −3.09 (±0.50)* | 0.014 |
| logC1 | 2.13 (±1.01) | −2.45 (±1.28) | −1.84 (±1.53) | −2.45 (±1.11) | −3.09 (±0.50)* | 0.028 |
| PGE2, μg/ml | ||||||
| logC5 | 3.83 (±3.81) | 6.23 (±3.21)* | 7.50 (±2.04)* | 3.88 (±3.50) | 2.67 (±2.61) | <0.001 |
| logC2 | 1.82 (±2.23) | 3.87 (±3.20)* | 3.49 (±3.46) | 2.62 (±2.89) | 1.44 (±1.55) | 0.027 |
| logC1 | 1.42 (±1.85) | 3.25 (±2.90)* | 3.40 (±3.34)* | 1.76 (±2.07) | 1.34 (±1.59) | 0.013 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PGE2 = prostaglandin E2.
Concentrations of tussive agents causing at least five coughs (C5), at least two coughs (C2), and at least one cough (C1). The lower the C5, C2, or C1, the greater the sensitivity of the cough reflex. Data are mean ± SD. P values <0.05 are shown in bold.
Significant differences compared with healthy control group (P < 0.05) based on analysis of variance.
Cough responses to citric acid
Citric acid logC5 in patients with COPD was lower than healthy control subjects but the difference was not quite significant (P = 0.064). Citric acid logC5 was significantly lower in subjects with asthma (P = 0.035) and refractory chronic cough (P < 0.001) than in healthy volunteers (Figure 1B). LogC2 and logC1 responses to citric acid exhibited similar patterns of response, but the differences between groups and the healthy control subjects only achieved statistical significance in patients with chronic cough.
Cough responses to PGE2
PGE2 was not as potent a tussive agent as either capsaicin or citric acid, evoking fewer coughs in all patient groups (see Figures E3A–E3C). PGE2 logC5 was significantly increased in patients with COPD (P = 0.003) and healthy smokers (P = 0.030) compared with healthy control subjects (Figure 1C). Interestingly, many subjects failed to reach C5 at any concentration, giving the data a bimodal distribution. LogC1 and logC2 responses to PGE2 exhibited similar patterns of response, with the largest differences between healthy control subjects and both patients with COPD (log C2, P = 0.066; logC1, P = 0.014) and healthy smokers (log C2, P = 0.017; logC1, P = 0.016).
Relationships between cough responses to different challenge agents
Combining data from all diagnostic groups, there was a positive correlation between capsaicin and citric acid logC5 (r = 0.643; P < 0.001). Given the bimodal nature of the PGE2 logC5, subjects were categorized for the purposes of analysis into those with PGE2 logC5 above or below the geometric mean of 111 μg/ml. Subjects with a high PGE2 logC5 had a significantly higher capsaicin logC5 (P < 0.001) and citric acid logC5 (P < 0.001) compared with low PGE2 logC5.
Despite the relationships between cough responses to different challenge agents, in the multinomial regression analysis the logC5 responses to capsaicin, citric acid, and PGE2 all independently predicted the diagnostic group (P = 0.015, P = 0.018, and P < 0.001, respectively).
