To the Editor:
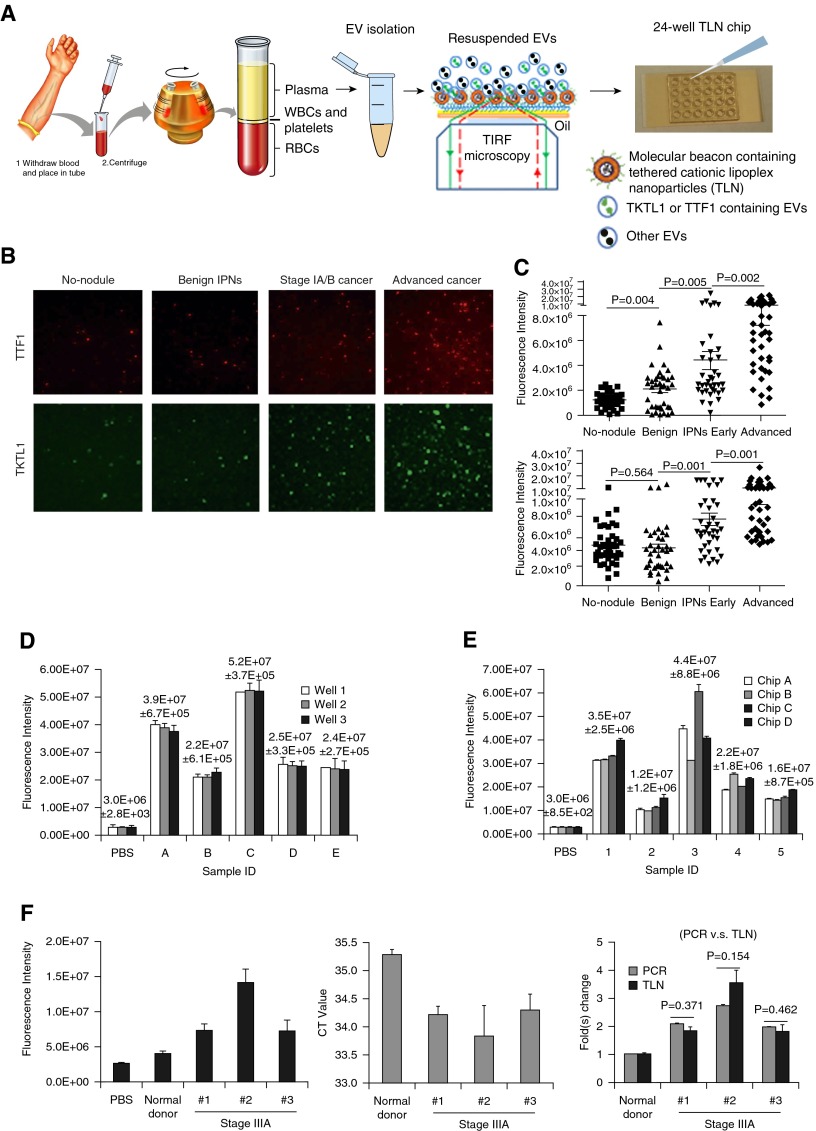
Lung cancer remains the leading cause of cancer-related deaths (1, 2). Low-dosage computerized tomography scanning can reduce lung cancer mortality in high-risk smokers (3); however, this type of scan is also accompanied by high false-positive rates. In addition, cost, concerns regarding overdiagnosis, and cumulative radiation exposure remain points of concern. Affordable and complementary noninvasive testing such as blood-based biomarkers could potentially increase the accuracy of early diagnosis of lung cancer. Several detection assays are currently being evaluated, including early CDT-lung (4), microRNA test (5), and a plasma protein classifier (6). Here, we describe a newly developed tethered lipoplex nanoparticle (TLN) biochip (7) that can both capture circulating extracellular vesicles (EVs) and detect RNA contents. Because many microRNAs in circulating EVs have been investigated as lung cancer biomarkers, with mixed performance and inconsistent results (8–11), here we chose to explore mRNA targets in blood EVs, which have not been well investigated for biomarker application. We first conducted next-generation sequencing to profile circulating RNA in plasma from surgically proven early-stage adenocarcinoma of the lung (n = 7) and benign granulomas of the lung (n = 10). We designed a molecular beacon for transketolase 1 (TKTL1), a glucose regulation gene identified from the next-generation sequencing study, as the most up-regulated mRNA for testing in our TLN biochip. We also designed a molecular beacon for thyroid transcription factor 1 (TTF1), a well-known up-regulated mRNA in lung cancer tissue (7–9). We tested these two mRNAs, using our TLN biochip to assess their feasibility as an assay for lung nodule assessment. Figure 1A shows the TLN concept and a 24-well TLN biochip on a glass slide. Individual molecular beacons (MBs) for the two mRNA targets were designed and encapsulated in cationic liposomal nanoparticles. These cationic lipoplex nanoparticles were tethered on the biochip, which can capture negatively charged EVs by electrical static interactions to form a larger nanoscale complex. This lipoplex–EV fusion leads to mixing of RNAs and MBs within the nanoscale confinement near the biochip interface. Total internal reflective fluorescence (TIRF) microscopy is capable of detecting a single biomolecule and measures signals smaller than 300 nm near the interface, which is where the tethered liposomal nanoparticles locate. The high sensitivity and near-interface detection makes TIRF microscopy a perfect combination with our TLN biochip for detecting the genetic materials in EVs as biomarkers. The well-to-well technical repeatability on the same chip revealed variation of 1–5%, as shown in Figure 1D, whereas the chip-to-chip technical repeatability revealed variation of 5–20% for most samples, as shown in Figure 1E.