Preclinical Studies
Effects of CS exposure on guinea pig cough responses to capsaicin and PGE2
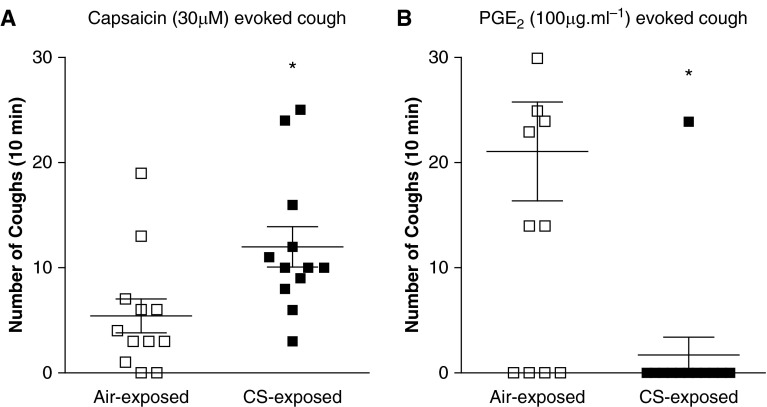
Consistent with the clinical data, the number of coughs evoked by capsaicin (30 μM) was increased in CS-exposed guinea pigs (mean, 12.0 ± 1.9), compared with air-exposed control animals (5.4 ± 1.6) (Figure 2A). By contrast, the number of coughs evoked by PGE2 (100 μg/ml1) was reduced with 1.7 ± 1.7 coughs in CS-exposed guinea pigs, compared with 21.1 ± 4.7 coughs in air-exposed comparators (Figure 2B). Of note, the number of coughs evoked by this concentration of PGE2 exhibited a bimodal distribution in air-exposed animals, with a subgroup of animals lacking any cough response.
Figure 2.
The numbers of coughs evoked by nebulized solutions of (A) capsaicin (5 min; n = 12) or (B) prostaglandin E2 (PGE2; 10 min; n = 10) in guinea pigs exposed to either air (open squares) or cigarette smoke (CS; solid squares) for 8 days. Coughs were recorded for 10 minutes from the start of nebulization. Data are presented as mean ± SE. *P < 0.05 as determined by Mann-Whitney U test.
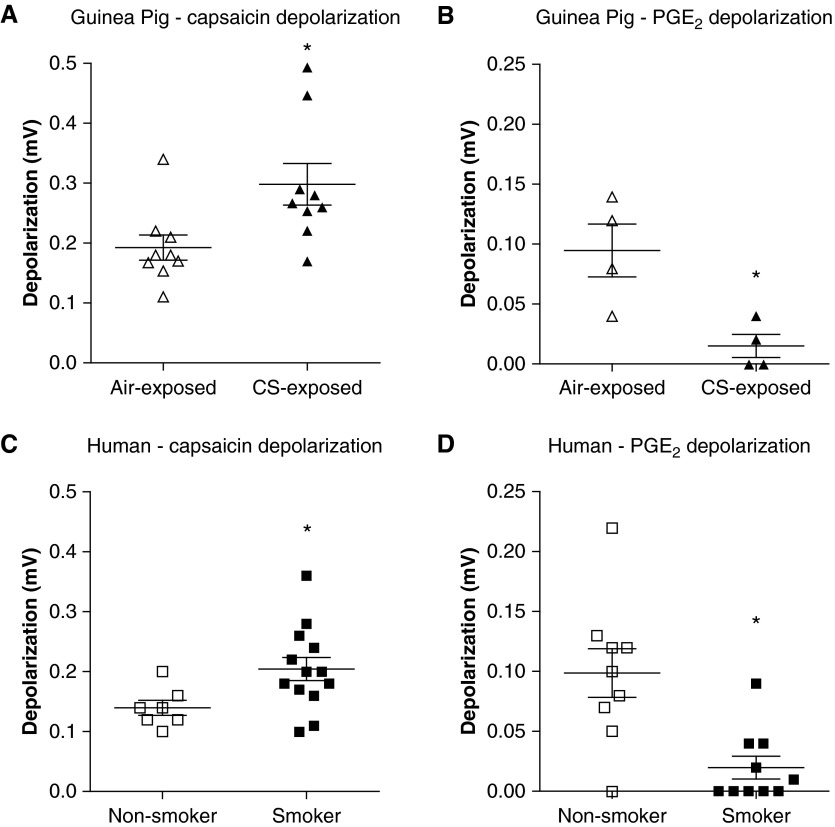
Effect of CS exposure on guinea pig isolated vagus nerve and isolated airway vagal neuron responses to capsaicin and PGE2
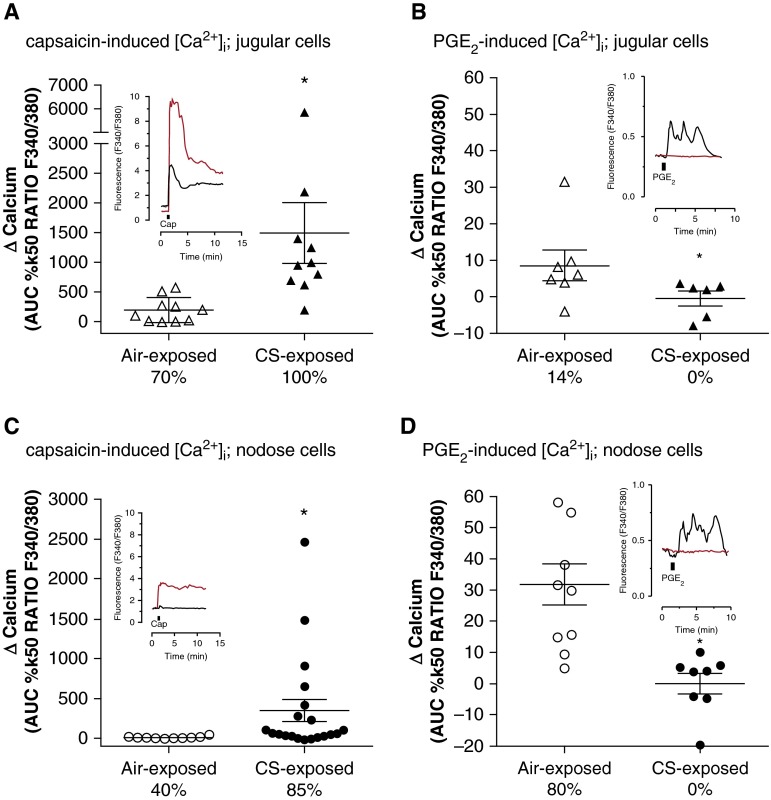
Isolated vagus nerves, which convey sensory nerves to the lungs but also other organs, from CS-exposed guinea pigs were depolarized by capsaicin (1 μM) to a greater extent than vagus nerve from air-exposed comparators (0.29 ± 0.03 compared with 0.19 ± 0.02 mV) (Figure 