Figure 1.
(A) Schematic demonstration of the procedure for cationic tethered lipoplex nanoparticle (TLN) assay. Extracellular vesicles (EVs) in plasma/serum were condensed by an exosome isolation kit and loaded onto the 24-well TLN biochip. EV transketolase 1 (TKTL1) and thyroid transcription factor 1 (TTF1) mRNAs in plasma from patients with lung cancer were detected using total internal reflective fluorescence (TIRF) microscopy. (B) Representative TIRF images of TTF1 and TKTL1 EV mRNA expression in the four patient cohorts. Images of the same size (30 × 30 μm) were cropped from original TIRF images and enlarged to the same size (80 × 80 μm), as shown. (C) Fluorescence intensities of EV TTF1 (top) and TKTL1 (bottom) mRNA expression calculated by Metlab software in different cohorts. (D) Well-to-well technical repeatability of TTF1 expression on the same chip (three wells each for five New York University Langone Medical Center plasma samples from patients with lung cancer). (E) Chip-to-chip technical repeatability of TTF1 expression on four chips for five The Ohio State University plasma samples from patients with lung cancer. (F) Comparison of TKTL1 mRNA expression by TLN and quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR). (Left) TKTL1 fluorescence intensities obtained by TLN biochip for one normal donor and three patients with stage IIIA lung cancer (20 μl plasma sample volume). (Middle) Cycle threshold (CT) values of TKTL1 mRNA detected by qRT-PCR for the same normal donor and patients with lung cancer (1 ml plasma was used for total EV RNA extraction). (Right) Comparison of TKTL1 mRNA fold changes by qRT-PCR and TLN assay. Data were normalized to the normal donor sample and presented as mean ± SD. Student’s t test was performed between different cohorts, and P < 0.05 was considered statistically significant. Error bars on all TLN data in D–F were averaged over 100 TLN-TIRF images; qRT-PCR data in F were from three independent experiments. IPN = indeterminate pulmonary nodule; PBS = phosphate-buffered saline; RBCs = red blood cells; WBCs = white blood cells.
Ethylenediaminetetraacetic acid plasma samples stored at −80°C from 38 individuals with benign lung nodules, 38 early stage I adenocarcinomas, 40 late-stage adenocarcinomas, and 40 patients without lung nodules were provided by the New York University Langone Medical Center. Figures 1B and 1C show representative TLN-TIRF images and bar charts, respectively, of EV TTF1 and TKTL1 expression for all four cohorts (N = 156). TTF1 showed an upward trend between cohorts with and without cancer and between patients with stage IA/B and advanced adenocarcinoma, whereas TKTL1 showed concentration differences between cohorts with and without cancer, with little difference between individuals with or without benign nodules. Because both TTF1 and TKTL1 were up-regulated in malignant nodules, overlap between the two mRNA expressions was observed. There was a distinction between patients with advanced cancer and nonpatients; however, there was overlap between patients with stage IA/B lung cancer and individuals with benign nodules.
The major TLN assay variation was attributable to the EV isolation from plasma samples. We found that sample volume had an effect on assay repeatability. The chip-to-chip variation could be greater than 20% when we used 20 μl plasma for EV isolation. When the volume was increased from 20 to 80 μl plasma, this variation was reduced, as shown in Figure 1E. We also found that the selection of a proper EV isolation kit and its operation protocol could affect the assay repeatability. A higher kit to sample ratio (1:3, instead of the manufacturer-suggested 1:4) can greatly reduce the sample preparation–induced assay variation.
The quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) analysis of cohorts with benign lung lesions and stage IA/B adenocarcinomas failed to show differences in TTF1 and TKTL1 concentrations as a result of very low target mRNA contents for PCR-based detection (high cycle threshold values), even when the volume of plasma used was increased from 20 to 200 μl. Although mRNAs have been routinely detected in tissue and cells by qRT-PCR, their detection in circulating EVs has been more challenging because mRNAs present in EVs are a mixture of intact and fragmented transcripts (12, 13). Because the qRT-PCR assay is designed to amplify and detect a larger portion of the transcripts (usually 100–150 nucleotides) and requires at least two sites for PCR primer recognition, the presence of smaller fragmented transcripts would interfere with the amplification process and require more template. MBs, in contrast, hybridize to 20–30 nucleotides of a specific mRNA, so it may detect intact and larger and smaller fragments of mRNA targets in EVs with only 20 μl plasma (7, 14). Figure 1F shows that both qRT-PCR (1 ml plasma) and the TLN (20 μl plasma) assay could provide similar information on TKTL1 levels in plasma EVs for blood samples from patients with late-stage lung cancer. However, TTF1 was still not detectable by qRT-PCR because of its much lower concentration in EVs.