3A). By contrast, PGE2 (10 μM) caused greatly reduced depolarization of vagus nerves from CS-exposed guinea pigs compared with air-exposed comparators (0.02 ± 0.01 compared with 0.10 ± 0.02 mV) (Figure 3B). In isolated jugular and nodose ganglion neurons that had projected termini to the airways, a similar profile was observed. Capsaicin (1 μM) induced much greater increases in intracellular calcium in jugular ganglion neurons from CS-exposed guinea pigs (1492.0 ± 512.3) than air-exposed guinea pigs (191.5 ± 66.9) (Figure 4A), whereas the increase in intracellular calcium on exposure to PGE2 (10 μM) in air-exposed guinea pigs (8.60 ± 4.16) was lost in neurons from CS-exposed guinea pigs (0.25 ± 2.00) (Figure 4B). Nodose ganglion neurons also exhibited an increase in capsaicin-induced intracellular calcium (347.2 ± 139.9 vs. 12.6 ± 4.4) in response to CS exposure (Figure 4C) and loss of response to PGE2 (−0.05 ± 3.3 vs. 31.76 ± 6.6) (Figure 4D).
Figure 3.
Depolarization of guinea pig and human vagus nerve. (A and B) Depolarization induced by capsaicin (n = 9) (A) or prostaglandin E2 (PGE2; n = 4) (B) of isolated vagus nerve taken from guinea pigs exposed to either air (open triangles) or cigarette smoke (CS; solid triangles) for 8 days. (C and D) Depolarization induced by capsaicin (n = 7 nonsmoker, 13 smoker) (C) or PGE2 (open squares, nonsmoker, n = 9; solid squares, smoker, n = 10) (D) of isolated vagus nerve taken from human smokers and nonsmokers (with no other respiratory disease). Data are presented as mean ± SE. *P < 0.05 as determined by Mann-Whitney U test.
Figure 4.
[Ca2+]i flux in guinea pig airway-terminating vagal ganglia neurons. Intracellular calcium increases induced by (A and C) capsaicin or (B and D) prostaglandin E2 (PGE2) in isolated airway-terminating (A and B) jugular or (C and D) nodose ganglia neurons taken from guinea pigs exposed to either air (open symbols) or cigarette smoke (CS; solid symbols) for 8 days. The percentage displayed denotes the proportion of neurons recorded from where the responses of the Δ[Ca2+]i was at least 10% of the internal control response. Data are presented as the mean ± SE; note different y-axis scales for [Ca2+]i flux with capsaicin application (A and C). *P < 0.05 as determined by Mann-Whitney U test. Representative traces (F340/F380 ratio, y-axis scale) of the data in each panel are shown in the respective inset graphs. These representative traces show [Ca2+]i recorded with Fura2 for airway neurons from air- (black line) or CS-exposed (red line) guinea pigs, where application of either capsaicin (1 μM) or PGE2 (10 μM) is indicated by the black bar. AUC = area under the curve.
Responses of human vagus nerve from smokers and nonsmokers to capsaicin and PGE2
Capsaicin responses were enhanced (from 0.13 ± 0.01 to 0.19 ± 0.01 mV) and PGE2 responses were diminished (from 0.1 ± 0.02 to 0.02 ± 0.01 mV) in human vagus obtained from smoking donors compared with nonsmoking donors (Figures 3C and 3D). For characteristics of donors, see Table E1.
Discussion
In recent years the role of airway innervation and how this may be altered in clinical disease has received very little attention. This study demonstrates for the first time that patients with COPD exhibit specific patterns of cough responses to inhaled irritants that are not only significantly different from healthy control subjects, but also distinct from those seen in healthy smokers, patients with asthma, and patients with refractory chronic cough, providing evidence for disease-specific changes in airway nerve function and the existence of neurophenotypes in respiratory patients that have previously gone unrecognized. Moreover, this pattern of neuronal dysfunction, characterized by enhanced responses to capsaicin and diminished responses to PGE2, can be observed in human vagal tissue and modeled in animals using subchronic CS exposure. Both in vitro and in vivo experiments show responses consistent with those seen in patients with COPD and provide evidence that CS is capable of inducing phenotypic switches in airway nerve function.
In agreement with other studies we demonstrated enhanced cough to capsaicin challenge following CS exposure (17, 24–28). Our data also suggest that CS exposure produces qualitative and quantitative changes in the responses of airway afferent nerves. For example, there were profound increases in the amplitude of the capsaicin responses particularly in jugular neurons. Interestingly, nodose ganglion neuron cell bodies from air-exposed animals were relatively less responsive to capsaicin; however, CS exposure was associated with a significant increase in the proportion of neurons capable of responding (112.5% increase in responding neurons in nodose compared with 42.8% in the jugular), consistent with a phenotypic switch, most likely caused by an increased expression or functionality of TRPV1 channels. The polymodal nature of the TRPV1 channel suggests that this will be accompanied by a substantial expansion in the range of stimuli to which nodose afferent fibers will respond, and thus a significant shift in the properties of these nerves. Although determination of the precise molecular mechanisms underlying these considerable alterations in nerve function was beyond the scope of these experiments, changes in levels of channel expression, membrane insertion, phosphorylation, and glycosylation could all explain the alterations in responses observed. However, many of the techniques used to assess these potential mechanisms require the availability of appropriate tools (e.g., selective antibodies) to study the TRPV1 protein that are currently unavailable, especially for use in guinea pigs.