The results from our TLN assay demonstrated concentration differences of TTF1 and TKTL1 targets between cohorts, particularly patients with or without lung cancer. We show that using the TLN biochip to detect EVs containing mRNA targets may be a feasible approach for the detection of RNA transcripts in circulation. To use the new TLN biochip as a viable tool to further develop EV-based blood biomarkers for cancer diagnosis, additional mRNA, microRNA, and long noncoding RNA targets will need to be identified and added to the TTF1/TKTL1 panel to enhance the performance. Because the TLN biochip is a multiwell device, multiplexing of many RNA targets can be easily achieved by placing different MBs in different wells. Larger-scale validation studies with larger patient cohorts at multiple sites also must be performed to further support a conclusion that EVs containing RNA targets in blood can serve as a viable biomarker in lung cancer. This new technology may potentially complement existing clinical assays and decrease the use of expensive and invasive testing.
Acknowledgments
Acknowledgment:
The authors acknowledge excellent laboratory support from David Baxter (Institute for Systems Biology) and Kelsey Scherler (Institute for Systems Biology).
Footnotes
Supported by grants from the National Cancer Institute, National Institutes of Health (R21 CA-179403), National Science Foundation (EEC-0914790), and Worly Lung Cancer Early Detection Research Fund to the Ohio State University, and the Early Detection Research Network, National Institutes of Health to the New York University Thoracic Oncology Research Laboratories (U01 CA-111295), U.S. Department of Defense research contracts W911NF-10-2-0111 and HDTRA1-13-C-0055 to the Institute for Systems Biology.
Author Contributions: L.J.L. and S.P.N.-S. initiated and designed the work; Z.Y. conducted tethered lipoplex nanoparticle assay with support from J.M. and K.J.K.; M.R. conducted polymerase chain reaction analysis; K.W. led next-generation sequencing with support from T.-K.K.; X.W., Y.H., and K.W. conducted RNAseq experiments; K.S. conducted immunohistochemistry; H.I.P. provided patient samples with support from C.G. and W.R.; and L.J.L. and Z.Y. wrote the manuscript with input from S.P.N.-S., K.W., L.Y., J.M., and H.I.P.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spira A, Ettinger DS. Extensive-stage small-cell lung cancer. Semin Surg Oncol. 2003;21:164–175. doi: 10.1002/ssu.10034. [DOI] [PubMed] [Google Scholar]
- 3.Patel AR, Wedzicha JA, Hurst JR. Reduced lung-cancer mortality with CT screening. N Engl J Med. 2011;365:2035. doi: 10.1056/NEJMc1110293. [DOI] [PubMed] [Google Scholar]
- 4.Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, Chapman CJ, Allen J, Wood WC, Sewell HF, et al. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila) 2011;4:1126–1134. doi: 10.1158/1940-6207.CAPR-10-0328. [DOI] [PubMed] [Google Scholar]
- 5.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C, Maisonneuve P, et al. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 6.Vachani A, Hammoud Z, Springmeyer S, Cohen N, Nguyen D, Williamson C, Starnes S, Hunsucker S, Law S, Li XJ, et al. Clinical utility of a plasma protein classifier for indeterminate lung nodules. Lung. 2015;193:1023–1027. doi: 10.1007/s00408-015-9800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Kwak KJ, Agarwal K, Marras A, Wang C, Mao Y, Huang X, Ma J, Yu B, Lee R, et al. Detection of extracellular RNAs in cancer and viral infection via tethered cationic lipoplex nanoparticles containing molecular beacons. Anal Chem. 2013;85:11265–11274. doi: 10.1021/ac401983w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazzoli R, Buttitta F, Di Nicola M, Malatesta S, Marchetti A, Rom WN, Pass HI. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J Thorac Oncol. 2013;8:1156–1162. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 10.Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, Liu Y, Chen X, Zhang CY, Gu H, et al. MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. doi: 10.1186/s12943-015-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing L, Su J, Guarnera MA, Zhang H, Cai L, Zhou R, Stass SA, Jiang F. Sputum microRNA biomarkers for identifying lung cancer in indeterminate solitary pulmonary nodules. Clin Cancer Res. 2015;21:484–489. doi: 10.1158/1078-0432.CCR-14-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 13.Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S, Zacharias W, Hao H, McMasters KM. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santangelo PJ, Nix B, Tsourkas A, Bao G. Dual FRET molecular beacons for mRNA detection in living cells. Nucleic Acids Res. 2004;32:e57. doi: 10.1093/nar/gnh062. [DOI] [PMC free article] [PubMed] [Google Scholar]