In marked contrast to capsaicin, the responses to PGE2 were lost with CS exposure, suggesting that although PGE2 evokes cough through PGE2 receptor 3 (EP3) activation and the subsequent engagement of TRP channels, such as TRPV1 (4), regulation of neuronal TRPV1 and EP3 receptors is independent. It is interesting to note that increased levels of endogenously produced PGE2 have been reported in the exhaled breath of ex-smokers with COPD compared with healthy ex-smokers (29) and in the sputum of current smokers both with and without COPD compared with healthy control subjects (30). PGE2 levels may also increase with increasing COPD stage (31). Thus reduced neuronal responses to PGE2 may result from down-regulation of EP3 receptors as a consequence of chronic exposure in the airways of smokers and patients with COPD.
Evidence of similar phenotypic switching of airway nerve function has previously been reported in guinea pigs sensitized and exposed to ovalbumin, finding increased gene expression of TRPV1 channels on single-cell polymerase chain reaction of airway nodose neurons innervating the trachea (32, 33). Although cough responses were not explored in that particular study, other investigators have reported heightened cough to inhaled capsaicin in guinea pigs exposed to allergen (34), but whether these findings translate to responses in humans remains unclear. The only human study assessing these mechanisms failed to demonstrate any change in capsaicin cough responses following house dust mite extract exposure in patients with asthma allergic to house dust mite (35). Importantly, in this study, we have demonstrated that functional changes in the cough reflex accompany CS-induced modifications of neuronal phenotype, and these translate to comparable changes in human nerve tissue and cough responses in patients with COPD.
This study is consistent with previous studies that have identified an increase in cough reflex sensitivity to capsaicin in patients with COPD (36, 37). However, this study has demonstrated for the first time that different airway diseases exhibit different patterns of sensitization/desensitization to a range of irritant stimuli, representing distinct neurophenotypes both between and within diagnostic groupings. This is of potential importance when testing novel interventions targeting neuronal sensitization aimed at improving symptoms including cough. If peripherally acting drugs are to be effective, these need to take account of these different neurophenotypes to identify subject groups in whom neuronal dysfunction might be the most relevant drivers of their symptoms.
This study has some limitations. We used the established C5 endpoint to assess cough in our clinical study; however, for capsaicin we have recently shown that continuing cough challenges beyond the C5 may provide better information for discriminating health from disease (38), and may also provide endpoints more readily compared with those used in animal studies. This study suggests there is value in exploring similar extended challenge protocols with other irritants beyond the standard endpoint. Additionally, our clinical study was only powered to detect differences of at least one doubling dose between groups and therefore cannot detect more subtle changes in cough responses. Healthy smokers and patients with asthma tended to have lower average capsaicin C5 values, whereas patients with COPD tended toward lower citric acid C5, but a larger sample size would have been needed for these differences to reach statistical significance. Of note, guinea pigs exposed to CS have been shown to exhibit enhanced cough responses to citric acid in previous studies (24). Finally, we were unable to include bradykinin in our panel of challenge agents. Although previous studies had demonstrated that inhaled bradykinin evoked coughing (39, 40), we were unable to obtain sufficiently high concentrations manufactured to good manufacturing practices standards.
To conclude, cigarette smoking is responsible for most cases of COPD. We have provided insights into the mechanisms by which CS induces neuronal dysfunction in an animal model and demonstrated how these translate to observations in subjects with established COPD. Additionally, our data support the concept of disease-specific neurophenotypes in airway diseases.
Acknowledgments
Acknowledgment
The authors thank all the subjects who participated in this study and also the National Institute of Health Research, South Manchester Respiratory & Allergy Clinical Research Facility who supported the clinical study.
Footnotes
Preclinical studies supported by a project grant from the Medical Research Council (MR/K020293/1; S.A.M. and E.D.) and by the North West Lung Centre Charity (M.A.W.). The human vagus experiments in this study were undertaken with the support of the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. Clinical study supported by Medical Research Council Clinician Scientist Award G0701918, Moulton Charitable Trust Project Grant, and performed with the support of the National Institute for Health Research South Manchester Clinical Research Facility.
Author Contributions: Conception and design, J.A.S., M.A.B., and M.G.B. Data generation and analysis, S.K., M.A.W., R.D., J.C., K.H., E.D., A.K., S.A.M., S.B., M.A.B., and J.A.S. Drafting of the paper, J.A.S., M.G.B., M.A.B., M.A.W., and S.K. Provided intellectual input and advice that was taken into consideration in the drafting of the manuscript, A.W. All authors reviewed the manuscript and approved the final draft.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201508-1602OC on January 7, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ford AC, Forman D, Moayyedi P, Morice AH. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61:975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janson C, Chinn S, Jarvis D, Burney P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J. 2001;18:647–654. doi: 10.1183/09031936.01.00098701. [DOI] [PubMed] [Google Scholar]
- 3.Sumner H, Woodcock A, Kolsum U, Dockry R, Lazaar AL, Singh D, Vestbo J, Smith JA. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:943–949. doi: 10.1164/rccm.201211-2000OC. [DOI] [PubMed] [Google Scholar]
- 4.Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- 6.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. J Physiol. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol. 1996;491:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol (1985) 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- 9.Mukhopadhyay I, Kulkarni A, Aranake S, Karnik P, Shetty M, Thorat S, Ghosh I, Wale D, Bhosale V, Khairatkar-Joshi N. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One. 2014;9:e97005. doi: 10.1371/journal.pone.0097005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2006;291:R454–R463. doi: 10.1152/ajpregu.00862.2005. [DOI] [PubMed] [Google Scholar]
- 11.Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 12.Khalid S, Dockry R, Holt K, Sumner H, Valdramidou D, Birrell MA, Belvisi MG, Woodcock A, Smith JA. Cough responses to tussive agents in health and disease [abstract] Thorax. 2011;66:S139. [Google Scholar]
- 13.Wortley MA, Grace MS, Dubuis ED, Maher SA, Khalid S, Smith JA, Birrell MA, Belvisi MG. Changes in vagal afferent responses and cough reflex sensitivity to PGE2 in smokers and COPD patients and in a guinea pig cigarette-smoke exposure model [abstract] Am J Respir Crit Care Med. 2012;185:A3565. [Google Scholar]
- 14.McGuinness K, Holt K, Smith J. Validation of the VitaloJAK 24hour ambulatory cough monitor [abstract] Thorax. 2012;67:A131. [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010;1:94–99. doi: 10.4103/0976-500X.72351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birrell MA, Bonvini SJ, Dubuis E, Maher SA, Wortley MA, Grace MS, Raemdonck K, Adcock JJ, Belvisi MG. Tiotropium modulates transient receptor potential V1 (TRPV1) in airway sensory nerves: a beneficial off-target effect? J Allergy Clin Immunol. 2014;133:679–687.e9. doi: 10.1016/j.jaci.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubuis E, Wortley MA, Grace MS, Maher SA, Adcock JJ, Birrell MA, Belvisi MG. Theophylline inhibits the cough reflex through a novel mechanism of action. J Allergy Clin Immunol. 2014;133:1588–1598. doi: 10.1016/j.jaci.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher SA, Birrell MA, Belvisi MG. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med. 2009;180:923–928. doi: 10.1164/rccm.200903-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubuis E, Grace M, Wortley MA, Birrell MA, Belvisi MG. Harvesting, isolation, and functional assessment of primary vagal ganglia cells. Curr Protoc Pharmacol. 2013;62:Unit 12.15. doi: 10.1002/0471141755.ph1215s62. [DOI] [PubMed] [Google Scholar]
- 20.Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, Maher SA, Freund-Michel V, Morice AH. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith J, Owen E, Earis J, Woodcock A. Cough in COPD: correlation of objective monitoring with cough challenge and subjective assessments. Chest. 2006;130:379–385. doi: 10.1378/chest.130.2.379. [DOI] [PubMed] [Google Scholar]
- 22.Smith J, Owen E, Earis J, Woodcock A. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117:831–835. doi: 10.1016/j.jaci.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Marsden PA, Smith JA, Kelsall AA, Owen E, Naylor JR, Webster D, Sumner H, Alam U, McGuinness K, Woodcock AA. A comparison of objective and subjective measures of cough in asthma. J Allergy Clin Immunol. 2008;122:903–907. doi: 10.1016/j.jaci.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Lewis CA, Ambrose C, Banner K, Battram C, Butler K, Giddings J, Mok J, Nasra J, Winny C, Poll C. Animal models of cough: literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm Pharmacol Ther. 2007;20:325–333. doi: 10.1016/j.pupt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Bergren DR. Chronic tobacco smoke exposure increases cough to capsaicin in awake guinea pigs. Respir Physiol. 2001;126:127–140. doi: 10.1016/s0034-5687(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson JA, Zackrisson C, Lundberg JM. Hyperresponsiveness to tussive stimuli in cigarette smoke-exposed guinea-pigs: a role for capsaicin-sensitive, calcitonin gene-related peptide-containing nerves. Acta Physiol Scand. 1991;141:445–454. doi: 10.1111/j.1748-1716.1991.tb09105.x. [DOI] [PubMed] [Google Scholar]
- 27.Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, Bonham AC. Passive smoke effects on cough and airways in young guinea pigs: role of brainstem substance P. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- 28.Lee LY, Gu Q, Lin YS. Effect of smoking on cough reflex sensitivity: basic and preclinical studies. Lung. 2010;188:S23–S27. doi: 10.1007/s00408-009-9191-1. [DOI] [PubMed] [Google Scholar]
- 29.Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58:585–588. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Profita M, Sala A, Bonanno A, Riccobono L, Ferraro M, La Grutta S, Albano GD, Montalbano AM, Gjomarkaj M. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. Am J Physiol Lung Cell Mol Physiol. 2010;298:L261–L269. doi: 10.1152/ajplung.90593.2008. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13:1014–1021. doi: 10.1111/j.1440-1843.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 32.Lieu TM, Myers AC, Meeker S, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol. 2012;302:L941–L948. doi: 10.1152/ajplung.00366.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang A, Uchida Y, Nomura A, Iijima H, Dong F, Zhang MJ, Hasegawa S. Effects of airway inflammation on cough response in the guinea pig. J Appl Physiol (1985) 1998;85:1847–1854. doi: 10.1152/jappl.1998.85.5.1847. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Fujimura M, Tachibana H, Myou S, Kasahara K, Yasui M. Characterization of increased cough sensitivity after antigen challenge in guinea pigs. Clin Exp Allergy. 2001;31:474–484. doi: 10.1046/j.1365-2222.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 35.Minoguchi H, Minoguchi K, Tanaka A, Matsuo H, Kihara N, Adachi M. Cough receptor sensitivity to capsaicin does not change after allergen bronchoprovocation in allergic asthma. Thorax. 2003;58:19–22. doi: 10.1136/thorax.58.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanc FX, Macedo P, Hew M, Chung KF. Capsaicin cough sensitivity in smokers with and without airflow obstruction. Respir Med. 2009;103:786–790. doi: 10.1016/j.rmed.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Hilton EC, Baverel PG, Woodcock A, Van Der Graaf PH, Smith JA. Pharmacodynamic modeling of cough responses to capsaicin inhalation calls into question the utility of the C5 end point. J Allergy Clin Immunol. 2013;132:847–855.e1–e5. doi: 10.1016/j.jaci.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Choudry NB, Fuller RW, Pride NB. Sensitivity of the human cough reflex: effect of inflammatory mediators prostaglandin E2, bradykinin, and histamine. Am Rev Respir Dis. 1989;140:137–141. doi: 10.1164/ajrccm/140.1.137. [DOI] [PubMed] [Google Scholar]
- 40.Fuller RW, Dixon CM, Cuss FM, Barnes PJ. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am Rev Respir Dis. 1987;135:176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]